Key Points
A deficiency in G6PT impairs neutrophil energy homeostasis characterized by reduced intracellular levels of G6P, ATP, lactate, and NADPH.
Impaired energy homeostasis and activation of the HIF-1α/PPAR-γ pathway underlie neutrophil dysfunction in GSD-Ib.
Abstract
Glycogen storage disease type Ib (GSD-Ib) is an autosomal-recessive syndrome characterized by neutropenia and impaired glucose homeostasis resulting from a deficiency in the glucose-6-phosphate (G6P) transporter (G6PT). The underlying cause of GSD-Ib neutropenia is an enhanced neutrophil apoptosis, but patients also manifest neutrophil dysfunction of unknown etiology. Previously, we showed G6PT interacts with the enzyme glucose-6-phosphatase-β (G6Pase-β) to regulate the availability of G6P/glucose in neutrophils. A deficiency in G6Pase-β activity in neutrophils impairs both their energy homeostasis and function. We now show that G6PT-deficient neutrophils from GSD-Ib patients are similarly impaired. Their energy impairment is characterized by decreased glucose uptake and reduced levels of intracellular G6P, lactate, adenosine triphosphate, and reduced NAD phosphate, whereas functional impairment is reflected in reduced neutrophil respiratory burst, chemotaxis, and calcium mobilization. We further show that the mechanism of neutrophil dysfunction in GSD-Ib arises from activation of the hypoxia-inducible factor-1α/peroxisome-proliferators–activated receptor-γ pathway.
Introduction
Glycogen storage disease type Ib (GSD-Ib; MIM232220) is an autosomal-recessive disorder characterized by impaired glucose homeostasis, neutropenia, and neutrophil dysfunction.1,2 GSD-Ib arises from SLC37A4 gene mutations that abolish the activity of the glucose-6-phosphate (G6P) transporter (G6PT), a ubiquitously expressed protein that spans the endoplasmic reticulum (ER) membrane.3 The primary role of G6PT is to translocate G6P from the cytoplasm into the ER lumen, where it is hydrolyzed into glucose and phosphate1,2 by either the liver/kidney/intestine-restricted glucose-6-phosphatase-α (G6Pase-α)4 or the ubiquitously expressed glucose-6-phosphatase-β (G6Pase-β).5 Mutations of G6Pase-α result in the metabolic disorder glycogen storage disease type Ia (GSD-Ia),1 and mutations of G6Pase-β result in a severe congenital neutropenia syndrome.1,6,7 GSD-Ib provides the link between these diseases, being a glycogen storage disease with neutropenia.1,2 GSD-Ia and GSD-Ib arise from disruption of the activity of the G6PT/G6Pase-α complex that is critical for the maintenance of interprandial glucose homeostasis.1,2 Neutropenia arises from disruption of the activity of an analogous G6PT/G6Pase-β complex5-8 that also hydrolyzes G6P to glucose.5 Therefore, in nongluconeogenic cells, such as neutrophils8-10 and macrophages,11 which lack the G6PT/G6Pase-α complex, the G6PT/G6Pase-β complex plays a critical role in meeting increased demands for glucose.1,2 This explains why both GSD-Ib9,12 and G6Pase-β–deficient6,8,10 patients manifest neutropenia caused by enhanced neutrophil ER stress, oxidative stress, and apoptosis, arising from loss of G6PT/G6Pase-β activity. An additional feature common to GSD-Ib and G6Pase-β deficiency is neutrophil dysfunction characterized by impaired respiratory burst, chemotaxis, and calcium mobilization activities.1,2 Several studies showed that bone marrows in some GSD-Ib13,14 and G6Pase-β–deficient6 patients exhibit neutrophil maturation arrest, which might contribute to neutrophil dysfunction observed clinically. In this study, we examine the maturation state and functionality of neutrophils in 15 human GSD-Ib patients. We show that the majority of GSD-Ib patients do manifest neutrophil maturation arrest but neutrophils from all patients are dysfunctional, indicating that maturation arrest is not the underlying cause of neutrophil dysfunction in GSD-Ib.
Neutrophils cannot produce glucose via gluconeogenesis,15 and their glucose is supplied by uptake from the blood mediated by the glucose transporters (GLUTs).16 Within neutrophils, glucose is metabolized by hexokinase (HK) to G6P.17,18 We have recently shown that there are 3 primary pathways competing for intracellular G6P/glucose in neutrophils, namely glycolysis, the hexose monophosphate shunt (HMS), and the cycling of G6P/glucose between the cytoplasm and ER.10 The latter pathway is mediated by the G6PT/G6Pase-β complex. In cycling, G6P enters the ER via G6PT, where it accumulates until it is hydrolyzed to glucose by G6Pase-β and returns to the cytoplasm. By limiting the cytoplasmic availability of G6P/glucose, cycling regulates the other cytoplasmic pathways for G6P metabolism. Consequently, a disruption of this cycling in G6Pase-β–deficient neutrophils results in impaired energy homeostasis and functionality.10 Previous studies have shown that G6PT-deficient neutrophils exhibit decreased glucose uptake19 and their intracellular levels of G6P are markedly lower20 compared with neutrophils in control subjects. We hypothesized that G6PT-deficient neutrophils would also exhibit impaired energy homeostasis, which would underlie, at least in part, neutrophil dysfunction in GSD-Ib.
Neutrophils play a key role in the early inflammatory response to infection and are highly dependent on anaerobic glycolysis for adenosine triphosphate (ATP) production.21 The transcription factor hypoxia-inducible factor-1α (HIF-1α) plays a vital role in the regulation of glycolytic capacity and energy metabolism in myeloid cells22,23 and is sensitive to the oxidative state of cells. In neutrophils, the HIF-1α pathway regulates survival24 and improves innate immune function.25 Interestingly, neutrophils exhibited impaired respiratory burst activity under hypoxia, a condition that increases HIF-1α protein levels.26 HIF-1α is also an upstream activator of peroxisome proliferator-activated receptor-γ (PPAR-γ),27 a nuclear receptor that regulates lipid and glucose metabolism and inflammation.28 We hypothesized that the HIF-1α/PPAR-γ pathway would be activated in neutrophils of immune-deficient GSD-Ib patients. Several observations already supported this hypothesis. G6PT-deficient neutrophils exhibit enhanced oxidative stress and have elevated levels of reactive oxygen species (ROS),9,12 which can stabilize and induce the HIF-1α protein.29 Neutrophils constitutively express PPAR-γ,30 and ligand-activation of PPAR-γ inhibits their chemotactic responses.30 Moreover, nitric-oxide–elicited PPAR-γ activation downregulates the expression of reduced NAD phosphate (NADPH) oxidase subunit, p47phox, leading to inhibition of respiratory burst that plays critical roles in antibacterial host defenses.31
In this report, we examine energy homeostasis and activation of the HIF-1α/PPAR-γ pathway in neutrophils of GSD-Ib patients. We show that G6PT-deficient neutrophils do exhibit impaired energy homeostasis, along with reduced expression and activation of p47phox, compared with neutrophils from healthy subjects. Moreover, the HIF-1α/PPAR-γ pathway is activated in G6PT-deficient neutrophils, leading to impairment in neutrophil respiratory burst, chemotaxis, and calcium mobilization activities. Together, these findings show that the G6PT-mediated G6P/glucose cycling is essential for neutrophil homeostasis and G6P metabolism and that a deficiency leads to impaired energy homeostasis and activation of the HIF-1α/PPAR-γ pathway that underlie, at least in part, neutrophil dysfunction in GSD-Ib.
