Abstract
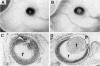
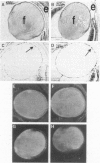
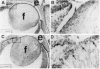
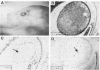
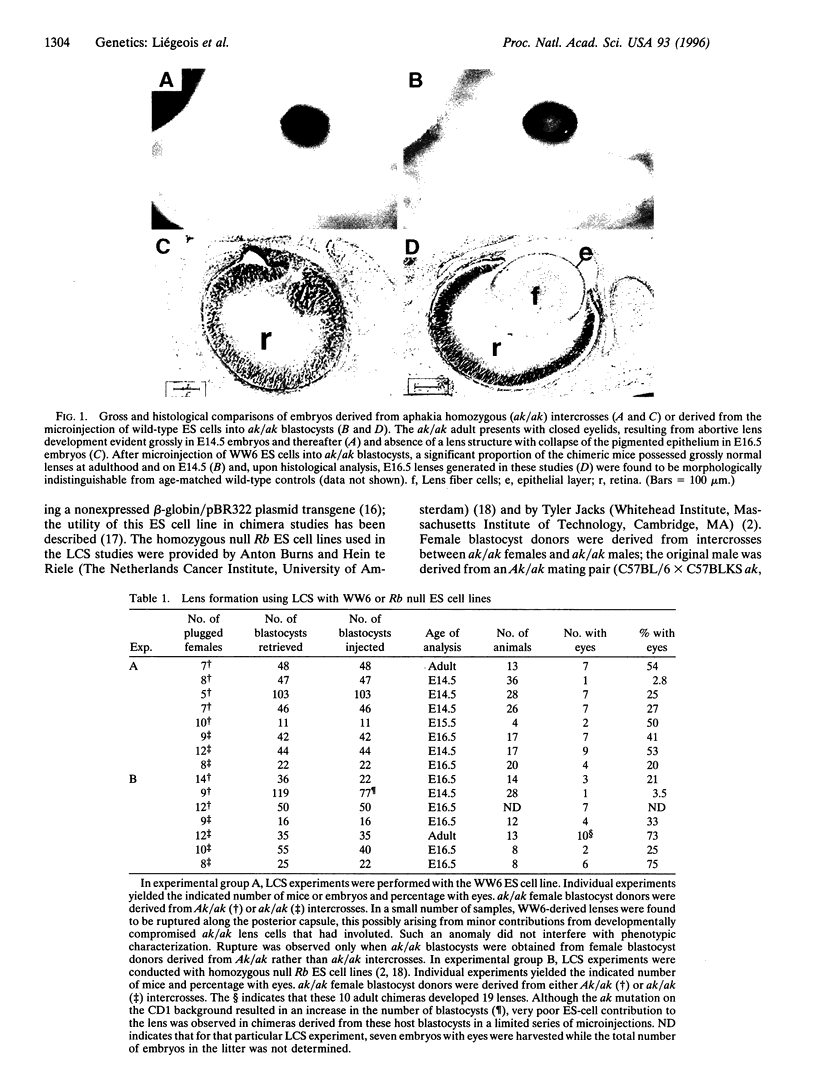
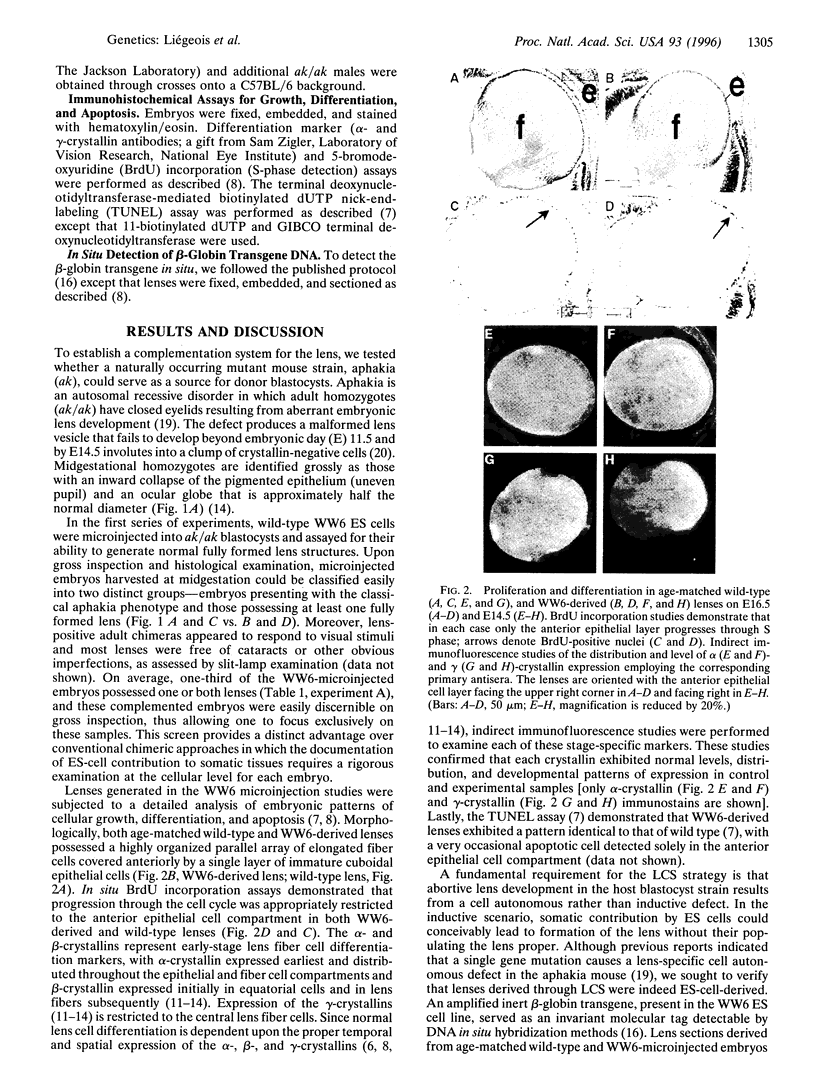
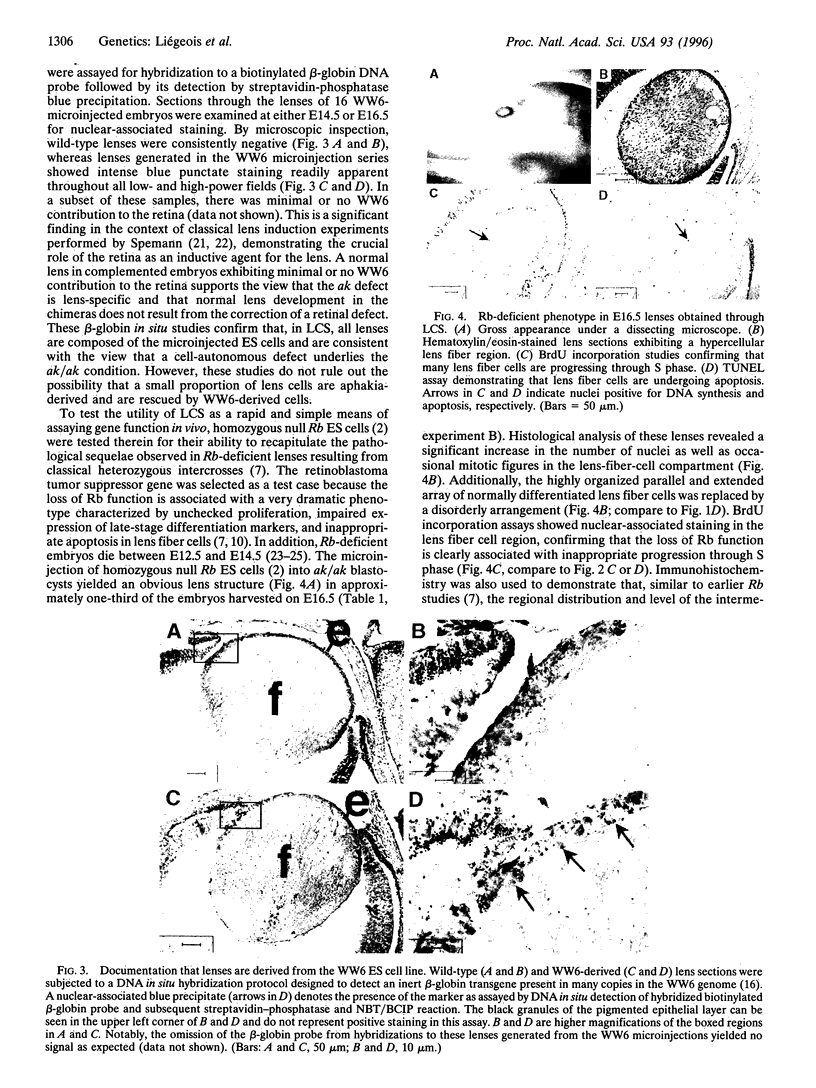
A genetic approach has been established that combines the advantages of blastocyst complementation with the experimental attributes of the developing lens for the functional analysis of genes governing cellular proliferation, terminal differentiation, and apoptosis. This lens complementation system (LCS) makes use of a mutant mouse strain, aphakia (ak), homozygotes of which fail to develop an ocular lens. We demonstrate that microinjection of wild-type embryonic stem (ES) cells into ak/ak blastocysts produces chimeras with normal ES-cell-derived lenses and that microinjection of Rb-/- ES cells generates an aberrant lens phenotype identical to that obtained through conventional gene targeting methodology. Our determination that a cell autonomous defect underlies the aphakia condition assures that lenses generated through LCS are necessarily ES-cell-derived. LCS provides for the rapid phenotypic analysis of loss-of-function mutations, circumvents the need for germ-line transmission of null alleles, and, most significantly, facilitates the study of essential genes whose inactivation is associated with early lethal phenotypes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen J., Lansford R., Stewart V., Young F., Alt F. W. