SUMMARY
Multiple sensory cues emanating from humans are thought to guide blood-feeding female mosquitoes to a host. To determine the relative contribution of carbon dioxide (CO2) detection to mosquito host-seeking behavior, we mutated the AaegGr3 gene, a subunit of the heteromeric CO2 receptor in Aedes aegypti mosquitoes. Gr3 mutants lack electrophysiological and behavioral responses to CO2. These mutants also fail to show CO2-evoked responses to heat and lactic acid, a human-derived attractant, suggesting that CO2 can gate responses to other sensory stimuli. While attraction of Gr3 mutants to live humans in a large semi-field environment was only slightly impaired, responses to an animal host were greatly reduced in a spatial-scale dependent manner. Synergistic integration of heat and odor cues likely drive host-seeking behavior in the absence of CO2 detection. We reveal a networked series of interactions by which multimodal integration of CO2, human odor, and heat orchestrates mosquito attraction to humans.
INTRODUCTION
Anthropophilic female mosquitoes possess a strong innate drive to find and blood-feed on humans, whose blood provides a protein source needed for egg development. As a consequence of such host-seeking behavior, many mosquito species including the yellow fever mosquito Ae. aegypti and the African malaria mosquito Anopheles gambiae efficiently spread blood-borne diseases such as dengue and malaria between humans. To orient towards hosts, female mosquitoes detect a variety of chemical and physical cues including body odor, CO2, moisture, heat, and visual contrast [reviewed in (Gibson and Torr, 1999)]. Of these sensory cues, the ~4% CO2 exhaled in breath is a potent behavioral activator and attractant for mosquitoes, and is often considered to be the most important sensory cue used by these disease vectors to find humans (Turner et al., 2011).
In a broad range of mosquito species, CO2 elicits a suite of context-dependent effects [reviewed in (Gillies, 1980)] that are thought to promote host-seeking behavior. Exposure to CO2 alone strongly activates mosquito flight, increasing the probability of flight take-off and locomotor activity in the absence of directional air currents (Erias and Jepson, 1991). Filamentous intermittent plumes of CO2 (Dekker et al., 2005; Geier et al., 1999; Healy and Copland, 1995) elicit stereotyped and sustained patterns of upwind flight toward the CO2 source (Dekker and Carde, 2011).
In Ae. aegypti, CO2 increases both the kinetics and fidelity of flight responses to host odor plumes, thereby enhancing source-finding (Dekker and Carde, 2011; Dekker et al., 2005). Several studies have also shown that CO2 enhances mosquito attraction to warmth (Burgess, 1959; Krober et al., 2010; Maekawa et al., 2011). Despite earlier attempts to quantify the overall effect of exhaled CO2 from breath on attraction to live humans (Snow, 1970), the scale over which CO2 acts and its relative importance compared to all the other sensory cues encountered during mosquito orientation towards a live host remains unclear.
Ae. aegypti maxillary palps house a specialized class of olfactory sensory neurons (OSNs) that are exquisitely sensitive to changes in CO2 concentration (Grant et al., 1995). In the vinegar fly Drosophila melanogaster, two gustatory receptors (DmelGr21a and DmelGr63a) function together to mediate CO2 detection (Jones et al., 2007; Kwon et al., 2007). Direct orthologues of DmelGr21a and DmelGr63a have been identified in a wide range of insect species including Ae. aegypti, where they are named Gr1 and Gr3, respectively (Robertson and Kent, 2009). In most non-Drosophila insect species examined, a Gr1 paralogue named Gr2 is present (Figure 1A). Functional studies of An. gambiae Gr1, Gr2 and Gr3 orthologues using heterologous expression in Drosophila (Lu et al., 2007), and transient knockdown studies in Ae. aegypti (Erdelyan et al., 2012) have suggested a conserved role for these genes as candidate CO2 receptors, but the functional requirement of one or more subunits for mosquito CO2 detection is unknown.
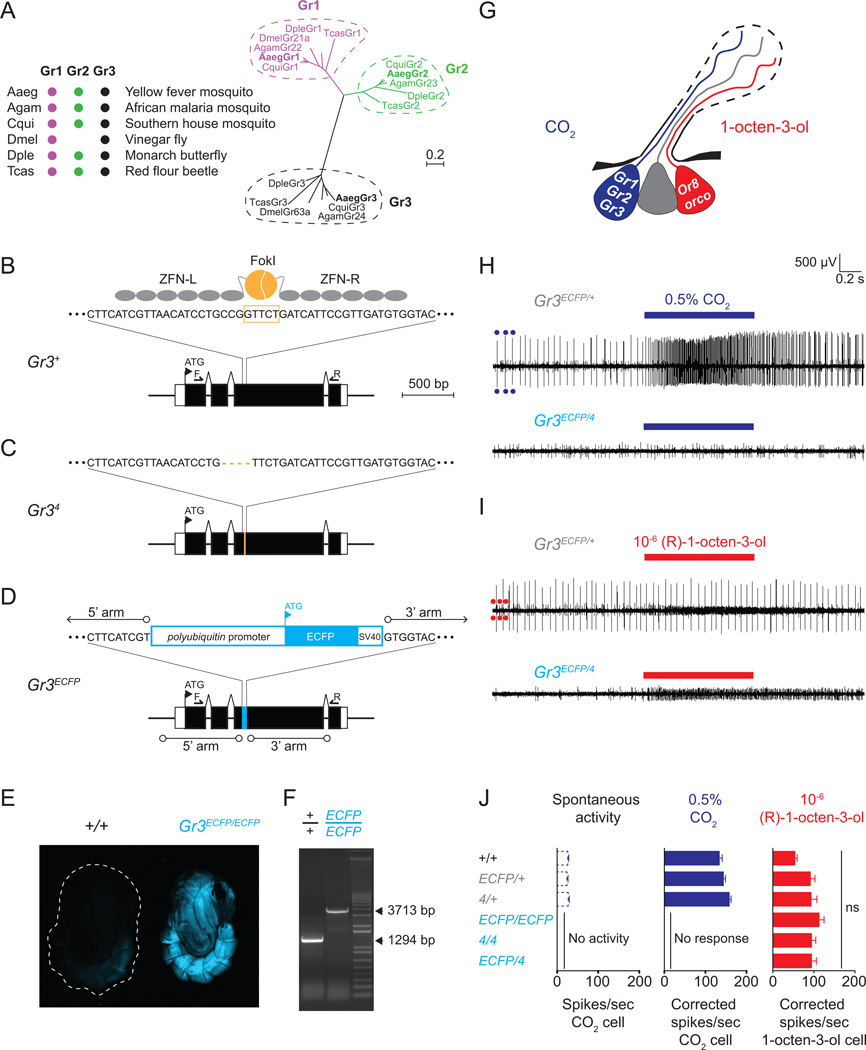
Figure 1. Electrophysiological characterization of Gr3 mutants generated by ZFN gene editing.
(A) Phylogenetic comparison of insect CO2 receptor genes. Aaeg: Aedes aegypti, Agam: Anopheles gambiae, Cqui: Culex quinquefasciatus, Dmel: Drosophila melanogaster, Dple: Danaus plexippus, Tcas: Tribolium castaneum. Branch lengths are proportional to sequence divergence. Scale bar, 0.2 amino acid replacements per site.
(B) The Ae. aegypti Gr3 genomic locus, with a schematic of the Gr3 ZFN pair binding to exon 3 DNA and the Fok1 cleavage site indicated in orange.
(C) Diagram of 4 bp deletion in the Gr34 genomic locus.
(D) Diagram of the mutant Gr3ECFP genomic locus.
(E) Representative fluorescent images of female wild-type and Gr3ECFP/ECFP mutants.
(F) PCR products generated across the ECFP insertion site using the F and R primers indicated by small arrows in B and D.
(G) Diagram of receptor expression and ligand-specificity of maxillary palp capitate peg sensillum OSNs.
(H) Representative spike traces during extracellular recordings from a Gr3 ECFP/+ heterozygous (top) and Gr3 ECFP/4 mutant (bottom) capitate peg sensillum evoked by 0.5% CO2. Dark blue circles mark large amplitude spikes from the CO2-sensitive OSN. Stimulus bar (blue): 1 sec.
(I) Representative spike traces from heterozygous and mutant basiconic sensilla evoked by 10−6 (R)-1-octen-3-ol. Red circles mark small amplitude spikes from the 1-octen-3-ol-sensitive OSN. Stimulus bar (red): 1 sec.
(J) Summary of spontaneous activity and CO2- and (R)-1-octen-3-ol-evoked responses in the indicated genotypes. Data are presented as mean ± s.e.m. Genotypes did not differ in their response to (R)-1-octen-3-ol (one-way ANOVA, p = 0.07 (ns, not significant; n=8–16 sensilla recorded from at least 3 mosquitoes per genotype).