Methods
Subjects
A total of 15 GSD-Ib patients (aged 5 to 40 years), 4 GSD-Ia patients (aged 18 to 43 years), and 13 healthy donors (HDs) (aged 17 to 42 years) were included in the study. All patients were diagnosed by standard clinical biochemical methods and confirmed by gene mutation analysis. All subjects were treated with daily granulocyte colony-stimulating factor (G-CSF; Amgen) therapy at the time of the studies with doses ranging from 1.0 to 4.8 µg/kg per day. Samples were obtained during research admissions when subjects were in good health. The study was approved by the Clinical Investigation Committee at the University of Florida, College of Medicine and at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH). Informed written consent and assent, when appropriate, was obtained from all subjects participating in the study. The study was conducted in accordance with the Declaration of Helsinki.
GSD-Ib is an ultrarare orphan disease with an incidence of 1:700 000,1,2 and access to patients is limited. Although the primary disease phenotypes are explained by the SLC37A4 mutations, patient-to-patient variability is expected as a result of the low number of patients available for the study and their background genetic variation.
Flow cytometry analysis
Heparinized human peripheral blood cells were erythrocyte-depleted and fixed in Lysis/Fix buffer (BD Biosciences). The resulting leukocytes were stained with a fluorescein-isothiocyanate–conjugated mouse monoclonal CD66b antibody (BD Pharmingen) and a phycoerythrin-conjugated CD16 antibody (eBiosciences). The levels of mature (CD66b+CD16hi) and immature (CD66b+CD16lo/−) neutrophils were analyzed by flow cytometry using a Guava EasyCyte Mini System (Millipore).
Isolation of nonapoptotic human blood neutrophils and analysis of neutrophil function
Heparinized human peripheral blood cells were erythrocyte-depleted with Ack lysing buffer (Quality Biologicals). The resulting leukocytes were incubated with annexin V Microbeads (Miltenyi Biotec) to deplete apoptotic cells as described previously.10 Neutrophils used for all assays were isolated from annexin V–depleted blood leukocytes using the CD66abce MicroBead Kit (Miltenyi Biotec). The viability of annexin V–depleted neutrophils was assessed using the Guava viacount reagent (Millipore) that contains 7-amino-actinomyocin D. The viable neutrophils were annexin V (−) and 7-amino-actinomyocin D (−). The purity and nuclear morphology of isolated neutrophils were examined on Hema-3–stained (Fisher Scientific) cytospin slides. Neutrophil respiratory burst, chemotaxis, and calcium flux were determined as described previously.8-10
Immunofluorescence microscopy
Neutrophils were plated onto glass slides by cytospin, fixed in paraformaldehyde, and incubated with Image-iFX signal enhancer (Invitrogen) as described previously.10 To examine translocation of GLUT1 and p47phox to the plasma membrane, fixed neutrophils were incubated with rabbit polyclonal antibodies against GLUT1 (Santa Cruz Biotechnology) and pan Cadherin (Abcam) or mouse monoclonal antibodies against p47phox (BD biosciences) and pan Cadherin. Neutrophils were then incubated with the appropriate immunoglobulin G antibody, conjugated with Alexa Fluor 488 or 555 (Invitrogen), mounted with an anti-fade medium containing 4′6-diamidino-2-phenylindole (DAPI) (Vector Laboratories), and visualized using a Zeiss Axiovert 200M inverted confocal microscope equipped with 40×/1.3 NA or 63×/1.4 numeric aperture (NA) oil objectives (Carl Zeiss Microimaging). Images were acquired using LSM 5 acquisition software. To detect HIF-1α, fixed neutrophils were permeabilized in 0.3% Triton X-100 and incubated with a mouse monoclonal antibody against HIF-1α (Novus Biologicals). Then, neutrophils were incubated with an anti–mouse immunoglobulin G antibody, conjugated with Alexa Fluor 488, mounted with anti-fade medium containing DAPI, and visualized using an EVOS fl inverted microscope equipped with 20×/0.4 and 40×/0.65 NA objectives (Advanced Microscopy Group). Images were acquired using AMG acquisition software.
The integrated fluorescence intensity of each stained cell and the integrated fluorescence intensity of protein translocated to the plasma membrane in the merged cells were quantified using ImageJ software (NIH). The intensity of unstained cells was used for background subtraction.
Quantitative real-time RT-PCR, western blot, and cytokine analyses
Messenger RNA (mRNA) expression was quantified by real-time reverse-transcription polymerase chain reaction (RT-PCR) in an Applied Biosystems 7300 Real-Time PCR System as described previously.10 The TaqMan probes used were gp91phox, Hs00166163_m1; p22phox, Hs03044361_m1; p47phox, Hs00165362_m1; GLUT1, Hs00892681_m1;HIF-1α, Hs00153153_m1; PPAR-γ, Hs01115513_m1; Hsp90, Hs00743767_sH, and β-actin, Hs99999903_m1. Data were analyzed using SDS (version 1.3) software (Applied Biosystems) and normalized to β-actin RNA.
Western blot analysis was performed as described previously.10,11 Mouse monoclonals used were gp91phox (BioLegend), p47phox (BD Biosciences), and HIF-1α (Novus Biologicals). Rabbit polyclonals used were HK3, GLUT1, p22phox, PPAR-γ (Santa Cruz Biotechnology), and Hsp90 (Cell Signaling). Protein expression was quantified by densitometry as described previously.10,11 Serum interleukin-4 (IL-4) was quantified using Quantikine enzyme-linked immunosorbent assay kits (R&D Systems).
Glucose uptake, G6P, lactate, ATP, and NADPH determination
Glucose uptake and determination of intracellular levels of G6P, lactate, and ATP were as described previously.10 To measure intracellular NADPH, 105 cells were lysed in 200 μL NAD phosphate (NADP)/NADPH extraction buffer as described in the instruction manual (BioVision). To decompose NADP, the extracted NADP/NADPH solution was incubated at 60°C for 30 minutes, followed by cooling on ice. Total NADP/NADPH and NADPH were measured by incubation with NADP cycling enzyme mix (BioVision) for 5 minutes at room temperature to convert NADP to NADPH, and the optical density at 450 nm was determined in a spectrophotometer.
Treating neutrophils with G-CSF, PPAR-γ agonist/antagonist, hypoxia, CoCl2, or 2-ME2
Neutrophils isolated from each human HD were suspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 5.6 mM glucose, pooled, and divided into several groups. Neutrophils in groups 1, 2, and 4 were incubated under normoxia (5% CO2/95% air). Neutrophils in groups 3 and 5 were incubated under either normoxia or hypoxia (5% CO2/5% O2/90% N2) (Hypoxia Incubator Chamber; STEMCELL Technologies).
The PPAR-γ agonist rosiglitazone (Cayman Chemical), the PPAR-γ antagonist GW9662 (Cayman Chemical), and the pharmacologic HIF-1α inhibitor 2-methoxyestradiol (2-ME2)32 (Sigma-Aldrich) were dissolved in 12.8 M dimethylsulfoxide (DMSO).