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. R., Maandag E. R., van Roon M., van der Lugt N. M., van der Valk M., Hooper M. L., Berns A., te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992 Sep 24;359(6393):328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- Fromm L., Shawlot W., Gunning K., Butel J. S., Overbeek P. A. The retinoblastoma protein-binding region of simian virus 40 large T antigen alters cell cycle regulation in lenses of transgenic mice. Mol Cell Biol. 1994 Oct;14(10):6743–6754. doi: 10.1128/mcb.14.10.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Zou Y. R., Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993 Jun 18;73(6):1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Ioffe E., Liu Y., Bhaumik M., Poirier F., Factor S. M., Stanley P. WW6: an embryonic stem cell line with an inert genetic marker that can be traced in chimeras. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7357–7361. doi: 10.1073/pnas.92.16.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Chang C. Y., Hu N., Wang Y. C., Lai C. C., Herrup K., Lee W. H., Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992 Sep 24;359(6393):288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Lo C. W. Localization of low abundance DNA sequences in tissue sections by in situ hybridization. J Cell Sci. 1986 Mar;81:143–162. doi: 10.1242/jcs.81.1.143. [DOI] [PubMed] [Google Scholar]
- Maandag E. C., van der Valk M., Vlaar M., Feltkamp C., O'Brien J., van Roon M., van der Lugt N., Berns A., te Riele H. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 1994 Sep 15;13(18):4260–4268. doi: 10.1002/j.1460-2075.1994.tb06746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon K. A., Chepelinsky A. B., Khillan J. S., Overbeek P. A., Piatigorsky J., Westphal H. Oncogenesis of the lens in transgenic mice. Science. 1987 Mar 27;235(4796):1622–1628. doi: 10.1126/science.3029873. [DOI] [PubMed] [Google Scholar]