Here we used genome editing to engineer Ae. aegypti unable to sense CO2, allowing us to uncover the relative contribution of CO2 detection to mosquito host-seeking behavior. We find that attraction to heat and lactic acid are absent in Gr3 mutants, suggesting that CO2 detection gates responses to these other sensory cues. Despite these dramatic deficits, Gr3 mutants show only mild impairment in their attraction to live human subjects in semi-field conditions. When we modeled host attraction using a live animal host presented at different spatial scales, the mutants showed no impairment in host-seeking in a small cage, but strong impairment in a large cage. Our results provide compelling evidence that multimodal integration of CO2 and other host sensory cues orchestrates mosquito attraction to humans.
RESULTS
Generation of targeted mutations in Gr3
Of the three Ae. aegypti CO2 receptors (Figure 1A), we chose to mutate Gr3 because mutations in the fly orthologue, DmelGr63a, were sufficient to disrupt CO2 reception (Jones et al., 2007). We generated null mutations in this gene by injecting a zinc-finger nuclease (ZFN) pair targeting exon 3 of Gr3 (Figure 1B) into pre-blastoderm stage mosquito embryos. Two independent Gr3 alleles were isolated and characterized, Gr34 and Gr3ECFP. Gr34 harbors a 4 bp deletion in exon 3 generated by non-homologous end-joining (Figure 1C), leading to a frameshift and multiple nonsense mutations. We also generated a homologous recombination allele Gr3ECFP (Figure 1D) by co-injecting a donor plasmid for homology-directed repair along with the ZFN mRNAs. In this allele, 33 bp of exon 3 were deleted, and a 2.45 kb cassette was inserted via ZFN-mediated homologous recombination. This insertion marked mutants visually with ECFP fluorescence (Figure 1E) and could be detected in the Gr3 locus by PCR (Figure 1F). After outcrossing each isolated allele to wild-type mosquitoes for at least five generations, we established heterozygous (Gr34/+ and Gr3ECFP/+), homozygous (Gr34/4 and Gr3ECFP/ECFP) and heteroallelic (Gr3ECFP/4) mutant lines for behavioral characterization.
Gr3 mutants cannot sense or respond behaviorally to CO2
CO2 is detected by OSNs of large-spiking amplitude that are housed within capitate peg sensilla on the Ae. aegypti maxillary palp, along with a small-spiking neuron responsive to 1-octen-3-ol, and a third neuron of unknown ligand specificity (Figure 1G) (Grant et al., 1995; McIver, 1972). Previous studies in An. gambiae demonstrated that Gr1, Gr2, and Gr3 are co-expressed in the CO2-responsive OSN and Or8/orco are co-expressed in the 1-octen-3-ol-responsive OSN (Jones et al., 2007; Lu et al., 2007)(Figure 1G). The organization and function of these OSNs is believed to be conserved in Ae. aegypti (Bohbot et al., 2007). To determine whether Gr3 mutants could sense CO2, we performed extracellular recordings of CO2-evoked activity from female capitate peg sensilla using single sensillum electrophysiology. Wild-type and heterozygous female mosquitoes responded robustly to a 1 second pulse of 0.5% CO2, whereas homozygous and heteroallelic mutants were totally unresponsive (Figure 1H). In the mutants, this lack of a CO2-evoked response was accompanied by the complete absence of spontaneous activity in this neuron. In contrast, the 1-octen-3-ol-sensitive cell of all genotypes responded similarly to a 1 second pulse of 10−6 (R)-1-octen-3-ol (Figure 1I), indicating that our extracellular recordings were targeted correctly to capitate peg sensilla. Summary data showing spontaneous and stimulus-evoked activity are in Figure 1J.
To test whether CO2-evoked behavior was affected in these mutants, we tracked the flying activity of groups of female mosquitoes using 3D multi-insect tracking in a large assay chamber. Following a period of base-line tracking, we introduced a pulse of CO2 into the airstream, which caused large bursts of flight activity in wild-type and heterozygous female mosquitoes (Figure 2A–C; top three rows). In contrast, homozygous and heteroallelic mutant mosquitoes remained indifferent to this stimulus (Figure 2A–C; bottom three rows).
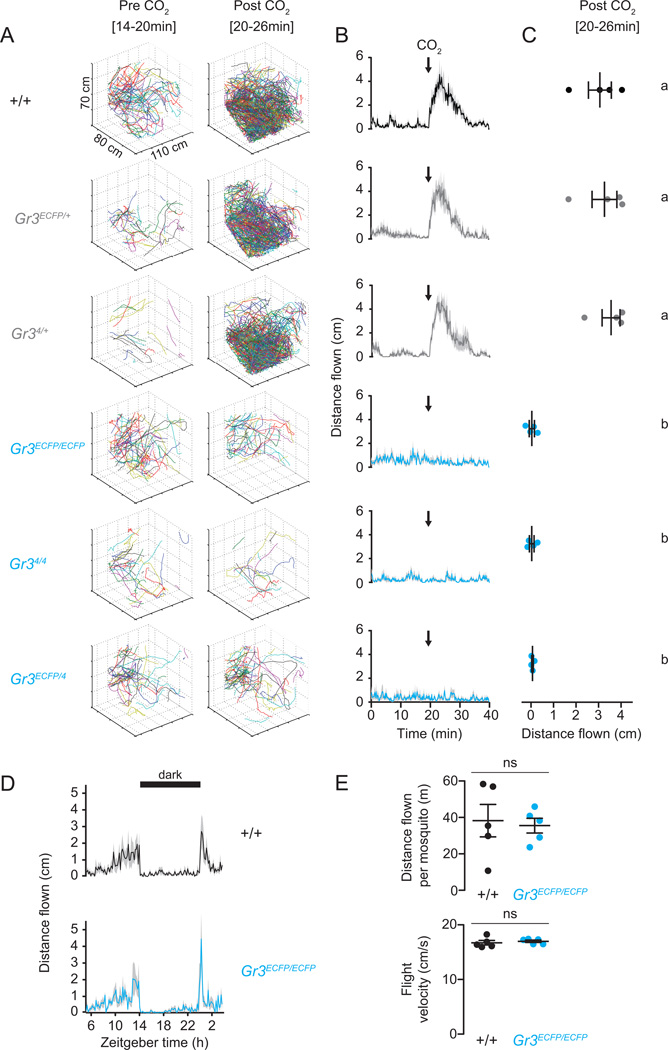
Figure 2. Ae. aegypti Gr3 mutants are not activated by CO2 and retain normal locomotor behavior.
(A–C) CO2-evoked flight activity of groups of female Ae. aegypti wild-type, heterozygous, and mutant mosquitoes tracked for 20 min before and after a 40 sec pulse of CO2 (n=4 replicates per genotype; n=20 mosquitoes/trial).
(A) Representative three-dimensional tracks of the indicated genotypes in the 6 min pre- (left) and post-application (right) of a 40 sec pulse of CO2.
(B) Distance flown in 1 sec bins per mosquito in response to stimulation with CO2 (black arrow). Data are shown as mean (solid line) ± s.e.m (grey shading).
(C) Mean distance flown per sec for mosquitoes post-application of CO2 (t=20–26min). Each replicate is indicated by a dot and mean ± s.e.m. as bars. Variation among genotypes was significant (one-way ANOVA, p < 0.0001). Genotypes marked with different letters are significantly different by post hoc Tukey’s HSD test (p < 0.001).
(D) Locomotor activity of wild-type and Gr3ECFP/ECFP mutant mosquitoes measured as cm/min/animal over a 23 hr period (dark period indicated by black bar) without supplemental CO2 (n=5 replicates per genotype; 20 females per replicate). Data are shown as mean (solid line) ± s.e.m (grey shading).
(E) Total distance flown per mosquito and average flight velocity of data in D. Each replicate is indicated by a dot and mean ± s.e.m. as bars (ns, not significant; t-test, p > 0.05).
To rule out non-specific locomotor defects in Gr3 mutants, we tracked the activity of wild-type mosquitoes and a representative homozygous mutant genotype, Gr3ECFP/ECFP over a 23 hour period. No significant differences in either flying distance tracked per mosquito or flight velocity were observed between genotypes and their daily rhythms of activity were qualitatively similar (Figure 2D–2E). We conclude that Gr3 mutants are selectively impaired in their response to CO2.
