Abstract
Aim:
We sought to investigate the effect of berbamine on the growth of human multiple myeloma cell line KM3 and elucidate the mechanism of its action.
Methods:
MTT assay was used to determine the inhibitory effect of berbamine alone or combined with chemotherapeutic drugs. Flow cytometry was performed to characterize cell cycle profile in response to berbamine treatment. Western blot was used to measure the protein levels of p65, IκB Kinase α (IKKα), TNFAIP3 (A20), IκBα, p-IκBα, cyclinD1, Bcl-2, BAX, Bcl-xL, Bid, and survivin.
Results:
Berbamine inhibits the proliferation of KM3 cells in a dose- and time-dependent manner. Combination of berbamine with dexamethasone (Dex), doxorubicin (Dox) or arsenic trioxide (ATO) resulted in enhanced inhibition of cell growth. Flow cytometric analysis revealed that KM3 cells were arrested at G1 phase and apoptotic cells increased from 0.54% to 51.83% for 36 h. Morphological changes of cells undergoing apoptosis were observed under light microscope. Berbamine treatment led to increased expression of A20, down-regulation of IKKα, p-IκBα, and followed by inhibition of p65 nuclear localization. As a result, NF-κB downstream targets such as cyclinD1, Bcl-xL, Bid and survivin were down-regulated.
Conclusion:
Berbamine inhibits the growth of KM3 cells by inducing G1 arrest as well as apoptosis. Berbamine blocks NF-κB signaling pathway through up-regulating A20, down-regulating IKKα, p-IκBα, and then inhibiting p65 nuclear translocation, and resulting in decreased expression of the downstream targets of NF-κB. Our results suggest that berbamine is a novel inhibitor of NF-κB activity with remarkable anti-myeloma efficacy.
Keywords: berbamine, multiple myeloma, nuclear factor-kappa B, apoptosis
Introduction
Multiple myeloma (MM) is the second most common hematological malignancy characterized by abnormal proliferation of plasma cells in bone marrow secreting monoclonal immunoglobulins. Prior treatment options for MM include chemotherapy, stem cell transplantation and immunotherapy with interferon-gamma1. Recently, novel agents such as bortezomib, thalidomide and lenalidomide were approved for MM treatment and have improved the complete remission rate and patient outcome2, 3. However, MM remains incurable, and development of drug resistance or intolerable toxicity emerges as a major problem especially for patients with refractory or relapsed MM. Therefore, design, identification and validation of novel chemicals with therapeutic potential are clearly needed for MM treatment.
Berbamine, a natural small molecular compound, is distilled from Chinese traditional medicine Berberis amurensis, which has been shown to improve normal hematopoiesis and immune function of cancer patients over the past decades4, 5. Our former studies showed that berbamine down-regulates p210 bcr-abl oncoprotein in K562 cells, and induces apoptosis through a caspase-3-dependent pathway6. In addition, berbamine was highly effective in suppressing the growth of imatinib-resistant cell line K562 (K562-r), human acute promyelocytic leukemia cell line NB4 and T-cell leukemia cell line Jurkat. Therefore, berbamine appears to have broad anti-leukemia activities with complex mechanism of actions7, 8. However, its activity against multiple myeloma cells remains to be determined.
It has been well established that constitutive activation of NF-κB is implicated in MM cells survival, proliferation and drug resistance, and NF-κB signaling pathway serves as a promising target for MM therapy9. Currently, dexamethasone (Dex), arsenic trioxide (ATO) and bortezomib exert their anti-MM activities mainly through inhibiting cytoplasm-to-nucleus translocation of NF-κB10, 11. Given that NF-κB plays a crucial role in MM pathogenesis, we wonder if berbamine can suppress proliferation and induce apoptosis in MM cells through modulating NF-κB activity.