Group-1 neutrophils were incubated for the indicated times in the presence of 100 ng/mL of G-CSF. Group 2 neutrophils were first incubated with 20 µM of rosiglitazone for 1 hour at 37°C, followed by treating with either 20 µM GW9662 or 0.02% (volume/volume) DMSO for 1 hour at 37°C. Chemotaxis was then performed for 1 hour in response to 10−7 M of N-formyl-methionyl-leucyl-phenylalanine (fMLP) in the presence of GW9662 or DMSO. Group 3 neutrophils were incubated for 2 hours at 37°C under hypoxia with either 20 µM GW9662 or 0.02% DMSO. Group 4 neutrophils were incubated for 2 hours at 37°C with 100 μM of the hypoxia-mimetic CoCl233 (Sigma-Aldrich), including either 20 µM GW9662 or 0.02% DMSO. HD neutrophils incubated for 2 hours at 37°C under normoxia with 0.02% DMSO were used as controls for groups 3 and 4 neutrophils. Group 5 neutrophils were pretreated for 1 hour at 37°C with 2 µM 2-ME2 or 0.02% DMSO under normoxia, followed by incubation for 2 hours at 37°C under hypoxia. HD neutrophils incubated at 37°C for 3 hours in 0.02% DMSO under normoxia were used as controls. Chemotaxis and calcium mobilization in response to 10−7 M fMLP and respiratory burst in response to 200 ng/mL of phorbol 12-myristate 13-acetate (PMA) were determined as described previously.8-10
The effects of GW9662 on the function of G6PT-deficient neutrophils suspended in the above medium were also examined. For chemotaxis analysis, G6PT-deficient neutrophils in response to fMLP was conducted in the presence of either 20 µM GW9662 or 0.02% DMSO for 1 hour at 37°C. For calcium mobilization and respiratory burst analyses,8-10 G6PT-deficient neutrophils were treated with either 20 µM GW9662 or 0.02% DMSO for 1 hour at 37°C.
Statistical analysis
The unpaired Student t test was performed using the GraphPad Prism Program, version 4 (GraphPad Software). Values were considered statistically significant at P < .05.
Results
The dysfunctional G6PT-deficient neutrophils vary greatly in their maturation states
A total of 15 human GSD-Ib patients on daily low-dose G-CSF therapy were included in this study (Table 1). A total of 13 normal HDs and 4 GSD-Ia patients (Table 1) were included as controls. The levels of total (CD66b+) and immature (CD66b+/CD16lo/−) neutrophils in the fixed, erythrocyte-depleted blood leukocytes were analyzed by flow cytometry (Figure 1A). Total blood neutrophil counts in GSD-Ib patients varied greatly, with 5 patients exhibiting mild neutropenia (bold values in Table 1). The immature neutrophils in HDs (n = 13) and GSD-Ia patients (n = 4) averaged 0.4% to 18% of total blood leukocytes. Among the 15 GSD-Ib patients, P13, P14, and P15 had normal levels (4.4% to 17%), 6 had intermediate levels (33% to 58%), and 6 had high levels (64% to 92%) of immature neutrophils (Table 1). The maturation states of neutrophils from GSD-Ib patients were confirmed by nuclear morphologic analysis of the Hema-3–stained cytospin slides (Figure 1B). Notably, no correlation existed between the age of the patients and the maturation states of their neutrophils.
Table 1.
Human GSD type I patients
| Patient ID | Age (y) | Sex | Gene/mutation | ANC* (×10−3/μL) | Total neutrophils CD66b+ (%)† | Immature neutrophils CD66b+-CD16−/lo (%)† | Immature neutrophils (%)‡ |
|---|---|---|---|---|---|---|---|
| GSD-Ib | SLC37A4 | ||||||
| P1 | 13 | M | p.G20D/? | 1.33 | 33.8 | 31.2 | 92.3 |
| P2 | 34 | F | p.R28C/p.G339C | 6.3 | 75.6 | 66.2 | 87.5 |
| P3 | 21 | M | p.G339C/c.1042_1043delCT | 1.86 | 59.2 | 43.9 | 74.2 |
| P4 | 17 | F | p.G363C/p.G363C | 2.16 | 53.6 | 38.7 | 72.2 |
| P5 | 16 | M | p.G363C/p.G363C | 2.12 | 60.1 | 41.2 | 68.6 |
| P6 | 9 | F | p.G68R/p.Q248X | 2.28 | 54.0 | 34.3 | 63.5 |
| P7 | 18 | M | c.381+1G>T/p.C183R | 1.99 | 65.5 | 38.0 | 58.0 |
| P8 | 5 | F | p.G339C/c.1042_1043delCT | 0.69 | 21.5 | 10.2 | 47.4 |
| P9 | 23 | F | c.381+1G>T/p.C183R | 1.46 | 51.6 | 22.4 | 43.4 |
| P10 | 16 | M | c.1042_1043delCT/c.1042_1043delCT | 0.95 | 26.6 | 10.3 | 40.2 |
| P11 | 9 | M | c.1042_1043delCT/c.1042_1043delCT | 0.85 | 27.6 | 9.2 | 33.3 |
| P12 | 25 | M | p.G68R/c.1103ins12 | 0.67 | 27.1 | 8.9 | 32.9 |
| P13 | 8 | F | p.G20D/? | 1.72 | 53.7 | 9.1 | 16.9 |
| P14 | 40 | M | p.G149E/p.G149E | 0.92 | 78.0 | 5.2 | 6.6 |
| P15 | 31 | F | p.G20D/p.G20D | 1.17 | 26.7 | 1.2 | 4.4 |
| GSD-Ia | G6PC | ||||||
| PA1 | 18 | M | p.F177C/p.Q347X | 2.8 | 58.1 | 4.9 | 8.4 |
| PA2 | 31 | M | p.R83C/p.R83C | 6.17 | 51.5 | 5.5 | 10.6 |
| PA3 | 32 | M | p.R83C/p.G188S | 4.58 | 51.7 | 5.6 | 10.9 |
| PA4 | 43 | M | p.R83C/p.Q347X | 6.56 | 66.0 | 3.6 | 5.45 |
ANC, absolute neutrophil count; F, female; M, male. Bold type represents patients exhibiting mild neutropenia.
Neutropenia is defined as an absolute neutrophil count <1.0 × 103 per μL.
The levels of total (CD66b+) and immature (CD66b+CD16−/lo) neutrophils in the fixed, erythrocyte-depleted blood leukocytes were analyzed by flow cytometry using CD66b and CD16 antibodies. The range of neutrophils in normal HDs (n = 13) was 47% to 62%.
The percentage of immature neutrophils represents the ratio of CD66b+CD16−/lo/CD66b+.
Figure 1.
G6PT-deficient neutrophils of GSD-Ib patients with varying maturation states were dysfunctional. (A) Analysis of the levels of total (CD66b+) and immature (CD66b+CD16−/lo) neutrophils in the fixed, erythrocyte-depleted blood leukocytes by flow cytometry using CD66b and CD16 antibodies. Representative profiles from HDs and GSD-Ib and GSD-Ia patients are shown. (B) Hema-3–stained cytospins of isolated peripheral blood neutrophils. (C) Viability of freshly isolated and annexin V–depleted neutrophils. Annexin V–depleted blood neutrophils were used for respiratory burst, chemotaxis, and calcium mobilization analyses. (D) Neutrophil respiratory burst activity in response to PMA. Representative experiments are shown. (E) Neutrophil concentration-dependent chemotaxis in response to fMLP. Data represent the mean ± standard error of the mean (SEM) of 5 patients (P1, P8, P10, P12, and P14) examined in separate experiments. **P < .005. (F) Calcium mobilization in response to 10−7 M of fMLP. Representative experiments are shown. ○, HDs; ●, GSD-Ib patients; ▲, GSD-Ia patients.
Peripheral blood neutrophils of GSD-Ib patients exhibit enhanced apoptosis (Figure 1C).9,12 To measure neutrophil function accurately, we depleted apoptotic cells from blood leukocytes by annexin V MicroBead Kit, followed by using the CD66abce MicroBead Kit to isolate neutrophils from annexin V-depleted blood leukocytes. Annexin V-depleted neutrophils from HDs and GSD-Ib patients were of similar viability (Figure 1C) and were used in the experiments described below.