CO2 detection can gate mosquito heat-seeking behavior
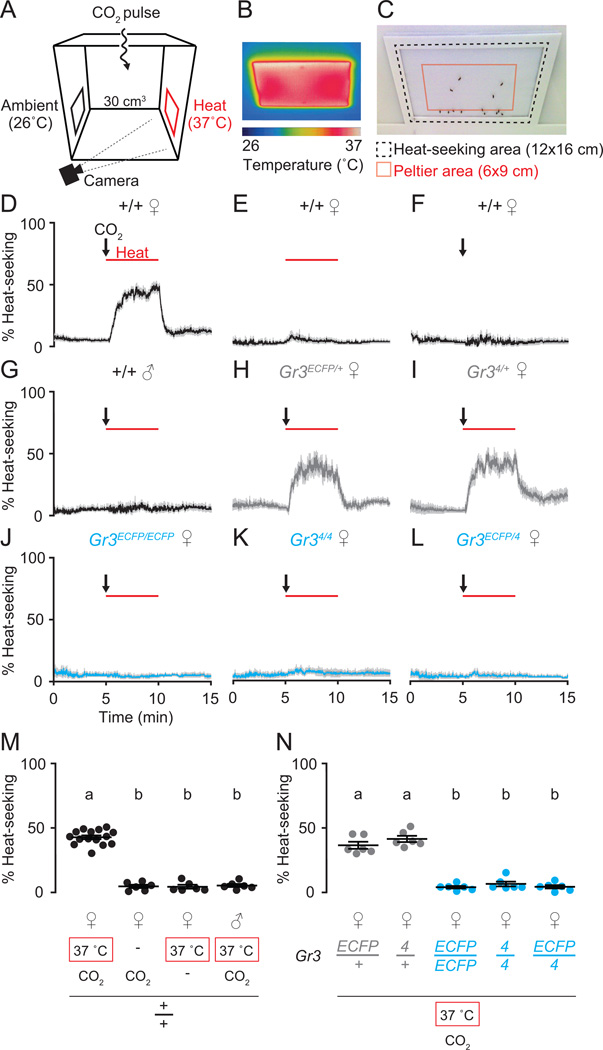
Warm-blooded hosts provide mosquitoes with thermal contrast that facilitates the localization of a suitable blood meal. To investigate the potential for an interaction between CO2 detection and heat perception, we developed a heat-seeking assay that scored the ability of mosquitoes to land on a 37°C target (Figure 3A–C). Trials with wild-type females revealed that mosquitoes only landed on the heated target when a pulse of CO2 was presented in the assay (Figure 3D–F). Wild-type males were not attracted using identical assay conditions (Figure 3G), showing that heat-seeking stimulated by CO2 is sexually dimorphic in Ae. aegypti.
Figure 3. CO2 detection gates heat-seeking behavior in female Ae. aegypti.
(A) Schematic of the mosquito heat-seeking assay.
(B) Thermal image of target Peltier heated to 37°C.
(C) Image indicating Peltier location (red outline) and area scored for heat-seeking (black dashed line).
(D–L) Mosquitoes of the indicated sex and genotype seeking an ambient or heated target, with or without a pulse of CO2. The 5 min 37°C stimulus is indicated by the red bar. The 20 sec pulse of CO2 is indicated by the black arrow. Data are shown as mean (solid line) ± s.e.m (grey shading) [n=6 trials, except (D) where n=15; 20–25 mosquitoes/trial].
(M–N) Quantification of heat-seeking during minutes 7–10 of assay. Each replicate is indicated by a dot and mean ± s.e.m. as bars. Variation among both stimulus regimes and mosquito genotypes were significant (one-way ANOVA, p < 0.0001 for both M and N). Data labeled with different letters are significantly different using Tukey’s HSD test comparing all pairs of means (p < 0.001).
To further verify that CO2 detection was required for heat-seeking behavior in female Ae. aegypti, we tested the heterozygous, homozygous, and heteroallelic Gr3 mutants in the same assay. Heterozygous genotypes landed on the heated target in a CO2-dependent manner (Figure 3H–I), but homozygous and heteroallelic Gr3 mutants did not land (Figure 3J–L), indicating that CO2 detection is required for this behavior. Heat-seeking data are summarized in Figure 3M–N.
CO2 detection enhances mosquito attraction to lactic acid and human scent
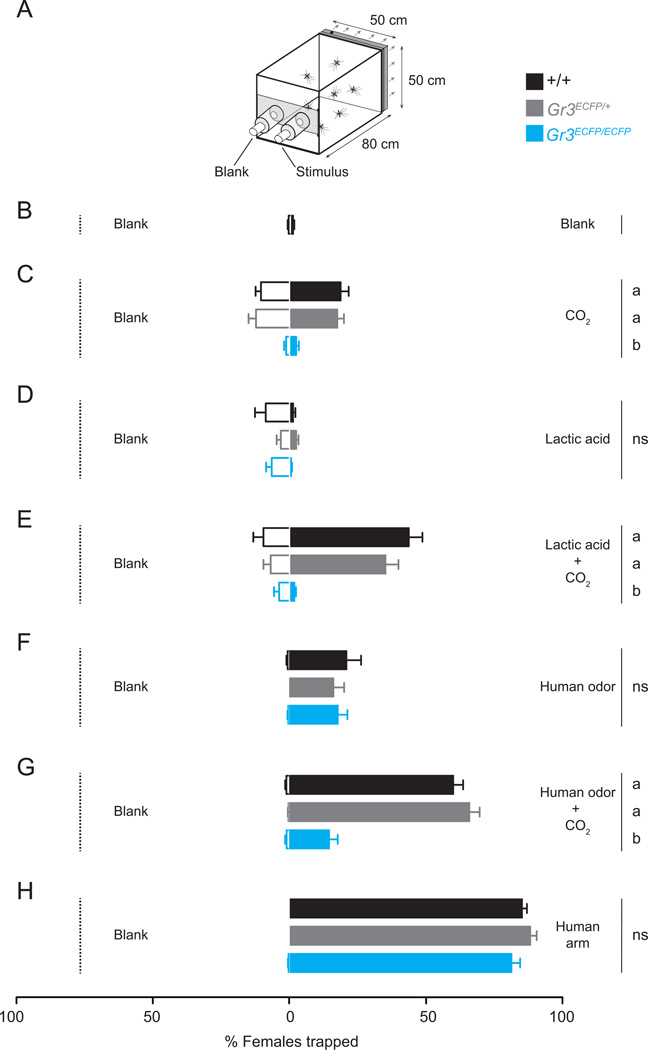
In addition to exhaled CO2, humans emit a blend of hundreds of volatile organic compounds in breath and skin odor (Gallagher et al., 2008). To evaluate whether CO2 detection influences the ability of Ae. aegypti to orient towards human odorants, we assayed attraction of wild-type, heterozygous, and homozygous Gr3 mutant female mosquitoes to various odor stimuli in the presence or absence of supplemental CO2 using a two-port olfactometer assay (Figure 4A). After characterizing background levels of attraction using blank trials that contained no stimuli (Figure 4B), we evaluated responses to CO2. We observed moderate numbers of wild-type and heterozygous Gr3 females trapped in response to stimulation with CO2 alone. However, attraction appeared to be non-directional with respect to the CO2 source in this assay (Figure 4C, Gr3+/+ and Gr3ECFP/+). This general increase in capture rate in both ports may reflect random exploratory behavior of females in the two-port assay chamber upon activation with CO2 in the absence of other olfactory cues. This contrasts with the known attraction of mosquitoes to CO2 in a wind tunnel (Dekker and Carde, 2011), likely because CO2 is not presented as a filament in our two-port assay. Responses to CO2 alone were abolished in Gr3ECFP/ECFP mutants, consistent with their inability to sense this stimulus (Figure 4C).
Figure 4. CO2 synergizes with host odor to drive mosquito attraction.
(A) Diagram of the two-port olfactometer.
(B–H) Response of the indicated genotypes to a choice of the blank port compared to the stimulus indicated at the right. Data are plotted as mean ± s.e.m (n=8–11 trials per condition; n=50 mosquitoes per trial). Significance was assessed with one-way ANOVA. Genotypes marked with different letters are significantly different by Tukey’s HSD test (p < 0.0001; ns, not significant; n=50 mosquitoes per trial).
We next evaluated responses to L-(+)-lactic acid, a volatile organic compound present in human breath and skin odor, which has previously been shown to synergize behaviorally with CO2 to enhance mosquito attraction (Acree et al., 1968; Erias and Jepson, 1991). L-(+)-lactic acid was not attractive to female mosquitoes when presented alone (Figure 4D). However, when L-(+)-lactic acid was presented with CO2, many wild-type and heterozygous females were attracted. Gr3ECFP/ECFP mutants did not respond in these same trials, indicating that CO2 detection is required for synergistic responses to lactic acid (Figure 4E).
To evaluate whether CO2 detection modulates perception of human skin odor, we examined mosquito responses to human odor collected on nylon sleeves. Human odor in the absence of CO2 elicited moderate attraction that was not significantly different among genotypes (Figure 4F). However, supplementation of CO2 to human odor enhanced levels of attraction observed for wild-type and heterozygous females, but not homozygous mutants (Figure 4G).