Materials and methods
Agents and antibodies
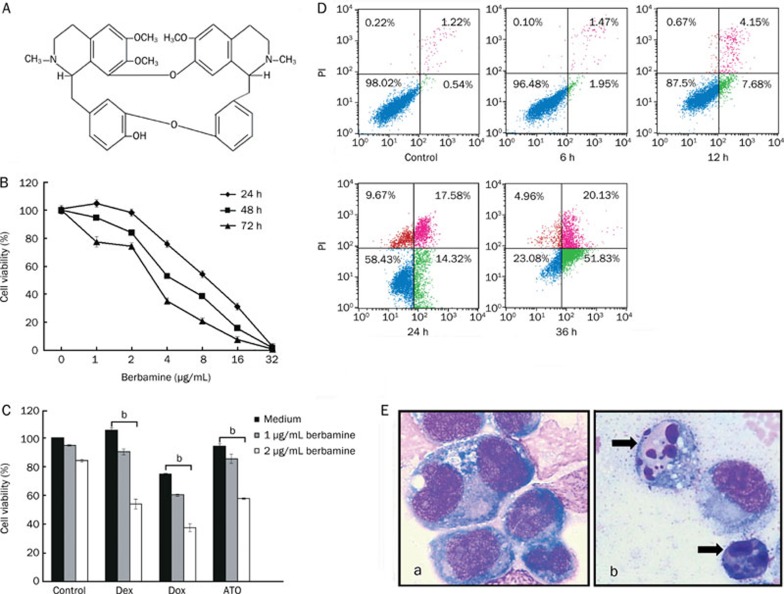
Berbamine (99% purity) is a natural compound from Berberis amurensis. The formula is presented in Figure 1A, and its molecular weight is 753.80. Berbamine was dissolved in phosphate-buffered saline (PBS) at a stock concentration of 1 mg/mL. Arsenic oxide stock solution (1 mg/mL) was stored at room temperature. Dexamethasone (Sigma, USA) was dissolved in DMSO and Doxorubicin(Dox) stock solution (ZheJiang HISUN Pharmaceutical Co Ltd, 5 mg/mL, in PBS) were stored at −20 °C. Mouse anti-p65, IκBα, Ikkα, A20, cyclin D1, Bcl-2, BAX, Bid, survivin, Histone 1 (H1) and β-actin were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Rabbit antibodies against Bcl-xL, p-IκBα were purchased from Cell Signaling Technology (Cell Signaling, USA).
Figure 1.
(A) The structural formula of berbamine (molecular weight: 753.80). (B) Berbamine inhibits the growth of KM3 cells. The experiment was done in triplicate and the cells were treated as described in Materials and methods. The total number of viable cells was determined by MTT assay. (C) Berbamine enhances the growth inhibitory effect of conventional chemotherapeutic agents. KM3 cells were cultured with control media and with 1 or 2 μg/mL berbamine in the absence or presence of Dex (1 μmol/L), Dox (0.25 μmol/L), and ATO (1 μmol/L) for 48 h and then subjected to MTT assay. B and C, data represent mean±SD of quadruplicate cultures. bP<0.05. (D) Berbamine induces cell apoptosis. KM3 cells were untreated or treated with 8 μg/mL berbamine for 6, 12, 24, and 36 h, respectively. Percentages of apoptotic cells (green color) were determined by staining with Annexin V and PI followed by flow cytometry. (E) Giemsa-Wright staining of KM3 cells cultured in absence (a) or presence of berbamine (b) for 24 h. Arrows indicate apoptotic cells.
Cell culture
Human multiple myeloma KM3 cells were obtained from Hematology Institute of Zhejiang University (Hangzhou, China). KM3 cells were cultured in RPMI 1640 medium (Invirogen, USA) supplemented with 10% fetal calf serum (FCS, Invirogen, USA), 2 mmol/L glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and incubated at 37 °C in a humidified atmosphere of 95% air/5% carbon dioxide.