- McAvoy J. W. Induction of the eye lens. Differentiation. 1980;17(3):137–149. doi: 10.1111/j.1432-0436.1980.tb01091.x. [DOI] [PubMed] [Google Scholar]
- Morgenbesser S. D., Schreiber-Agus N., Bidder M., Mahon K. A., Overbeek P. A., Horner J., DePinho R. A. Contrasting roles for c-Myc and L-Myc in the regulation of cellular growth and differentiation in vivo. EMBO J. 1995 Feb 15;14(4):743–756. doi: 10.1002/j.1460-2075.1995.tb07053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenbesser S. D., Williams B. O., Jacks T., DePinho R. A. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 1994 Sep 1;371(6492):72–74. doi: 10.1038/371072a0. [DOI] [PubMed] [Google Scholar]
- Pan H., Griep A. E. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994 Jun 1;8(11):1285–1299. doi: 10.1101/gad.8.11.1285. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19(3):134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Varnum D. S., Stevens L. C. Aphakia, a new mutation in the mouse. J Hered. 1968 Mar-Apr;59(2):147–150. doi: 10.1093/oxfordjournals.jhered.a107667. [DOI] [PubMed] [Google Scholar]
- Williams B. O., Schmitt E. M., Remington L., Bronson R. T., Albert D. M., Weinberg R. A., Jacks T. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 1994 Sep 15;13(18):4251–4259. doi: 10.1002/j.1460-2075.1994.tb06745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Zwaan J. Immunofluorescent studies on aphakia, a mutation of a gene involved in the control of lens differentiation in the mouse embryo. Dev Biol. 1975 Jun;44(2):306–312. doi: 10.1016/0012-1606(75)90401-7. [DOI] [PubMed] [Google Scholar]
- Zwaan J. The appearance of alpha-crystallin in relation to cell cycle phase in the embryonic mouse lens. Dev Biol. 1983 Mar;96(1):173–181. doi: 10.1016/0012-1606(83)90320-2. [DOI] [PubMed] [Google Scholar]