To assess a more complex multi-sensory stimulus, we tested mosquito attraction to a live human arm inserted into the stimulus port. Very high levels of attraction were observed for all mosquito genotypes to a live human arm in this assay (Figure 4H). We speculate that additional stimuli presented by the human arm, including odorants not trapped on nylon sleeves, moisture, heat, and visual cues overcome the behavioral defect seen with simpler stimulus combinations in the mutants.
Mosquito attraction to humans in a semi-field environment is diminished but not abolished in Gr3 mutants
To obtain a more biologically relevant estimate of the contribution that CO2 detection makes to mosquito host attraction, we tested the ability of Gr3 mutants and wild-type females to locate and land on live human volunteers in the James Cook University semi-field cage in Northern Queensland, Australia (Figure 5A). This semi-field cage contains the underside of an elevated Queenslander style house, including furniture used as resting sites of Ae. aegypti, and a lush tropical backyard that mimics the native habitat of Ae. aegypti in this region (Ritchie et al., 2011). Equal numbers of wild-type and mutant females were released into the semi-field cage during each trial for simultaneous comparisons of mosquito host-seeking behavior.
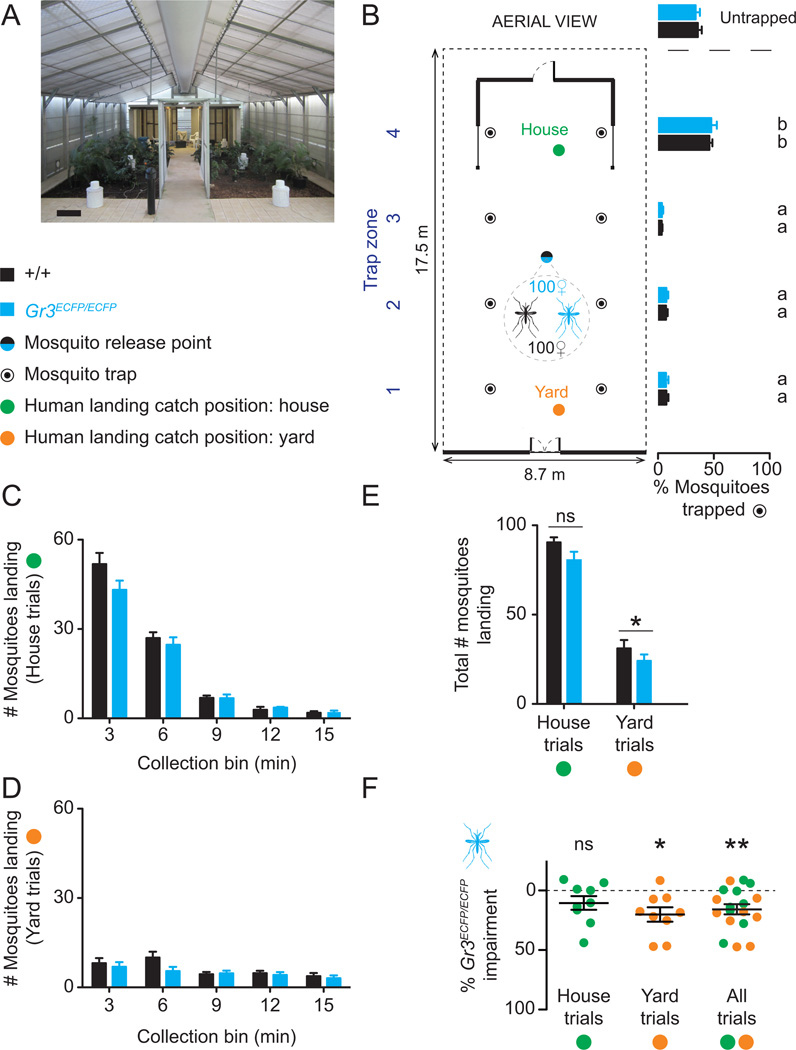
Figure 5. Gr3 mutants have diminished responses to live humans in a semi-field environment.
(A) Interior view semi-field cage. Scale bar: 0.5 m.
(B) Schematic of the semi-field cage showing the average distribution of a 50:50 mix of wild-type and Gr3ECFP/ECFP mutant female mosquitoes (n=100 per genotype) in the cage in the absence of humans (n=5 trials). Distribution varied significantly according to trap zone, but not genotype (two way-ANOVA, P<0.0001 for trap zone and P=0.773 for genotype). Different letters indicate significantly different means by post hoc Tukey’s HSD test.
(C–D) Number of mosquitoes of the indicated genotype landing on human subjects in 3 min bins during 15 min at house (C) or yard (D) position (n=9 trials per position).
(E) Total number of mosquitoes landing in human landing catch trials with human volunteers at house or yard position. Paired t-tests were used to assess statistical significance in comparisons between genotypes (ns, not significant, p=0.09; * p=0.018).
(F) Relative impairment in host-seeking of Gr3ECFP/ECFP mutants compared to wild-type mosquitoes (one-sample t-test relative to zero; ns, not significant, p = 0.105; * p = 0.011; ** p = 0.002).
In B–F, all data are plotted as mean ± s.e.m.
To examine the distribution of the released mosquito population over the semi-field cage without humans present, we used mosquito traps to capture females in four designated trap zones (Figure 5B left). The majority of the mosquitoes of both genotypes was trapped near the house and there were no differences between the numbers of each mosquito genotype trapped in each zone (Figure 5B right). Therefore under semi-field conditions both wild-type and Gr3 mutant mosquitoes retained an innate preference for human habitation. The mutants showed similar dispersal across the semi-field cage relative to wild-type mosquitoes, consistent with their performance in flight tracking assays (Figure 2D–E).
To test mosquito attraction to humans, we counted the number of wild-type and mutant mosquitoes landing on a seated volunteer positioned either within the house or yard of the semi-field cage (Figure 5B, green and orange circles, respectively). The mosquito traps were inaccessible during human landing catch trials. Slightly fewer Gr3 mutants landed on humans compared to wild-type mosquitoes during the initial two collection bins of house and yard trials (Figure 5C–D). These small cumulative differences were maintained over the duration of each assay, leading to fewer Gr3 mutants being collected in total on average compared to wild-type mosquitoes at either human landing catch position (Figure 5E). Overall, landing varied significantly according to genotype (P=0.043), and position in the cage (P< 0.0001), but there was no interaction between these two variables (P=0.713) using a two way-ANOVA. Variation in mosquito landings between human volunteers was not significant. Overall, Gr3ECFP/ECFP mosquitoes were impaired by approximately 15% relative to wild-type mosquitoes in the semi-field cage (Figure 5F). Thus, a loss of CO2 detection diminishes but does not abolish the ability of mosquitoes to find humans in the semi-field cage.
Effects of CO2 sensation on mosquito-host seeking behavior are dependent on spatial scale
Host sensory cues such as heat, CO2, human odor, moisture and visual contrast are most potent when a mosquito is close to the human host and decline at a distance. We therefore reasoned that the host-seeking defects in Gr3 mutants would depend on spatial scale, such that a more dramatic defect would be apparent if the mutants were tested in a larger semi-field cage. Because to our knowledge no larger semi-field facilities currently exist, we modeled host-seeking behavior at two different spatial scales with live animal hosts in laboratory cages of different sizes (Figure 6A–B).