Cell viability assay
The inhibitory effect of berbamine on growth of KM3 cells was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described earlier7. Briefly, KM3 cells (8×103 per well) were incubated with increasing concentrations of berbamine (1−32 μg/mL) for 24, 48, or 72 h and then pulsed with 20 μL of 5 mg/mL MTT for the last 4 h, 200 μL DMSO was then added to dissolve the formazan crystals. Dye absorbance in viable cells was measured at 570 nm, and then the inhibitory concentration of 50% (IC50) was calculated.
Effect of combined drug treatment on cell growth
To determine the cytotoxicity after exposure to berbamine alone or combined with other chemotherapeutic agents, KM3 cells were cultured with media containing 1 or 2 μg/mL berbamine in the absence or presence of Dex (1 μmol/L), Dox (0.25 μmol/L) and ATO (1 μmol/L) for 48 h, respectively. Cell growth was then assessed by MTT assay.
Cell cycle analysis and assessment of apoptosis
Flow cytometry (FCM) was used to determine the effect of berbamine on the cell cycle and apoptosis. Briefly, KM3 cells were treated with 4 μg/mL berbamine for the various times, fixed in 70% cold ethanol overnight, stained with 50 μg/mL propidium iodide (PI, Sigma, USA) for 30 min at 4 °C, and then assessed with a FACSCan flow cytometer (Becton Dickinson). G0–G1, S, and G2–M indicate the cell phases. Apoptosis was determined based on morphologic changes, briefly, KM3 cells were treated with 8 μg/mL Berbamine for 24 h, and cytospin slides were then prepared and stained with Giemsa-Wright staining as described previously. The cell morphology was evaluated under light microscope. To confirm the results of morphologic analysis, apoptotic cells were quantified by annexin V-FITC and PI double staining using Annexin-V-FLUOS staining kit (Immunotech, France), and apoptosis was then quantified by FCM.
Immunofluorescence assay
Berbamine-treated cells were fixed in freshly prepared 4% paraformaldehyde in PBS for 30 min at room temperature and then permeabilized with 0.2% Triton X-100 for 10 min. After blocking with 2% bovine serum albumin (BSA), slides were incubated with p65 antibody (dilution, 1:50) for overnight at 4 °C, washed 3 times in PBS-T and reacted with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (IgG, 1:100) for 1 h and counterstained for nuclei with 10 μg/mL 4,6-diamidino-2-phenylindole (DAPI) for 5 min. Stained slides were observed and photographed using a Zeiss Axiophot epifluorscence microscope (Oberkochen, Germany).
Western blot analysis
KM3 cells were treated with 8 μg/mL berbamine, and collected at 0, 6, 12, and 24 h, respectively. Total cellular protein was extracted using MPER mammalian protein extraction reagent (Pierce Chemical Co) according to the instruction manual. The nuclear extracts were prepared according to Schreiber et al12. Briefly, cells were washed and suspended in hypotonic lysis buffer containing protease inhibitors for 30 min, and then 10% Nonidet P-40 was added, shaking for 10 s. The homogenates were centrifuged and the nuclear pellets were re-suspended in ice-cold extraction buffer. After vigorously rocked at 4 °C for 15 min, the extracts were centrifuged, and supernatants containing nuclear extracts were secured. Total cellular proteins or nuclear protein extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to phenylmethlsulfonyl fluoride (PVDF) membrane (BioRad, USA), and immunoblotted with primary antibodies overnight at 4 °C. After three time washes with TBS-T, the membranes were probed with horseradish peroxidase(HRP)-conjugated secondary antibodies for 1 h at room temperature, and reacted with SuperSignal Weat Pico Chemiluminescent Substrate (Pierce Chemical Co).
Statistical analysis
The data were expressed as mean±standard deviation (SD) and analyzed by the SPSS 10.0 software to evaluate the statistical difference. Statistical significance was defined as P<0.05.