In neutrophils from HDs or GSD-Ia patients, superoxide production was markedly increased by exposure to PMA, whereas in nonapoptotic neutrophils from GSD-Ib patients, the PMA-stimulated superoxide production was reduced, regardless of the maturation states of the patients’ neutrophils (Figure 1D). Neutrophils from HDs or GSD-Ia patients exhibited a greater chemotactic response to fMLP than neutrophils from GSD-Ib patients (Figure 1E). The neutrophil chemotactic responses of 5 GSD-Ib patients, harboring 4% to 58% of immature neutrophils, varied only slightly (Figure 1E), again suggesting the minor role of the maturation state played in neutrophil chemotaxis. Similarly, calcium mobilization in response to fMLP was impaired in G6PT-deficient neutrophils relative to control neutrophils, whereas GSD-Ia neutrophils exhibited normal calcium mobilization activity (Figure 1F).
Reduced glucose uptake and decreased intracellular G6P, lactate, and ATP in G6PT-deficient neutrophils
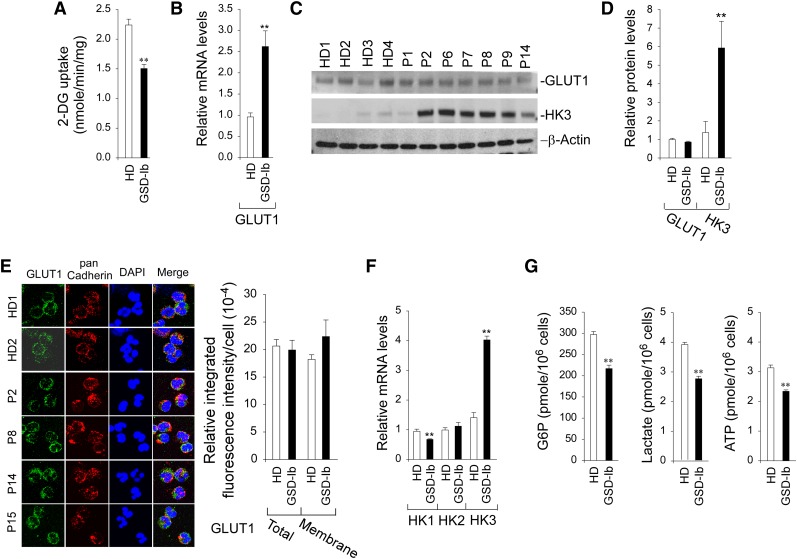
In neutrophils, glucose is mainly supplied from the blood via GLUT1-facilitated uptake.16,34 Within neutrophils, glucose is phosphorylated to G6P by HK.17,18 Therefore, glucose uptake can be controlled by glucose transport and phosphorylation. Annexin V–depleted neutrophils from HDs took up 2-deoxy-D-glucose (2-DG) at a rate 1.5-fold greater than neutrophils from GSD-Ib patients (Figure 2A). The GLUT1 mRNA levels in G6PT-deficient neutrophils were significantly higher than those in HD neutrophils (Figure 2B). However, western blots showed that GLUT1 protein levels in GSD-Ib and HD neutrophils were similar (Figure 2C-D). Confocal microscopic analysis confirmed that membrane-associated GLUT1 was similar between HD and GSD-Ib neutrophils (Figure 2E). The expression of HK1 mRNA is downregulated and HK2 is unchanged, but the expression of HK3, the major HK isozyme in neutrophils,35,36 is increased fourfold in G6PT-deficient neutrophils compared with controls (Figure 2F). Western blots confirmed a 4.3-fold increase in HK3 protein levels in G6PT-deficient neutrophils (Figure 2C-D).
Figure 2.
Analysis of 2-DG uptake, the expression of GLUT1 and HK, and levels of intracellular G6P, lactate, and ATP in G6PT-deficient neutrophils of GSD-Ib patients. Annexin V–depleted peripheral blood neutrophils isolated from HDs and GSD-Ib patients were used in the study. For quantitative RT-PCR, data represent the mean ± SEM for HDs (n = 10) and GSD-Ib patients (n = 12). (A) Uptake of 2-DG. Data represent the mean ± SEM for HDs (n = 3) and GSD-Ib patients (n = 3). (B) Quantification of mRNA of GLUT1 by real-time RT-PCR. (C) Western blot analysis of protein extracts using antibodies against GLUT1 and HK3. Each lane contains 50 μg of protein. (D) Quantification of GLUT1 and HK3 protein levels by densitometry. Data represent the mean ± SEM for HDs (n = 8) and GSD-Ib patients (n = 7). (E) Confocal analysis of GLUT1 (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) at original magnification ×630. (F) Quantification of HK1, HK2, and HK3 mRNA by real-time RT-PCR. (G) Quantification of G6P, lactate, and ATP. Data represent the mean ± SEM for HDs (n = 13) and GSD-Ib patients (n = 12). **P < .005.
We have previously shown that a disruption of the G6PT/G6Pase-β–mediated G6P/glucose cycling in G6Pase-β–deficient neutrophils results in impaired energy homeostasis, characterized by reduced intracellular levels of G6P, ATP, and lactate.10 We now show that G6PT-deficient neutrophils exhibited a similar impairment. The intracellular levels of G6P, lactate, and ATP in neutrophils from GSD-Ib patients (n = 12) were 71.9%, 74.4%, and 68.4%, respectively, of the levels in the control subjects (n = 13) (Figure 2G). In summary, G6PT-deficient neutrophils exhibited impaired glucose uptake and harbored reduced intracellular levels of G6P, lactate, and ATP compared with HD neutrophils.
Reduced NADPH and impaired activation of NADPH oxidase in G6PT-deficient neutrophils
NADPH oxidase plays critical roles in antibacterial host defenses.37-39 The enzyme substrate, NADPH, is produced from G6P via the HMS pathway.17 Consistent with the decrease in intracellular G6P, intracellular levels of NADPH in annexin V–depleted G6PT-deficient neutrophils were 66.3% of the levels in HD neutrophils (Figure 3A).
Figure 3.
Analysis of NADPH and the expression of NADPH oxidase in G6PT-deficient neutrophils of GSD-Ib patients. Annexin V–depleted peripheral blood neutrophils isolated from HDs and GSD-Ib patients were used in the study. (A) Levels of neutrophil NADPH. Data represent the mean ± SEM for HDs (n = 7) and GSD-Ib patients (n = 6). (B) Quantification of gp91phox, p22phox, and p47phox mRNA by real-time RT-PCR. Data represent the mean ± SEM for HDs (n = 10) and GSD-Ib patients (n = 12). (C) Western blot analysis of protein extracts using antibodies against gp91phox, p22phox, p47phox, or β-actin. Each lane contains 50 μg of protein. (D) The relative protein levels of gp91phox, p22phox, and p47phox were quantified by densitometry. Data represent the mean ± SEM for HDs (n = 6) and GSD-Ib patients (n = 6). (E) Confocal analysis of p47phox (green fluorescence), pan Cadherin membrane staining (red fluorescence), and DAPI nuclei staining (blue fluorescence) at original magnification ×630 and quantification of the relative integrated fluorescence intensity by ImageJ. The p47phox translocation from the cytoplasm to the plasma membrane was demonstrated by colocalization of p47phox with the plasma membrane marker pan Cadherin. **P < .005.
NADPH oxidase is a multicomponent enzyme system composed of 2 transmembrane proteins, gp91phox and p22phox, and several cytosolic proteins.37,38 For activation of NADPH oxidase activity, the cytosolic subunit, p47phox must translocate from the cytoplasm to the plasma membrane.40 In G6PT-deficient neutrophils, gp91phox mRNA levels were increased and p22phoxmRNA levels were unchanged compared with HD neutrophils (Figure 3B). Hayee et al41 have shown that gp91phox is hypoglycosylated in neutrophils of GSD-Ib patients but the abnormal glycosylation does not interfere with electron transport or assembly of the oxidase complex in a cell-free system. Western blots showed that levels of both glycosylated and hypoglycosylated gp91phox existed in HD and GSD-Ib neutrophils and their levels varied greatly (Figure 3C). In agreement with Hayee et al,41 levels of glycosylated gp91phoxin G6PT-deficient neutrophils were significantly lower than that in HD neutrophils (Figure 3D). Western blots confirmed that neutrophil p22phox protein levels were similar between HDs and GSD-Ib patients (Figure 3D).