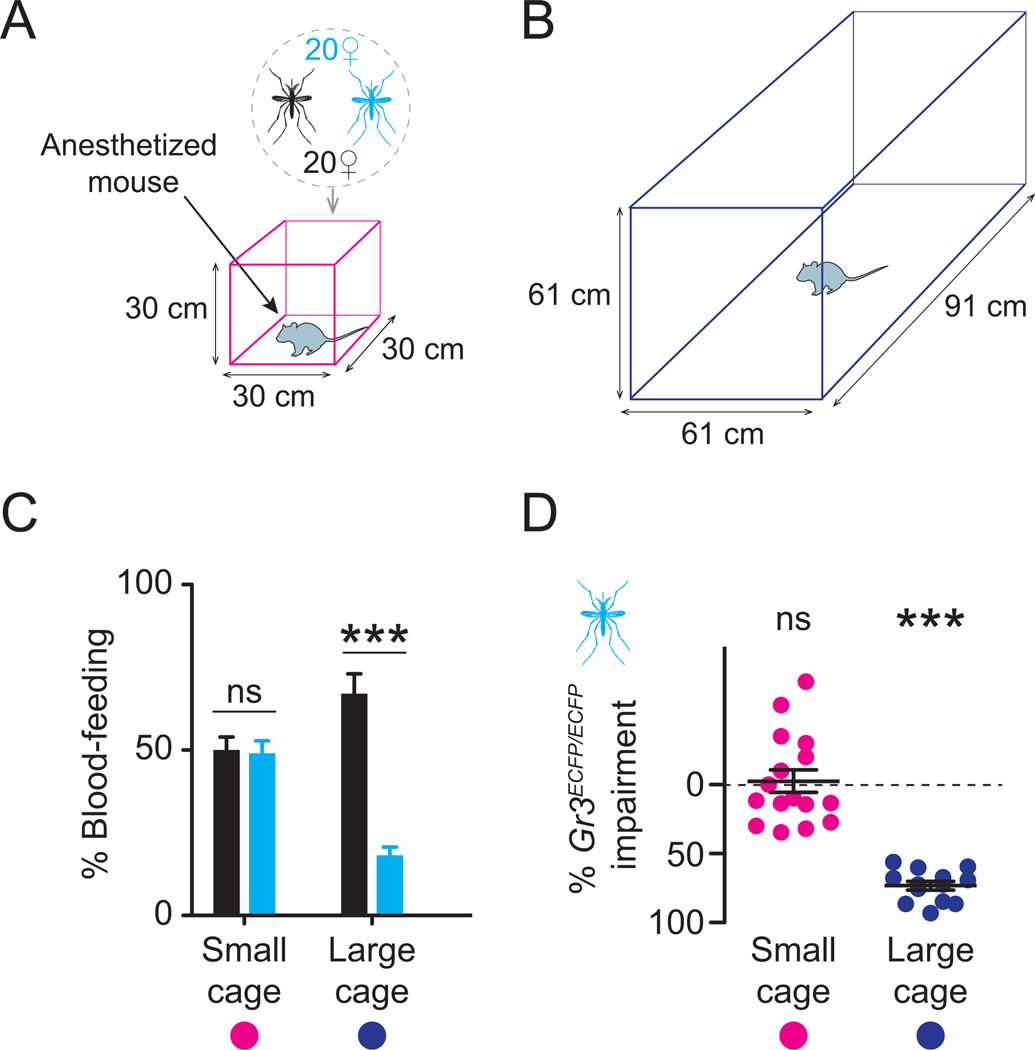
Figure 6. Spatial scale-dependent responses of Gr3 mutants to live animal hosts.
(A–B) Diagrams of small (A) and large (B) cage assays.
(C–D) Per cent blood-feeding of the indicated genotype (C) and impairment of Gr3ECFP/ECFP mutants relative to wild-type (D).
Significance was assessed in C with paired t-tests and in D with a one-sample t-test relative to zero (ns, not significant; *** p < 0.001).
All data are plotted as mean ± s.e.m (small cage: n=16 trials; large cage: n=13 trials; n=20 mosquitoes/genotype/trial).
In these assays, mixed populations of equal numbers of wild-type and Gr3ECFP/ECFP mosquitoes were released into each cage, and assayed for their ability to blood-feed on an anesthetized mouse. Similar numbers of wild-type and Gr3ECFP/ECFP females blood-fed during trials in the small (0.027 m3) cage, but significantly fewer Gr3 mutant females blood-fed relative to wild-type mosquitoes in the larger (0.339 m3) cage (Figure 6C). On average, blood-feeding rates in Gr3ECFP/ECFP females were unimpaired in the small cage consistent with semi-field cage results, but impaired by approximately 70% relative to wild-type mosquitoes in the larger cage (Figure 6D). Two way-ANOVA on blood-feeding rates revealed a highly significant interaction (P< 0.0001) between mosquito genotype and cage size, suggesting that the effects of CO2 sensation on host-seeking behavior are dependent on spatial scale. We note that presence of wild-type mosquitoes did not rescue the major deficits in host-seeking seen in Gr3ECFP/ECFP mutants in the large cage (Figure 6C). This rules out the possibility that motor, auditory, or pheromonal cues from wild-type mosquitoes influence the behavior of mutant mosquitoes in this assay.
Sensory stimuli synergize in multiple binary combinations to drive mosquito blood-feeding behavior
Given that CO2 is a major host-seeking cue and strongly influences mosquito perception of heat and odorants, we were intrigued by the nearly normal attraction of homozygous Gr3 mutants to live humans in a semi-field environment. To ask how the phenotypic deficits of Gr3 mutants may be compensated during attraction to live hosts, we developed an artificial membrane-feeding assay (Figure 7A) to test the contribution of individual and combined sensory cues including CO2, heat, and human odor to mosquito blood-feeding behavior.
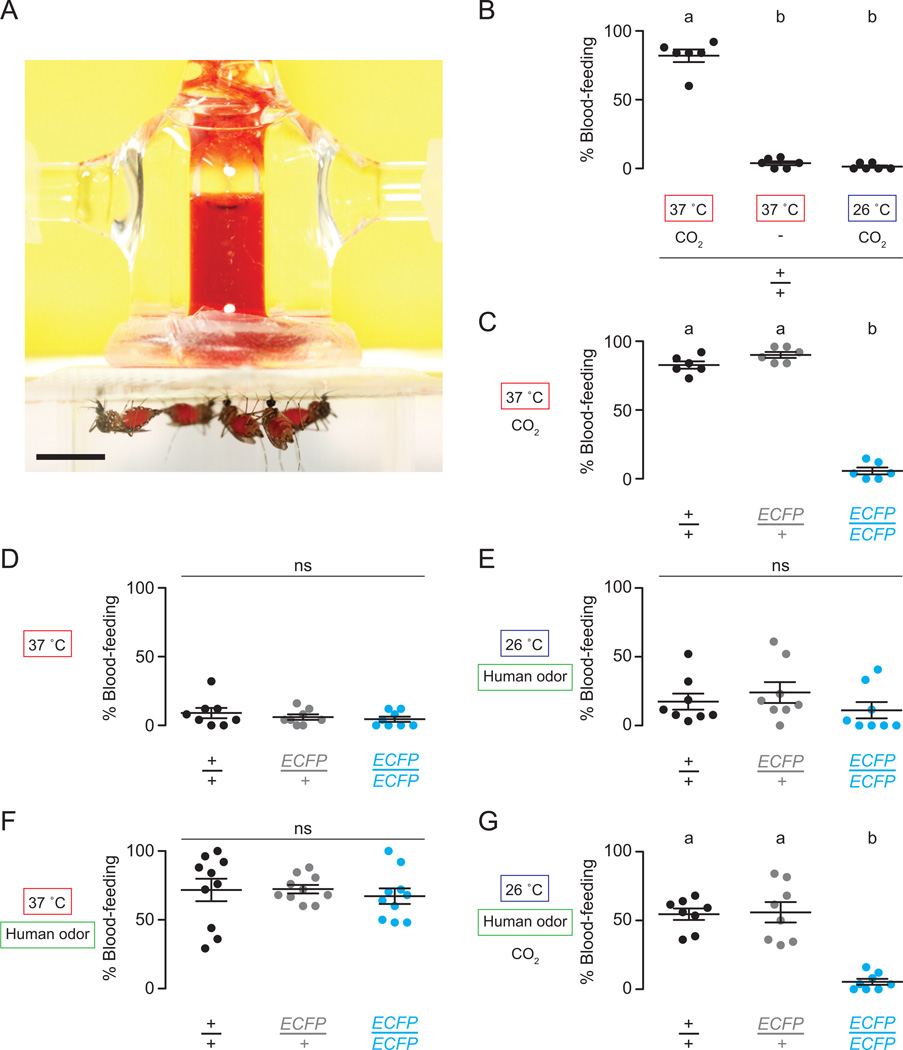
Figure 7. Multiple host sensory cues combine to elicit mosquito blood-feeding behavior.
(A) Image of female Ae. aegypti mosquitoes blood-feeding from a glass membrane feeder. Scale bar, 0.5 cm.
(B) Blood-feeding responses of wild-type mosquitoes with different blood-temperatures and ±CO2.
(C–G) Blood-feeding responses of the indicated genotypes with different blood-temperatures ±CO2 ±human odor.
In B–G, significance was assessed with one-way ANOVA. Data labeled with different letters are significantly different using Tukey’s HSD test (p < 0.001; ns, not significant). Replicate data points and mean ± s.e.m. are shown (n=6–10 trials/genotype and condition; n=25 mosquitoes per trial).
To test whether heat and CO2 are integrated to drive blood-feeding behavior, we manipulated the temperature of blood inside the membrane feeder to reflect human body (37°C) or ambient (26°C) temperatures in the presence or absence of CO2. Wild-type and heterozygous mosquitoes blood-fed from membrane feeders only when the blood was heated to 37°C and CO2 was added to the assay (Figure 7B). Homozygous Gr3 mutants rarely blood-fed under these conditions (Figure 7C).
We then tested whether human odor presented with heat can rescue blood-feeding in Gr3 mutants. Very few females of any genotype blood-fed from a heated membrane feeder in the absence of other cues (Figure 7D). Human odor when applied alone to a membrane feeder filled with ambient-temperature blood also elicited low levels of blood-feeding (Figure 7E). However, when we applied human odor to the membrane of heated feeders, we observed high rates of blood-feeding in all genotypes (Figure 7F).