Results
Effects of Berbamine on the growth of KM3 cells
To determine whether berbamine has growth inhibitory effect on myeloma cells, KM3 cells were treated with berbamine at various concentrations for 24, 48, and 72 h, respectively, and then cell viability was assessed by MTT assays. As shown in Figure 1B, berbamine inhibit the growth of KM3 cells in a dose- and time-dependent manner, and the IC50 values were 8.17 μg/mL, 5.09 μg/mL, and 3.84 μg/mL for treatment of 24, 48, and 72 h, respectively. In contrast, IC50 value of berbamine for normal hematopoietic cells was 185.20 μg/mL at 48 h, as described previously6. Thus, these data suggest that berbamine specifically induces cytotoxicity in MM cells but not in normal cells.
Berbamine potentiated the anti-MM effect of chemotherapeutic agents
To determine whether low-dose berbamine could enhance the anti-MM effect of conventional chemotherapeutic drugs, KM3 cells were treated with 1 or 2 μg/mL berbamine combined with 1 μmol/L Dex, 0.25 μmol/L Dox, and 1 μmol/L ATO for 48 h, respectively. As shown in Figure 1C, Dex, Dox, ATO and berbamine inhibited cell growth minimally when used alone, cell viability from 74.8% to 105.1% respectively; however, these agents combined with 2 μg/mL berbamine led to dramatic growth inhibition, cell viability from 37.5% to 57.7% respectively. Taken together, these data suggested that berbamine could enhance the anti-MM effect of chemotherapeutic angents incluing Dex, Dox, and ATO.
Berbamine induces apoptosis of KM3 cells
We next examined whether berbamine-mediated anti-myeloma activity was associated with its ability to inducing apoptosis. Annexin V staining is a well-recognized marker to detect apoptotic cells. Flow cytometric analysis showed that KM3 cells underwent apoptosis in a time-dependent manner after exposing to 8 μg/mL berbamine. As shown in Figure 1D, the percentage of apoptotic cells was 0.54% at 0 h, 1.95% at 6 h, 7.68% at 12 h, 14.32% at 24 h, and increased to 51.83% at 36 h. After treatment with 8 μg/mL berbamine for 24 h, morphological changes such as chromatin condensation, nuclear fragmentation, and apoptotic body formation, which are characteristic of apoptosis, were also observed (Figure 1E).
Berbamine inhibits nuclear translocation of NF-κB
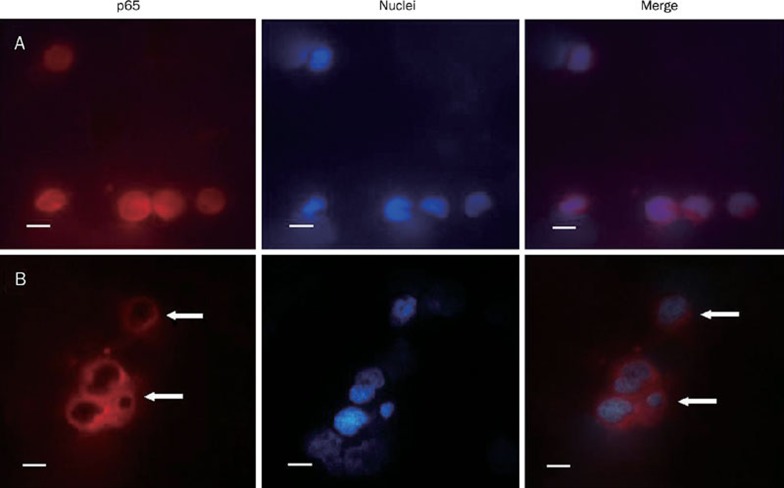
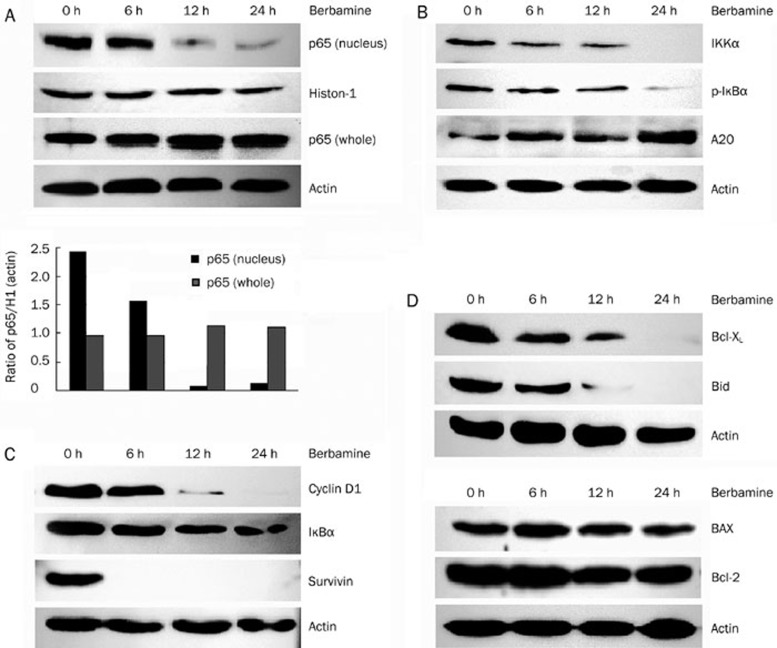