Quantitative real-time RT-PCR (Figure 3B) and western blots (Figure 3C-D) showed that the expression of p47phox was decreased in G6PT-deficient neutrophils compared with the controls. Confocal microscopic analysis showed that the amounts of p47phox translocated to the plasma membrane were markedly decreased in G6PT-deficient neutrophils (Figure 3E). Therefore, the impaired respiratory burst inherent of G6PT-deficient neutrophils is associated with impairment of both the expression of p47phox and the activation of NADPH oxidase.
Activation of the HIF-1α/PPAR-γ pathway in G6PT-deficient neutrophils
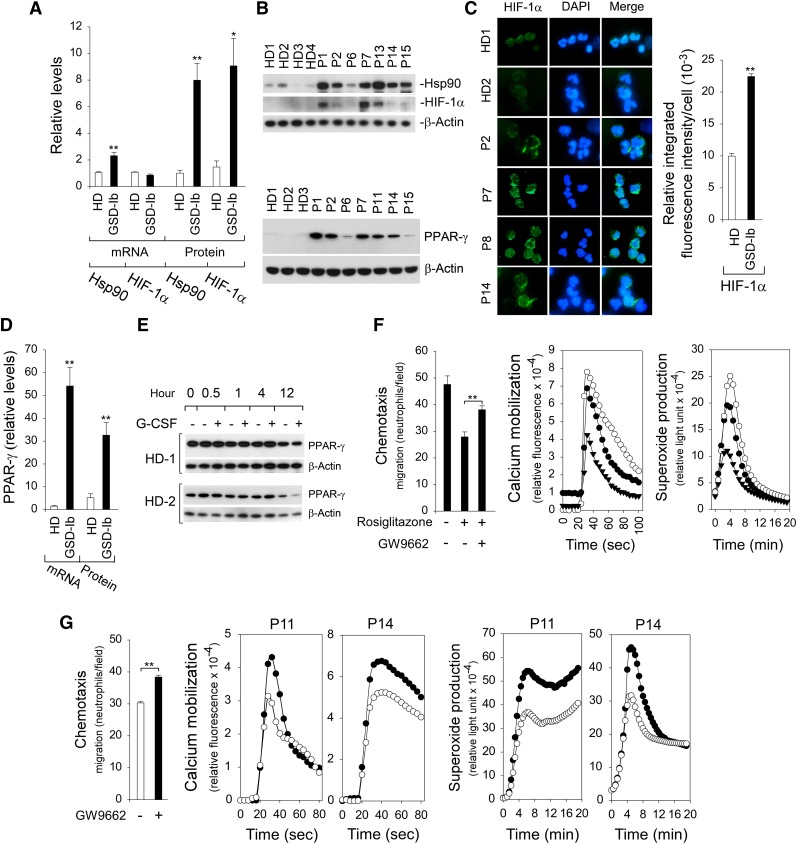
The regulation of HIF-1α occurs primarily at the level of protein stabilization.22 In addition to the hypoxia environment, HIF-1α can be activated by various stimuli, including ROS22,29 and Hsp90.42,43 Studies have shown that G6PT-deficient neutrophils exhibit elevated levels of ROS.9,12 We now show that levels of Hsp90 transcript and protein (Figure 4A-B) were markedly increased in annexin V–depleted G6PT-deficient neutrophils when compared with controls. The expression of neutrophil HIF-1α mRNA was similar between GSD-Ib patients and HDs (Figure 4A). In contrast, HIF-1α protein levels in G6PT-deficient neutrophils were 6.2-fold higher than the levels in HD neutrophils (Figure 4A-B). Immunofluorescence analysis confirmed the increase in the HIF-1α protein in G6PT-deficient neutrophils (Figure 4C).
Figure 4.
Analysis of levels of Hsp90, HIF-1α, and PPAR-γ in G6PT-deficient neutrophils and the effects of PPAR-γ antagonist/agonist on neutrophil function. Annexin V–depleted peripheral blood neutrophils isolated from HDs and GSD-Ib patients were used in the study. (A) Quantification of Hsp90 and HIF-1α mRNA levels by real-time RT-PCR and protein levels by densitometry. Data for RT-PCR represent the mean ± SEM for HDs (n = 10) and GSD-Ib patients (n = 12), and data for protein levels represent mean ± SEM for HDs (n = 11) and GSD-Ib patients (n = 7). (B) Western blot analysis of protein extracts using antibodies against Hsp90, HIF-1α, PPAR-γ, or β-actin. Data represent the mean ± SEM for HDs (n = 11) and GSD-Ib patients (n = 7). (C) Immunofluorescence of HIF-1α (green fluorescence) and DAPI nuclei staining (blue fluorescence) at original magnification ×400 and quantification of the relative integrated fluorescence intensity by ImageJ. (D) Quantification of PPAR-γ mRNA levels by real-time RT-PCR and protein levels by densitometry. Data for RT-PCR represent the mean ± SEM for HDs (n = 10) and GSD-Ib patients (n = 12), and data for PPAR-γ protein represent the mean ± SEM for HDs (n = 11) and GSD-Ib patients (n = 7). (E) Western blot analysis of the effects of G-CSF on PPAR-γ expression in HD neutrophils after in vitro culturing. (F) Effects of PPAR-γ agonist rosiglitazone and antagonist GW9662 on function of neutrophils isolated from HDs. Three independent experiments were conducted with similar results. Chemotaxis was examined in response to 10−7 M fMLP. Data represent the mean ± SEM. Calcium mobilization was examined in response to 10−7 M fMLP. Representative profiles are shown. Respiratory burst was examined in response to PMA. Representative profiles are shown. ○, control; ▲, PPAR-γ agonist rosiglitazone; ●, PPAR-γ agonist rosiglitazone followed by antagonist GW9662. (G) Effects of PPAR-γ antagonist GW9662 on function of G6PT-deficient neutrophils isolated from 2 GSD-Ib patients. Two independent experiments using neutrophils isolated from P11 and P14 were conducted. Chemotaxis was examined in response to 10−7 M fMLP. Data represent the mean ± SEM of both patients. Calcium mobilization was examined in response to 10−7 M fMLP. Respiratory burst was examined in response to PMA. ○, control; ●, PPAR-γ antagonist GW9662. **P < .005, *P < .05.
HIF-1α is an activator of PPAR-γ.27 The levels of PPAR-γ mRNA (Figure 4D) and protein (Figure 4B,D) in neutrophils of GSD-Ib patients were elevated 36-fold and 6.3-fold, respectively, over the levels in neutrophils of HDs. Studies have shown that in sepsis patients, an increase in neutrophil PPAR-γ levels is mediated by induction of IL-4.30 This was not the case in GSD-Ib patients, where serum levels of IL-4 were similar to the levels in HDs (not shown). GSD-Ib patients included in this study are under G-CSF therapy. We therefore examined the expression of PPAR-γ in HD neutrophils after culturing in vitro in medium containing G-CSF. Figure 4E shows that G-CSF has little effect on PPAR-γ expression in HD neutrophils upon short exposure (0 to 4 hours), whereas at longer exposures (12 hours) there may be decreased expression.