To determine whether CO2 and odor cues can also synergize to drive blood-feeding in the absence of heat, we applied human odor to a membrane feeder maintained at ambient conditions and added CO2. High numbers of wild-type and heterozygous mosquitoes, but very few homozygous Gr3 mutants, blood-fed on this room-temperature blood (Figure 7G). Taken together, these results indicate that CO2, odor, and heat synergize in all possible binary combinations to drive Ae. aegypti blood-feeding behavior.
DISCUSSION
Here we used ZFN-mediated targeted mutagenesis and homologous recombination to target the Ae. aegypti Gr3 gene to generate mosquitoes unable to detect or behaviorally respond to volatile CO2. Interestingly, mutating only Gr3 while sparing Gr1 and Gr2 was sufficient to disrupt CO2 responses. This suggests that this subunit plays an essential role not compensated for by the two remaining CO2 receptor subunits. The stoichiometry of the CO2 receptors in Aedes, Anopheles, and Drosophila currently remains unknown. In Drosophila, it has been shown that the two CO2 receptor subunits together reconstitute a functional receptor (Jones et al., 2007; Kwon et al., 2007). Ectopic expression of An. gambiae CO2 receptor subunits in Drosophila OSNs suggests that Gr1 and Gr3, as well as the combination of Gr1, Gr2, and Gr3 form a functional CO2 receptor (Lu et al., 2007). In Ae. aegypti, transient RNAi knockdown experiments that assessed mosquito orientation towards a CO2 source suggested that Gr1 and Gr3 but not Gr2 play a role in CO2 reception (Erdelyan et al., 2012). The respective functional contribution of Gr1 and Gr2 to CO2 detection therefore remains unclear and awaits further genetic and biochemical characterization. It will be interesting to determine if all three subunits are functionally required to detect CO2 or if Gr1 and Gr2 are functionally redundant. Further, it is not known if one CO2 receptor subunit functions as a non-ligand-selective co-receptor while others interact with the ligand, as is the case with insect odorant receptors and ionotropic receptors (Benton et al., 2009; Larsson et al., 2004).
Several volatile odorants have recently been shown to antagonize, activate, or hyper-activate the CO2 neuron in a variety of mosquito species, in some instances leading to behavioral disorientation (Lu et al., 2007; Tauxe et al., 2013; Turner et al., 2011). The availability of Gr3 mutants will allow us to ask whether these compounds act through the CO2 receptor complex, or through other uncharacterized receptors expressed in this neuron. The behavioral effects of recently described CO2 receptor antagonists (Tauxe et al., 2013) are unlikely to be mediated by the CO2 receptor complex because CO2-insensitive Gr3 mutants remain attracted to live humans in our semi-field assays.
Our behavioral data support the conclusion that multimodal integration of CO2, heat and odor drives host-seeking and blood-feeding behavior in Ae. aegypti. We show that CO2 gates mosquito perception of both heat and the mono-molecular attractant lactic acid. This binary synergism extended to other combinations of sensory stimuli, including human skin odor with heat or CO2. This synergism of multiple sensory cues may explain why attraction to live humans was diminished but not abolished in Gr3 mutants in semi-field and two-port olfactometer trials. Interestingly, we also observed that synergy between CO2 and host odor was sufficient to induce wild-type female mosquitoes to blood-feed on unheated blood. Consistent with this notion, Ae. aegypti have been observed to blood-feed on cold-blooded animals such as lizards when warm-blooded vertebrates were unavailable (Woke, 1937).
The absence of a major deficit in Gr3 mutant attraction to live humans in the semi-field cage tested here does not preclude the possibility that more pronounced effects may have been observed if the human host was presented in a larger semi-field cage. Indeed, we determined that the effects of CO2 detection on host-seeking behavior appear to be dependent on spatial scale by tests with animal hosts presented in small or large cages. Synergism between multiple remaining sensory cues including visual contrast, moisture, heat and volatile odorants from the skin and breath may explain why attraction to live humans was diminished but not abolished in Gr3 mutants in our assays.
When combined, heat and human odors have been found to influence crosswind flight behavior and increase source-finding and landing responses in female An. gambiae (Spitzen et al., 2013). Although it is not known over what distance mosquitoes perceive heat, it is generally assumed that thermal convection currents emanating from human skin rapidly dissipate to reach equilibrium with ambient temperature conditions within ≤1.5 m of the host (Lewis et al., 1969; Spitzen et al., 2013). Synergistic interactions between human odor or CO2 with heat may potentially act over short distances to guide landing behavior as mosquitoes approach hosts.
Previous studies suggested that Ae. aegypti is attracted to both stationary and moving visual contrast, and that the activating effects of human scent combined with visual feedback allow mosquitoes to navigate upwind towards hosts (Kennedy, 1940). In addition, it is possible that diurnal patterns of flight periodicity in Ae. aegypti (Trpis et al., 1973) may randomly bring females into the effective range of host cues where multimodal integration may then occur. The role of mosquito vision in host-seeking and its interactions with odors warrants further investigation.
Multisensory integration allows organisms to form salient percepts from combined sensory stimuli to drive context-dependent behaviors. An example of such integration in humans is the fusion of smell and taste of food or drink into the single percept of flavor [reviewed in (Small, 2012)]. Neither chemosensory input in isolation fully encodes the experience of the food or beverage, which may explain why humans with olfactory dysfunction report difficulties with enjoyment of food and wine (Keller and Malaspina, 2013). Another benefit of multi-sensory integration is improvement in the reliability of signal detection. In noisy auditory environments, verbal comprehension can be improved dramatically by the practice of lip-reading, thus combining auditory and visual perception to enhance the accuracy of signal detection [reviewed in (Campbell, 2008)]. For the female mosquito, such multisensory integration may be crucial for efficient host-seeking in a cluttered sensory environment. In the case of female mosquitoes living near human settlements, there are likely to be many sensory distractors in the environment that emit heat, human scent, or CO2 in isolation. By requiring the simultaneous presentation of at least two such cues before host-seeking behavior is triggered, the mosquito increases her chances of high-fidelity host localization. Furthermore, body odor is itself a complex blend of multiple odorants that acts to shape both mosquito host preference (DeGennaro et al., 2013) and the tendency of Ae. aegypti and An. gambiae to bite certain regions of the body including the lower legs, ankles, and feet [reviewed in (Smallegange et al., 2011)].
What are the neural mechanisms employed by the female mosquito to integrate sensory cues to trigger attraction to humans? One can envision peripheral mechanisms by which CO2 or heat directly sensitize peripheral odor-detecting OSNs, but no such mechanism has yet been described in insects. Conversely, odors have been shown to affect responses of the CO2 OSN (Lu et al., 2007; Tauxe et al., 2013; Turner et al., 2011), but this modulation required very high odor concentrations unlikely to be attained in human skin odor. A more plausible mechanism would be central sensory integration of these cues, as is known to occur for human taste and smell integration in the anterior ventral insula [reviewed in (Small, 2012)]. One can imagine odor and CO2 synergy occurring early in sensory processing pathways, because both send afferents to the antennal lobe (Anton et al., 2003). Much less is known about what brain areas process heat cues, so it seems plausible that higher brain centers are responsible for the synergistic interactions reported here. It is intriguing that male mosquitoes do not show synergy between CO2 and heat sensation. We presume that this is not due to defects in primary detection of the cues, but more likely due to differences in wiring the male brain or its susceptibility to modulation by multiple sensory inputs. The availability of genetically-encoded calcium sensors and novel circuit tracing methods (Ruta et al., 2010) opens up the possibility of carrying out functional imaging in the mosquito brain to reveal the anatomical sites and mechanisms of multi-sensory integration.
The ability of Ae. aegypti to improve host source localization via multimodal integration of sensory cues could act to increase the probability that the female mosquito obtains a blood meal, a behavior inextricably linked to mosquito reproduction. In light of such sensory redundancy, the application of novel chemicals targeting a single sensory pathway including CO2 (Tauxe et al., 2013; Turner et al., 2011) or odorant reception (Jones et al., 2011) may be less effective in perturbing mosquito behavior than simultaneous disruption of multiple sensory pathways. Such synergistic approaches to manipulate mosquito sensory perception may ultimately yield a powerful strategy to deter mosquitoes from finding humans.
EXPERIMENTAL PROCEDURES
Mosquito rearing and maintenance
Ae. aegypti wild-type (Orlando) and Gr3 mutant strains were maintained and reared at 25–28°C, 70–80% relative humidity, with a photoperiod of 14 hr light:10 hr dark (lights on 8AM) as previously described (DeGennaro et al., 2013). Adult mosquitoes were provided constant access to 10% sucrose solution for feeding. Females were provided with a blood source for egg production. All laboratory blood-feeding procedures with mice and humans and use of humans in behavioral assays were approved and monitored by The Rockefeller University Institutional Animal Care and Use Committee and Institutional Review Board, protocols 11487 and LVO-0652, respectively. Semi-field cage experiments performed with human subjects at James Cook University were approved and monitored by the JCU Institutional Biosafety Committee and Human Research Ethics Committee (Ethics approval H4450). All human subjects gave their informed consent to participate in laboratory and semi-field experiments. Female mosquitoes used for electrophysiological and behavioral assays were 5–10 days old and mated but not blood-fed. Before behavioral assays mosquitoes were sexed and sorted under hypothermia (4°C) or aspiration and fasted for 14–24 hr in the presence of a water source.