Since NF-κB is a survival determinant of myeloma cells, we reasoned that apoptosis of KM3 cell was due to blockade of NF-κB signaling pathway. To test this, immuno-fluorescence assay was performed to analyze the expression and localization of p65 subunit of NF-κB. The p65 was localized in both cytoplasm and nucleus in untreated KM3 cells. After treatment with 8 μg/mL berbamine for 24 h, p65 expression was decreased significantly in the nucleus, while its expression in the cytoplasm was little affected (Figure 2). Consistently, Western blot showed that p65 protein level was decreased in the nuclear fraction in a time-dependent fashion, while total p65 level was little if any changed (Figure 3A). Taken together, these data suggest that berbamine inhibits the nuclear translocation of p65 without affecting its total protein expression.
Figure 2.
Berbamine causes inhibition of translocation of p65 into the nucleus. KM3 cells were incubated with (B) or without (A) 8 μg/mL berbamine for 24 h and then analyzed by immunofluorescence assay (×40), p65 was stained red and nucleus was stained blue with DAPI. Scale bars, 10 μm.
Figure 3.
(A) Effect of berbamine on the constitutive expression of p65. KM3 cells were treated with 8 μg/mL berbamine for the indicated times, nuclear and whole cell lysates were prepared to check the level of p65 by Western blotting. (B) Effects of berbamine on IκBα phosphorylation. KM3 cells were treated with 8 μg/mL berbamine for the indicated times, and cytoplasmic extracts were prepared to check the level of A20, IKKα and p-IκBα by Western blotting. (C, D) Berbamine down-regulates the expression of NF-κB-regulated gene. KM3 cells were treated with 8 μg/mL berbamine for the indicated time, and cell extracts were prepared to check the level of cyclin D1, IκBα, survivin and Bcl-2 family proteins by Western blotting. Representative of one experiment, two additional studies yield equivalent results.
Berbamine inhibits phosphorylation of IκBα
IκB kinase α (IKKα) phosphorylates IκBα, a specific NF-κB inhibitor, which was then degraded through an ubiquitin-proteasome pathway to allow p65 translocate into the nucleus13. To gain insight into inhibition of p65 nuclear translocation by berbamine, IKKα and IκBα phosphorylation were examined by Western blot. We found that the expression of IKKα was dramatically decreased and phosphorylated IκBα (p-IκBα) was nearly abrogated 24 h after berbamine treatment (Figure 3B), whereas total IκBα was little changed (Figure 3C). A20, a deubiquitinating enzyme which reduces the amplitude of ubiquitin and thereby inhibits NF-κB signaling pathway, was also upregulated (Figure 3B). These findings suggest that berbamine inhibits the phosphorylation of IκBα through down-regulating IKKα and up-regulating A20.