To further test the hypothesis that dysfunctional chemotaxis, calcium mobilization, and respiratory burst activities in neutrophils of GSD-Ib patients are mediated by PPAR-γ, we examined the effects of PPAR-γ agonist rosiglitazone and/or antagonist GW9662 on the function of freshly isolated HD neutrophils. The chemotaxis and calcium mobilization in response to fMLP and the PMA-stimulated superoxide production in HD neutrophils were markedly inhibited by rosiglitazone but reversed upon the addition of GW9662 (Figure 4F). Importantly, in G6PT-deficient neutrophils, fMLP-mediated chemotaxis and calcium mobilization, as well as the PMA-stimulated superoxide production, were markedly improved by the addition of PPAR-γ antagonist GW9662 (Figure 4G).
To demonstrate that inhibition of neutrophil function by PPAR-γ is mediated via HIF-1α signaling, neutrophil function was examined in HD neutrophils that had increased HIF-1α and PPAR-γ protein levels induced by exposure to either hypoxic conditions (Figure 5A) or the hypoxia mimetic CoCl233 (Figure 5B). Neutrophil chemotaxis, calcium mobilization, and superoxide production were all inhibited by hypoxia (Figure 5A) or CoCl2 (Figure 5B). These functional impairments were reversed upon the addition of a PPAR-γ antagonist GW9662 that also decreased the protein levels of HIF-1α and PPAR-γ (Figure 5A-B). HIF-1α is an activator of PPAR-γ,27 but activation of PPAR-γ also increases the expression of HIF-1α,44 suggesting that the expression of HIF-1α and PPAR-γ are codependent.
Figure 5.
Inhibition of neutrophil function by PPAR-γ is mediated via HIF-1α signaling. Annexin V–depleted peripheral blood neutrophils isolated from HDs were used in the study. (A) Effects of the PPAR-γ antagonist GW9662 on chemotaxis, calcium mobilization, and respiratory burst activities of neutrophils exposing to hypoxic conditions, and western blot analysis of protein extracts using antibodies against HIF-1α, PPAR-γ, or β-actin. Three independent experiments were conducted with similar results. ○, normoxia; ●, hypoxia; ▾, hypoxia and PPAR-γ antagonist GW9662. (B) Effects of the PPAR-γ antagonist GW9662 on chemotaxis, calcium mobilization, and respiratory burst activities of neutrophils exposed to the hypoxia mimetic CoCl2, and western blot analysis of protein extracts using antibodies against HIF-1α, PPAR-γ, or β-actin. Three independent experiments were conducted with similar results. ○, control; ●,CoCl2; ▾, CoCl2 and PPAR-γ antagonist GW9662. (C) Effects of HIF-α inhibitor 2-ME2 on chemotaxis, calcium mobilization, and respiratory burst activities of neutrophils exposed to hypoxic conditions, and western blot analysis of protein extracts using antibodies against HIF-1α, PPAR-γ or β-actin. Three independent experiments were conducted with similar results. ○, normoxia; ●, hypoxia; ▾, hypoxia and 2-ME2. Chemotaxis was examined in response to 10−7 M fMLP. Data represent the mean ± SEM. Calcium mobilization was examined in response to 10−7 M fMLP. Representative profiles are shown. Respiratory burst was examined in response to PMA. Representative profiles are shown. **P < .005, *P < .05.
To further demonstrate that activation of the HIF-1α/PPAR-γ pathway impairs neutrophil function, HD neutrophils were exposed to 2-ME2, a pharmacologic inhibitor of HIF-1α activity.32 As in the previous experiment, the hypoxia-mediated inhibition of neutrophil calcium mobilization and superoxide production was associated with increased levels of HIF-1α and PPAR-γ (Figure 5C) and the functional impairment was reversed upon the addition of 2-ME2 that also reduced the levels of both proteins (Figure 5C). Studies have shown that microtubules control neutrophil motility and disruption of microtubules impairs neutrophil chemotaxis.45 2-ME2 disrupts microtubules, an action required for HIF-1α downregulation.32 Consequently, the hypoxia-mediated inhibition of neutrophil chemotaxis was not reversed by 2-ME2 treatment (Figure 5C).
Discussion
The G6PT and G6Pase-β activities are known to be functionally codependent.1,2 One role of the G6PT/G6Pase-β complex is to maintain intracellular glucose homeostasis in neutrophils6-10 and macrophages11 during periods of increased energy demand. Recent studies showed that the loss of the G6PT/G6Pase-β activity in GSD-Ib9,12 and G6Pase-β deficiency6,8,10 leads to increased neutrophil ER stress, oxidative stress, and apoptosis, leading to neutropenia (Figure 6). Beyond neutropenia, GSD-Ib– and G6Pase-β–deficient patients also manifest neutrophil dysfunction characterized by impairments in respiratory burst, chemotaxis, and calcium mobilization.1,2 The underlying mechanism of neutrophil dysfunction is not understood well. There are 3 primary pathways competing for intracellular G6P/glucose in neutrophils, namely glycolysis, the HMS, and the G6PT/G6Pase-β–mediated cycling of G6P/glucose between the cytoplasm and ER10 (Figure 6). We have shown that disruption of G6P/glucose cycling in G6Pase-β deficiency leads to impaired neutrophil energy homeostasis, which underlies, at least in part, the neutrophil dysfunction seen in this disorder.10 We hypothesized that G6PT-deficient neutrophils would also exhibit impaired energy homeostasis. In this study, we show that neutrophils from GSD-Ib patients exhibit reduced glucose uptake and decreased intracellular levels of G6P, ATP, lactate, and NADPH (Figure 6).
Figure 6.
Proposed mechanisms that underlie neutrophil dysfunction in GSD-Ib. Glucose transported into the cytoplasm via GLUT1 is metabolized by HK to G6P, which participates in 3 major pathways: glycolysis, the HMS, and ER cycling. In cycling, G6P enters the ER via G6PT, where it can accumulate until it is hydrolyzed to glucose by G6Pase-β and transported back into the cytoplasm. By limiting the cytoplasmic glucose/G6P availability, cycling regulates the other 2 cytoplasmic pathways for G6P metabolism. Disruption of ER cycling in G6PT-deficient neutrophils results in reduced glucose uptake and impaired energy homeostasis and functionality. The underlying cause of neutropenia in GSD-Ib is enhanced neutrophil ER stress and oxidative stress.10 The increases in Hsp90 and ROS in G6PT-deficient neutrophils stabilize HIF-1α, an upstream activator of PPAR-γ. The increase in PPAR-γ downregulates neutrophil respiratory burst, chemotaxis, and calcium mobilization activities. GLUT1, responsible for the transport of glucose in and out of the cell, is shown embedded in the plasma membrane. The G6PT, responsible for the transport of G6P into the ER, and G6Pase-β, responsible for hydrolyzing G6P to glucose and phosphate, are shown embedded in the ER membrane. Thick arrows indicate the changes caused by a defect in G6PT activity.