Phylogenetic analysis
Amino acid alignment and phylogenetic analysis of insect CO2 receptor genes was performed with MegAlign version 8.0.2 (DNASTAR) using the ClustalW and neighbor-joining methods. The phylogenetic tree was visualized as a radial phylogram using Dendroscope version 3.2.8 (Huson and Scornavacca, 2012).
ZFN-targeted mutagenesis and homologous recombination
ZFNs targeting Ae. aegypti Gr3 were produced by the CompoZr Custom ZFN Service (Sigma-Aldrich Life Science). Details of the design of the ZFNs and isolation of deletion and homologous recombination mutants are in the Supplemental Experimental Procedures.
Single sensillum electrophysiology
Extracellular recordings of CO2 (Matheson Tri-Gas) and (R)-1-octen-3-ol (C.A.S. 3687-48-7, Bedoukian) responses from female maxillary palp capitate peg sensilla of the indicated genotype were carried out as previously described (DeGennaro et al., 2013; Pellegrino et al., 2010). All capitate peg sensilla sampled from wild-type and Gr3 heterozygous mosquitoes responded to both (R)-1-octen-3-ol and CO2. Corrected spike increases were calculated by subtracting the spontaneous activity for 1 sec before stimulus application from the activity evoked during 1 sec of stimulus. Spontaneous activity was determined by counting spikes for 1 sec prior to the application of paraffin oil (Sigma-Aldrich) to the same sensillum. A total of 8–16 sensilla per genotype and stimulus were recorded from at least 3 mosquitoes.
Three-dimensional multi-insect tracking system
A multi-insect 3D tracking system was custom-designed and built in collaboration with SciTrackS GmbH. Details of the fabrication and operation of the system are in the Supplemental Experimental Procedures.
Heat-seeking assay
For each 15-min trial, 20–25 mosquitoes were introduced into a custom-made Plexiglass box (30 × 30 × 30 cm) and allowed to acclimate for 5 min. Two Peltier elements (6 × 9 cm surface area; Tellurex) located on opposite walls of the enclosure, and covered with white paper and white plastic mesh, were used to present heat stimuli. During acclimation, both Peltiers were kept at ambient temperature (26°C) via controllers (Oven Industries) commanded by a custom MATLAB script. Throughout the trial, carbon-filtered air was pumped into the box via a diffusion pad (59–144, Flystuff.com) installed on the ceiling of the enclosure. After acclimation, 10% CO2 was added to the air stream for 20 sec via a solenoid valve (Parker-Hannifin) to increase CO2 concentration 1000 ppm above background levels. At this time, one Peltier was warmed to 37°C for 5 min and subsequently cooled to ambient for the remaining 5 min of the trial. The side of the heated Peltier was randomized across trials. Mosquito landings at the Peltier were monitored by fixed cameras (FFMV-03M2M-CS, Point Grey Research) acquiring images at 1Hz. We noted that mosquitoes were attracted to the Peltier surface as well as to adjacent surfaces, presumably due to heat dissipation and convective currents. Therefore, images were analyzed using custom MATLAB scripts to count heat-seeking mosquitoes within a fixed target region comprising a 12 cm × 16 cm area around the center of the Peltier. Peltier surface temperatures were confirmed using a thermal imaging camera (E60, FLIR).
Two-port olfactometer assays
Two-port olfactometer assays were performed as previously described (DeGennaro et al., 2013). Experimental details are in the Supplemental Experimental Procedures.
Semi-field cage and human landing catch assays
Semi-field cage experiments were conducted at the James Cook University Mosquito Research Facility Semi-Field System (Ritchie et al., 2011) in Cairns, Queensland, Australia from January 11, 2013 – February 5, 2013. Mated, nulliparous 5–10 day females were used for these assays. Prior to use, mosquitoes were sexed via aspiration, and fasted for 16 hr in holding containers with access to water only. To populate the semi-field cage for human landing catch trials, at 10AM each day, a 50:50 mix of 100 each wild-type Orlando and Gr3ECFP/ECFP mutant female mosquitoes was released from a central release point in the cage (Figure 5A and 5B). Mosquitoes were allowed to disperse across the cage and acclimate to semi-field conditions for 5 hr. At 3PM each day, a human volunteer entered the cage and sat down for a period of 15 min at one of two pre-designated human landing catch positions: either within the house structure or 11 m away in the yard of the cage (Figure 5B). During each trial, volunteers wore full-length white lab coats to standardize visual cues, and collected mosquitoes landing on their exposed legs and ankles using a hand-held mechanical aspirator (model 2809C, BioQuip Products). Mosquitoes were aspirated during five consecutive 3 min collection windows across each 15 min trial period using separate aspirator cartridges (2809V, BioQuip Products). The number of wild-type and Gr3ECFP/ECFP mutant mosquitoes landing on each volunteer was then scored post hoc by assaying tissue homogenates from each collected mosquito for ECFP fluorescence using a POLARstar Omega microplate reader (BMG Labtech) equipped with a 440/485 nm excitation-emission filter set. To prepare samples for analysis, whole mosquitoes were homogenized at 4°C in 2 mL microcentrifuge tubes containing 100 µl PBS pH 7.2 (Gibco) with 2 mm borosilicate beads (Sigma-Aldrich) using a Qiagen TissueLyser II (Qiagen) set to maximum speed for 5 min. Samples were centrifuged at 12,100 × g at 4°C for 5 min to pellet debris and obtain a soluble protein fraction that was assayed in triplicate for ECFP fluorescence on black 384 well small volume HiBase polystyrene microplates (784076, Greiner Bio-One). Three adult male volunteers (ages 27–46) participated in human landing catch trials. Each volunteer was tested 3 times in each of the two house and yard positions (n=9 trials total per position). A single experimental replicate was performed each day.
In separate experiments, the distribution of wild-type and Gr3ECFP/ECFP mosquitoes in the cage in the absence of humans was determined using eight Biogents Sentinel (BGS) Mosquito traps (Biogents GmbH) devoid of chemical lures and symmetrically placed, two in each of four trap zones, across the semi-field cage (Figure 5A–B). During BGS trials (n=5), each mixed population of 100 females per strain was released at 9AM and mosquitoes were allowed to distribute across the cage for 1 hr. After this acclimation period, traps were uncovered and turned on until 5PM the same day. The BGS trap socks containing catches were then collected, emptied, and mosquitoes genotyped as described above.
Between experimental replicates, the semi-field cage was further depleted of all remaining mosquitoes by a mass-trapping procedure using 1 hr human landing catches, mechanical aspiration, and continuous BGS trap runs. Cage depletion between replicates was verified in the morning prior to initiating the next experimental replicate by the prerequisite condition that no mosquito landings were observed during two mock 15 min human landing catch periods at both human landing catch positions in the semi-field cage.
Host-seeking assays with mice
Assays were performed in small (30 cm × 30 cm × 30 cm; DP1000, MegaView) or large (91 cm × 61 cm × 61 cm; BioQuip Products) screened mosquito cages. For each trial, a 50:50 mix of 20 each wild-type and Gr3ECFP/ECFP females was released and acclimated for 5 min. After this period, a single female BALB/c mouse (Harlan Laboratories) anaesthetized with ketamine:xylazine was introduced into the center of the cage and mosquitoes given the opportunity to blood-feed for 10 min. The number of mosquitoes of each genotype that blood-fed on the mouse was scored post-hoc by ECFP fluorescence with a Nikon SMZ1500 dissecting microscope fitted with a CHGFI Intensilight Illuminator (Nikon Instruments Inc.). A separate mouse was used in each experimental replicate. Humans were not present during acclimation or feeding to eliminate human host cues.
Membrane-feeding assay
A membrane-feeding assay was assembled using six custom 20 mm flanked glass jacketed membrane feeders (JHU-0804-081MS, Chemglass Life Sciences) connected in series via silicone tubing to a digital 12 L water bath (VWR International) set to 37°C. Technical details of the construction and operation of the assay are in Supplemental Experimental Procedures.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism Software version 5.0b (GraphPad Software, Inc.).
Supplementary Material
HIGHLIGHTS.