Berbamine down-regulates the expression of NF-κB-regulated gene products
Once activated, NF-κB functions as a transcription factor to transactivate a set of target genes which are essential to regulate cell survival, proliferation and apoptosis14. To determine which NF-κB downstream targets are involved in berbamine-induced apoptosis, we used western blot to evaluate the protein level of some established NF-κB targets. As shown in Figure 3C and Figure 3D, upon treatment with 8 μg/mL berbamine for 24 h, Bcl-xL, Bid, cyclin D1 and survivin were down-regulated, while levels of IκBα, Bcl-2 and BAX were not affected markedly. These data suggest that berbamine induces apoptosis in myeloma cells through selectively inhibiting certain NF-κB-regulated anti-apoptotic gene expression.
Berbamine induces cell cycle arrest at G1 phase
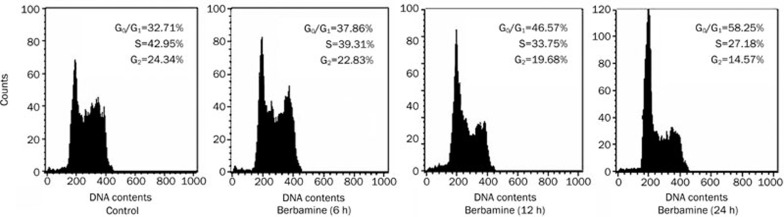
Since Cyclin D1 is required for the G1/S cell cycle transition, we sought to evaluate alteration in cell cycle progression when cyclin D1 was down-regulated. As expected, we found that the percentage of G1 phase were increased from 32.71% to 58.25%, and accordingly S phase were decreased from 42.95% to 27.18% in KM3 cells treated with 4 μg/mL berbamine for 24 h (Figure 4). Thus, these data demonstrate that berbamine inhibits the proliferation of myeloma cells by inducing G1/S phase arrest.
Figure 4.
Effect of berbamine on cell cycle progression. KM3 cells were untreated or treated with 4 μg/mL berbamine for 6, 12, and 24 h, respectively. Cells were washed, fixed, stained with PI, and then DNA histogram was analyzed by flow cytometry.
Discussion
Since NF-κB activation plays a key role in MM pathogenesis, agents targeting NF-κB have shown promising therapeutic efficacies in MM treatment15. For example, bortezomib, a 26S proteasome inhibitor capable of preventing proteasomal degradation of IκB, demonstrates remarkable anti-MM activity11. However, about 30% patients fail to respond to bortezomib and emergence of intolerable neurotoxicity is not uncommon in clinical setting16, 17. Such limitations highlight the urgent need for identification of novel compounds displaying potent anti-MM activities and less toxicities.
This report is to identify berbamine as a novel inhibitor of NF-κB with anti-MM activity. Berbamine alone preferentially induces growth inhibition and apoptosis in myeloma but not in normal blood cells, as evidenced by IC50 that is ∼50 folds lower for myeloma cells. Interestingly, we also demonstrated that, low-dose berbamine was able to enhance the anti-MM effects triggered by chemotherapeutic agents including Dex, Dox, and ATO. Therefore, incorporating berbamine into current chemotherapeutic protocols may serve as a promising strategy to overcome drug resistance and improve response rate in MM treatment.
Our results suggested that inhibition of NF-κB activity contributes to the cell growth inhibition and apoptosis induced by berbamine. The mechanism whereby berbamine inhibits NF-κB activity was further investigated. First, nuclear translocation of p65, a critical step required for NF-κB activation, was inhibited upon berbamine treatment. Next, we defined signaling events upstream p65, ie inhibition of IκB phosphorylation resulting from IKKα down-regulation and simultaneously up-regulation of another NF-κB inhibitor, A20. Whether differential expression of IKKα and A20 induced by berbamine represents distinct levels in regulation of NF-κB activity remains to be characterized. A20 is known to target receptor interacting protein (RIP) for proteasomal degradation and suppress IKK activation18. Therefore, it is likely that berbamine inhibits IKKα expression through A20-RIP interaction.