One reasonable explanation of the cytoplasmic G6P/glucose deficiency in G6PT-deficient neutrophils is that ER cycling and blood glucose uptake are coregulated by HK3. Both G6PT- and G6Pase-β–deficient neutrophils exhibit reduced glucose uptake and decreased intracellular levels of G6P, ATP, and lactate. Although GLUT1 levels were decreased in G6Pase-β–deficient neutrophils,10 GLUT1 expression in G6PT-deficient neutrophils was similar to that of HD neutrophils, suggesting mechanisms beyond transporter availability are involved. Glucose uptake can also be controlled by glucose phosphorylation. The major HK isozyme in neutrophils is HK3,36 which also plays the role of protecting cells from oxidant-induced cell death.46 The expression of HK3 is upregulated by HIF-1α,46 and consistent with this we observed that both HK3 mRNA and protein are increased 4-fold in G6PT-deficient neutrophils. In ER cycling, G6P enters the ER via G6PT, where it accumulates until it is hydrolyzed by G6Pase-β to glucose. The ER-regenerated glucose then diffuses across the ER membrane into the cytoplasm, where it can be rephosphorylated by HK to G6P and re-enter glycolysis or HMS pathway or be recycled again via the ER. In neutrophils of normal subjects, one role of HK3 is to maintain a glucose concentration gradient across the ER membrane necessary for glucose to diffuse from the ER into the cytoplasm. In G6PT-deficient neutrophils, ER cycling is blocked and upregulation of HK3 fails to increase the glucose gradient across the ER to rectify the cytoplasmic deficiency. Because an increase in HK activity will also increase the glucose gradient across the cell membrane, increasing the rate of GLUT-facilitated diffusion, this raises the possibility that ER cycling also regulates glucose uptake from the plasma membrane mediated by GLUT. Studies have shown that GLUT-mediated uptake of blood glucose also increases when cytoplasmic levels of lactate and ATP increase.47,48 In G6PT-deficient neutrophils, with lower-than-normal levels of ATP and lactate, this mechanism of increasing glucose uptake is also not available, exacerbating the low cytoplasmic G6P/glucose levels caused by the absence of ER cycling.
In addition to impaired energy homeostasis, we provide evidence that activation of the HIF-1α/PPAR-γ pathway leads to impairments in respiratory burst, chemotaxis, and calcium mobilization in G6PT-deficient neutrophils (Figure 6). GSD-Ib patients included in this study were under G-CSF therapy, which improves neutrophil counts and decreases the number and severity of bacterial infections.14,49 Therefore, one concern is that the G-CSF therapy may impact the findings from the patient neutrophils to the extent that G-CSF by itself might be activating the HIF-1α/PPAR-γ pathway. We now show that PPAR-γ expression is not affected by G-CSF treatment in HD neutrophils. Although we cannot demonstrate this in G6PT-deficient neutrophils without interfering with patients’ standard of care, it is reasonable to suggest that G-CSF does not drive PPAR-γ expression. Instead the HIF-1α/PPAR-γ pathway in G6PT-deficient neutrophils is stimulated in response to the increase in ROS9,12 and Hsp90 (this study), which are inducers of HIF-1α via protein stabilization.29,42,43 HIF-1α is an upstream activator of PPAR-γ.27 Studies have shown that in monocytes/macrophages, PPAR-γ activation downregulates the expression of p47phox,31 an activating subunit of NADPH oxidase.37-40 Sepsis patients have increased serum IL-4 levels that activate PPAR-γ expression, leading to impaired neutrophil chemotaxis.30 We show that the expression of both HIF-1α and PPAR-γ in G6PT-deficient neutrophils is also markedly stimulated compared with HD neutrophils but independent of IL-4. Moreover, the expression and membrane translocation of p47phox is reduced in G6PT-deficient neutrophils. We further show that freshly isolated human neutrophils exposed to a PPAR-γ agonist have reduced respiratory burst, chemotaxis, and calcium mobilization activities, but these activities are restored by the addition of a countering PPAR-γ antagonist. Moreover, exposing G6PT-deficient neutrophils to a PPAR-γ antagonist improves their function.
HIF-1α regulates the expression of multiple genes, but the function of this transcription factor is complex and not fully understood. For instance, myeloid-lineage–specific knockout mice have reduced neutrophil survival24 and decreased bactericidal activity25 but normal neutrophil endothelial transcytosis or oxidative burst function.25 However, under hypoxia, a condition that increases HIF-1α protein levels, neutrophils are still negatively impacted, with impaired respiratory burst activity.26 We now show that in both HD and G6PT-deficient neutrophils, activation of PPAR-γ, a nuclear receptor that acts downstream of HIF-1α,27 leads to neutrophil dysfunction characterized by impairments in respiratory burst, chemotaxis, and calcium mobilization activities. We further provide evidence demonstrating that inhibition of neutrophil function by PPAR-γ is mediated via HIF-1α signaling. In HD neutrophils, the increase in HIF-1α protein levels either by hypoxia or the hypoxia mimetic CoCl233 reduces respiratory burst, chemotaxis, and calcium mobilization activities, but these activities are restored upon the addition of a PPAR-γ antagonist. Furthermore, downregulation of HIF-1α activity by 2-ME232 reverses the hypoxia-mediated inhibition of neutrophil calcium mobilization and respiratory burst activities.
In conclusion, we have shown that G6PT expression is important for neutrophil energy homeostasis and that the G6PT defect leads to reduced glucose uptake and decreased intracellular levels of G6P, ATP, lactate, and NADPH. The disruption in neutrophil energy homeostasis leads to impaired neutrophil function. We have also shown that the expression and membrane translocation of the NADPH oxidase subunit p47phox is downregulated in G6PT-deficient neutrophils, explaining why respiratory burst activity is impaired. Finally, we show that the HIF-1α/PPAR-γ pathway that directly impacts neutrophil chemotaxis, respiratory burst, and calcium mobilization is activated in G6PT-deficient neutrophils. Taken together, our results demonstrate that the underlying cause of neutrophil dysfunction in GSD-Ib arises from impaired neutrophil energy homeostasis and activation of the HIF-1α/PPAR-γ pathway. The insight into the etiology of neutrophil dysfunction in GSD-Ib should facilitate the development of novel therapies for this disorder.
Acknowledgments
This research was supported by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. Additional support was provided by the following philanthropic funds managed through the University of Florida Foundation: Jonah Pournazarian Type Ib GSD Research Fund, the Efforts for Ellie Fund, Jamie Konieczka Type Ib GSD Research Fund, Jerry Kaczur’s Fund, and the Type Ib Glycogen Storage Disease Research Fund. Additional support was also provided by the next generation of BioGreen21 project (PJ008196/PJ008127) from the Rural Development Administration, Republic of Korea.
Footnotes
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.S.J. designed and performed the research, analyzed data, and wrote the paper; D.A.W. and Y.M.L. performed the research and analyzed data; B.C.M. analyzed data and wrote the paper; and J.Y.C. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janice Y. Chou, Building 10, Room 9D42, NIH, 10 Center Dr, Bethesda, MD 20892-1830; e-mail: chouja@mail.nih.gov.