Aedes aegypti Gr3 mutants do not detect or behaviorally respond to volatile CO2
CO2 detection can gate mosquito responses to heat and lactic acid
Attraction to live hosts is diminished, but not abolished in Gr3 mutants
Multimodal integration of CO2, heat, and odor cues drives mosquito attraction
ACKNOWLEDGMENTS
We thank Matthew DeGennaro, Jeff Liesch, Carolyn McBride, and other members of the Vosshall Lab for helpful discussions and comments on the manuscript; Deborah Beck, Allison Goff, Emma Mullen, Jason Pitts, Laura Seeholzer, and Sarah Yeoh-Wang for expert technical assistance; Lindsay Bellani for collaborating to establish the membrane feeding assay, and Scott Dewell for bioinformatic support. At James Cook University, Jessica Dickie, Chris Paton, and Michael Townsend provided valuable support with Australian importation, regulatory inspections, and mosquito rearing, and Mark Pearson facilitated sample processing. Zach Adelman of Virginia Tech University provided plasmids. C.J.M. was supported by a Marie-Josée and Henry Kravis Postdoctoral Fellowship and a Human Frontier Science Program Long-Term Postdoctoral Fellowship. B.J.M. is a Jane Coffin Childs Postdoctoral Fellow. L.B.V. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: C.J.M designed and carried out all the experiments in the paper except those in Figures 2 and 3, which were conducted by B.J.M. and R.A.C., respectively with assistance from C.J.M. S.A.R. coordinated the semi-field cage trials in Figure 5. C.J.M., R.A.C., B.J.M., and L.B.V. composed the figures. C.J.M. and L.B.V. together wrote the paper.
REFERENCES
- Acree F, Jr, Turner RB, Gouck HK, Beroza M, Smith N. L-Lactic acid: a mosquito attractant isolated from humans. Science. 1968;161:1346–1347. doi: 10.1126/science.161.3848.1346. [DOI] [PubMed] [Google Scholar]
- Anton S, van Loon JJ, Meijerink J, Smid HM, Takken W, Rospars JP. Central projections of olfactory receptor neurons from single antennal and palpal sensilla in mosquitoes. Arthropod Struct Dev. 2003;32:319–327. doi: 10.1016/j.asd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot J, Pitts RJ, Kwon HW, Rutzler M, Robertson HM, Zwiebel LJ. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol Biol. 2007;16:525–537. doi: 10.1111/j.1365-2583.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess L. Probing behavior of Aedes aegypti (L.) in response to heat and moisture. Nature. 1959;184:1968–1969. [Google Scholar]
- Campbell R. The processing of audio-visual speech: empirical and neural bases. Philos Trans R Soc Lond B Biol Sci. 2008;363:1001–1010. doi: 10.1098/rstb.2007.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, Vosshall LB. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T, Carde RT. Moment-to-moment flight manoeuvres of the female yellow fever mosquito ( Aedes aegypti L.) in response to plumes of carbon dioxide and human skin odour. J Exp Biol. 2011;214:3480–3494. doi: 10.1242/jeb.055186. [DOI] [PubMed] [Google Scholar]
- Dekker T, Geier M, Carde RT. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol. 2005;208:2963–2972. doi: 10.1242/jeb.01736. [DOI] [PubMed] [Google Scholar]
- Erdelyan CN, Mahood TH, Bader TS, Whyard S. Functional validation of the carbon dioxide receptor genes in Aedes aegypti mosquitoes using RNA interference. Insect Mol Biol. 2012;21:119–127. doi: 10.1111/j.1365-2583.2011.01120.x. [DOI] [PubMed] [Google Scholar]
- Erias AE, Jepson PC. Host location by Aedes aegypti (Diptera: Culicidae): a wind tunnel study of chemical cues. Bull Entomol Res. 1991;81:151–160. [Google Scholar]
- Gallagher M, Wysocki CJ, Leyden JJ, Spielman AI, Sun X, Preti G. Analyses of volatile organic compounds from human skin. Br J Dermatol. 2008;159:780–791. doi: 10.1111/j.1365-2133.2008.08748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier M, Bosch OJ, Boeckh J. Influence of odour plume structure on upwind flight of mosquitoes towards hosts. J Exp Biol 202. 1999;Pt 12:1639–1648. doi: 10.1242/jeb.202.12.1639. [DOI] [PubMed] [Google Scholar]
- Gibson G, Torr SJ. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med Vet Entomol. 1999;13:2–23. doi: 10.1046/j.1365-2915.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Gillies MT. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bull Entomol Res. 1980;70:525–532. [Google Scholar]
- Grant AJ, Wigton BE, Aghajanian JG, O'Connell RJ. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J Comp Physiol [A] 1995;177:389–396. doi: 10.1007/BF00187475. [DOI] [PubMed] [Google Scholar]
- Healy TP, Copland MJ. Activation of Anopheles gambiae mosquitoes by carbon dioxide and human breath. Med Vet Entomol. 1995;9:331–336. doi: 10.1111/j.1365-2915.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Huson DH, Scornavacca C. Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Syst Biol. 2012;61:1061–1067. doi: 10.1093/sysbio/sys062. [DOI] [PubMed] [Google Scholar]
- Jones PL, Pask GM, Rinker DC, Zwiebel LJ. Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci U S A. 2011;108:8821–8825. doi: 10.1073/pnas.1102425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Keller A, Malaspina D. Hidden consequences of olfactory dysfunction: a patient report series. BMC Ear Nose Throat Disord. 2013;13:8. doi: 10.1186/1472-6815-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JS. The visual responses of flying mosquitoes. Proc Zool Soc Lond. 1940;A109:221–242. [Google Scholar]
- Krober T, Kessler S, Frei J, Bourquin M, Guerin PM. An in vitro assay for testing mosquito repellents employing a warm body and carbon dioxide as a behavioral activator. J Am Mosq Control Assoc. 2010;26:381–386. doi: 10.2987/10-6044.1. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2reception in Drosophila. Proc Natl Acad Sci U S A. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lewis HE, Foster AR, Mullan BJ, Cox RN, Clark RP. Aerodynamics of the human microenvironment. Lancet. 1969;1:1273–1277. doi: 10.1016/s0140-6736(69)92220-x. [DOI] [PubMed] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJ, Takken W, Carlson JR, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa E, Aonuma H, Nelson B, Yoshimura A, Tokunaga F, Fukumoto S, Kanuka H. The role of proboscis of the malaria vector mosquito Anopheles stephensi in host-seeking behavior. Parasit Vectors. 2011;4:10. doi: 10.1186/1756-3305-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SB. Fine structure of pegs on the palps of female culicine mosquitoes. Can J Zool. 1972;50:571–576. doi: 10.1139/z72-078. [DOI] [PubMed] [Google Scholar]
- Pellegrino M, Nakagawa T, Vosshall LB. Single sensillum recordings in the insects Drosophila melanogaster and Anopheles gambiae. J Vis Exp. 2010:1–5. doi: 10.3791/1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SA, Johnson PH, Freeman AJ, Odell RG, Graham N, Dejong PA, Standfield GW, Sale RW, O'Neill SL. A secure semi-field system for the study of Aedes aegypti. PLoS Negl Trop Dis. 2011;5:e988. doi: 10.1371/journal.pntd.0000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Kent LB. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J Insect Sci. 2009;9:19. doi: 10.1673/031.009.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- Small DM. Flavor is in the brain. Physiol Behav. 2012;107:540–552. doi: 10.1016/j.physbeh.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Smallegange RC, Verhulst NO, Takken W. Sweaty skin: an invitation to bite? Trends Parasitol. 2011;27:143–148. doi: 10.1016/j.pt.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Snow WF. The effect of a reduction in expired carbon dioxide on the attractiveness of human subjects to mosquitoes. Bull Entomol Res. 1970;60:43–48. [Google Scholar]
- Spitzen J, Spoor CW, Grieco F, ter Braak C, Beeuwkes J, van Brugge SP, Kranenbarg S, Noldus LP, van Leeuwen JL, Takken W. A 3D analysis of flight behavior of Anopheles gambiae sensu stricto malaria mosquitoes in response to human odor and heat. PLoS One. 2013;8:e62995. doi: 10.1371/journal.pone.0062995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauxe GM, Macwilliam D, Boyle SM, Guda T, Ray A. Targeting a Dual Detector of Skin and CO2to Modify Mosquito Host Seeking. Cell. 2013;155:1365–1379. doi: 10.1016/j.cell.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trpis M, McClelland GAH, Gillett JD, Teesdale C, Rao TR. Diel periodicity in the landing of Aedes aegypti on man*. Bull World Health Organ. 1973;48:623–629. [PMC free article] [PubMed] [Google Scholar]
- Turner SL, Li N, Guda T, Githure J, Carde RT, Ray A. Ultraprolonged activation of CO2-sensing neurons disorients mosquitoes. Nature. 2011;474:87–91. doi: 10.1038/nature10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woke PA. Cold-blooded vertebrates as hosts for Aedes aegypti Linn. J Parasitol. 1937;23:310–311. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.