After translocated into the nucleus, the activated NF-κB binds to specific DNA sequence and activates transcription of its target genes19, 20, most of which are involved in cell proliferation and counteracting apoptosis. In this study, we found that NF-κB target genes, such as Bcl-xL, Bid, cyclin D1 and survivin are highly expressed in KM3 cells, indicating an anti-apoptosis role of NF-κB in myeloma. As a result, berbamine down-regulated Bcl-xL, Bid and survivin, which contributes to berbamine-induced apoptosis. Additionally, Berbamine induces cell cycle arrest in G1 phase through down-regulating the expression of cyclin D1. Thus, the molecular consequences of inhibiton of NF-κB are linked to the anti-MM activity conferred by berbamine.
In summary, our results provide evidence supporting the use of berbamine as a novel NF-κB inhibitor with remarkable anti-MM efficacy in vitro. Its effect on other MM cell lines and primary MM cells warrants further investigation. Importantly, the safety, pharmacokinetic properties and in vivo efficacy of berbamine should be addressed in animal models or phase I clinical trials. Designing chemically synthetic compounds based on berbamine may represent a promising strategy to develop novel anti-myeloma drugs.
Author contribution
Xiao-ying ZHAO designed research; Yun LIANG performed research; Rong-zhen XU contributed new analytical tools and reagents; Lei ZHANG analyzed data; Yun LIANG wrote the paper.
References
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:15–7. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002;2:927–37. doi: 10.1038/nrc952. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–18. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- Ge MZ, Liu X, Zhang Y. An experiment on immune protection unction of berbamine in mice injury by irradiation. Immunol J. 1998;4:238–40. [Google Scholar]
- Liu X, Zhou ZR. The effect of berbamine on the immunoregulation of BALB/c mice. J China Med Univ. 1996;25:229–31. [Google Scholar]
- Xu RZ, Dong QH, Yu YZ, Zhao XY, Gan XX, Wu D, et al. Berbanine: A novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity. Leuk Res. 2006;30:17–23. doi: 10.1016/j.leukres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Zhao XY, He ZW, Wu D, Xu RZ. Berbamine selectively induces apoptosis of human acute promyelocytic leukemia cells via surviving-mediated pathway. Chin Med J. 2007;120:802–6. [PubMed] [Google Scholar]
- Wei YL, Xu L, Liang Y, Xu XH, Zhao XY. Berbanine exhibits potent antitumor effects on imatinib-resistant CML cells in vitro and in vivo. Acta Pharmacol Sin. 2009;30:451–7. doi: 10.1038/aps.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, et al. NF-κB as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–47. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS., Jr Inhibition of NF-κB activity by thalidomide through suppression of IκB kinase activity. J Biol Chem. 2001;276:22382–7. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma. Cancer Res. 2001;61:3071–6. [PubMed] [Google Scholar]
- Feinman R, Koury J, Thames M, Barlogie B, Epstein J, Siegel DS. Role of NF-kappaB in the rescue of multiple myeloma cells from glucocorticoid-induced apoptosis by bcl-2. Blood. 1999;93:3044–52. [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–81. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-kappaB is the answer-role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–82. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Baldwin AS., Jr NF-KappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–9. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- San Miguel J, Bladé J, Boccadoro M, Cavenagh J, Glasmacher A, Jagannath S, et al. A practical update on the use of bortezomib in the management of multiple myeloma. Oncologist. 2006;11:51–61. doi: 10.1634/theoncologist.11-1-51. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–20. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- Hutti JE, Turk BE, Asara JM, Ma A, Cantley LC, Abbott DW. IκB Kinase β phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-κB Pathway. Mol Cell Biol. 2007;27:7451–61. doi: 10.1128/MCB.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Peet GW, Balzarano D, Li X, Massa P, Barton RW, et al. Novel NEMO/IκB kinase and NF-κB target genes at the Pre-B to immature B cell transition. J Biol Chem. 2001. 276. pp. 18579–90. [DOI] [PubMed]