References
- 1.Chou JY, Jun HS, Mansfield BC. Glycogen storage disease type I and G6Pase-β deficiency: etiology and therapy. Nat Rev Endocrinol. 2010;6(12):676–688. doi: 10.1038/nrendo.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou JY, Jun HS, Mansfield BC. Neutropenia in type Ib glycogen storage disease. Curr Opin Hematol. 2010;17(1):36–42. doi: 10.1097/MOH.0b013e328331df85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan C-J, Lin B, Chou JY. Transmembrane topology of human glucose 6-phosphate transporter. J Biol Chem. 1999;274(20):13865–13869. doi: 10.1074/jbc.274.20.13865. [DOI] [PubMed] [Google Scholar]
- 4.Lei K-J, Shelly LL, Pan C-J, Sidbury JB, Chou JY. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science. 1993;262(5133):580–583. doi: 10.1126/science.8211187. [DOI] [PubMed] [Google Scholar]
- 5.Shieh J-J, Pan C-J, Mansfield BC, Chou JY. A glucose-6-phosphate hydrolase, widely expressed outside the liver, can explain age-dependent resolution of hypoglycemia in glycogen storage disease type Ia. J Biol Chem. 2003;278(47):47098–47103. doi: 10.1074/jbc.M309472200. [DOI] [PubMed] [Google Scholar]
- 6.Boztug K, Appaswamy G, Ashikov A, et al. A syndrome with congenital neutropenia and mutations in G6PC3. N Engl J Med. 2009;360(1):32–43. doi: 10.1056/NEJMoa0805051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein C. Genetic defects in severe congenital neutropenia: emerging insights into life and death of human neutrophil granulocytes. Annu Rev Immunol. 2011;29:399–413. doi: 10.1146/annurev-immunol-030409-101259. [DOI] [PubMed] [Google Scholar]
- 8.Cheung YY, Kim SY, Yiu WH, et al. Impaired neutrophil activity and increased susceptibility to bacterial infection in mice lacking glucose-6-phosphatase-β. J Clin Invest. 2007;117(3):784–793. doi: 10.1172/JCI30443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SY, Jun HS, Mead PA, Mansfield BC, Chou JY. Neutrophil stress and apoptosis underlie myeloid dysfunction in glycogen storage disease type Ib. Blood. 2008;111(12):5704–5711. doi: 10.1182/blood-2007-12-129114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jun HS, Lee YM, Cheung YY, et al. Lack of glucose recycling between endoplasmic reticulum and cytoplasm underlies cellular dysfunction in glucose-6-phosphatase-β-deficient neutrophils in a congenital neutropenia syndrome. Blood. 2010;116(15):2783–2792. doi: 10.1182/blood-2009-12-258491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun HS, Cheung YY, Lee YM, Mansfield BC, Chou JY. Glucose-6-phosphatase-β, implicated in a congenital neutropenia syndrome, is essential for macrophage energy homeostasis and functionality. Blood. 2012;119(17):4047–4055. doi: 10.1182/blood-2011-09-377820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuijpers TW, Maianski NA, Tool AT, et al. Apoptotic neutrophils in the circulation of patients with glycogen storage disease type 1b (GSD1b). Blood. 2003;101(12):5021–5024. doi: 10.1182/blood-2002-10-3128. [DOI] [PubMed] [Google Scholar]
- 13.Ambruso DR, McCabe ER, Anderson D, et al. Infectious and bleeding complications in patients with glycogenosis Ib. Am J Dis Child. 1985;139(7):691–697. doi: 10.1001/archpedi.1985.02140090053027. [DOI] [PubMed] [Google Scholar]
- 14.Calderwood S, Kilpatrick L, Douglas SD, et al. Recombinant human granulocyte colony-stimulating factor therapy for patients with neutropenia and/or neutrophil dysfunction secondary to glycogen storage disease type 1b. Blood. 2001;97(2):376–382. doi: 10.1182/blood.v97.2.376. [DOI] [PubMed] [Google Scholar]
- 15.Stjernholm RL, Burns CP, Hohnadel JH. Carbohydrate metabolism by leukocytes. Enzyme. 1972;13(1):7–31. doi: 10.1159/000459647. [DOI] [PubMed] [Google Scholar]
- 16.Pessin JE, Bell GI. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- 17.Beutler E. Composition and metabolism of neutrophils. In: Lichman MA, Buetler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, editors. Williams Hematology. 6th ed. New York: McGraw Hill; 2001. pp. 745–752. [Google Scholar]
- 18.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(Pt 12):2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 19.Bashan N, Potashnik R, Hagay Y, Moses SW. Impaired glucose transport in polymorphonuclear leukocytes in glycogen storage disease Ib. J Inherit Metab Dis. 1987;10(3):234–241. doi: 10.1007/BF01800068. [DOI] [PubMed] [Google Scholar]
- 20.Verhoeven AJ, Visser G, van Zwieten R, Gruszczynska B, Tien Poll-The DW, Smit GP. A convenient diagnostic function test of peripheral blood neutrophils in glycogen storage disease type Ib. Pediatr Res. 1999;45(6):881–885. doi: 10.1203/00006450-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 2011;18(9):1457–1469. doi: 10.1038/cdd.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bárdos JI, Ashcroft M. Negative and positive regulation of HIF-1: a complex network. Biochim Biophys Acta. 2005;1755(2):107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Walmsley SR, Print C, Farahi N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201(1):105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peyssonnaux C, Datta V, Cramer T, et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115(7):1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGovern NN, Cowburn AS, Porter L, et al. Hypoxia selectively inhibits respiratory burst activity and killing of Staphylococcus aureus in human neutrophils. J Immunol. 2011;186(1):453–463. doi: 10.4049/jimmunol.1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan J, Suter M, Windak R, et al. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 2009;9(6):512–524. doi: 10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 29.Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275(33):25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 30.Reddy RC, Narala VR, Keshamouni VG, Milam JE, Newstead MW, Standiford TJ. Sepsis-induced inhibition of neutrophil chemotaxis is mediated by activation of peroxisome proliferator-activated receptor-gamma. Blood. 2008;112(10):4250–4258. doi: 10.1182/blood-2007-12-128967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Knethen A, Brüne B. Activation of peroxisome proliferator-activated receptor gamma by nitric oxide in monocytes/macrophages down-regulates p47phox and attenuates the respiratory burst. J Immunol. 2002;169(5):2619–2626. doi: 10.4049/jimmunol.169.5.2619. [DOI] [PubMed] [Google Scholar]
- 32.Mabjeesh NJ, Escuin D, LaVallee TM, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3(4):363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Y, Hilliard G, Ferguson T, Millhorn DE. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem. 2003;278(18):15911–15916. doi: 10.1074/jbc.M300463200. [DOI] [PubMed] [Google Scholar]
- 34.Schuster DP, Brody SL, Zhou Z, et al. Regulation of lipopolysaccharide-induced increases in neutrophil glucose uptake. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L845–L851. doi: 10.1152/ajplung.00350.2006. [DOI] [PubMed] [Google Scholar]
- 35.Rijksen G, Staal GE, Beks PJ, Streefkerk M, Akkerman JW. Compartmentation of hexokinase in human blood cells. Characterization of soluble and particulate enzymes. Biochim Biophys Acta. 1982;719(3):431–437. doi: 10.1016/0304-4165(82)90230-6. [DOI] [PubMed] [Google Scholar]
- 36.Federzoni EA, Valk PJ, Torbett BE, et al. PU.1 is linking the glycolytic enzyme HK3 in neutrophil differentiation and survival of APL cells. Blood. 2012;119(21):4963–4970. doi: 10.1182/blood-2011-09-378117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397(2):342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 38.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16(1):42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30(3):279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 41.Hayee B, Antonopoulos A, Murphy EJ, et al. G6PC3 mutations are associated with a major defect of glycosylation: a novel mechanism for neutrophil dysfunction. Glycobiology. 2011;21(7):914–924. doi: 10.1093/glycob/cwr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katschinski DM, Le L, Heinrich D, et al. Heat induction of the unphosphorylated form of hypoxia-inducible factor-1alpha is dependent on heat shock protein-90 activity. J Biol Chem. 2002;277(11):9262–9267. doi: 10.1074/jbc.M110377200. [DOI] [PubMed] [Google Scholar]
- 43.Davenport EL, Morgan GJ, Davies FE. Untangling the unfolded protein response. Cell Cycle. 2008;7(7):865–869. doi: 10.4161/cc.7.7.5615. [DOI] [PubMed] [Google Scholar]
- 44.Zhou J, Zhang W, Liang B, et al. PPARgamma activation induces autophagy in breast cancer cells. Int J Biochem Cell Biol. 2009;41(11):2334–2342. doi: 10.1016/j.biocel.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoo SK, Lam PY, Eichelberg MR, Zasadil L, Bement WM, Huttenlocher A. The role of microtubules in neutrophil polarity and migration in live zebrafish. J Cell Sci. 2012;125(Pt 23):5702–5710. doi: 10.1242/jcs.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyatt E, Wu R, Rabeh W, Park HW, Ghanefar M, Ardehali H. Regulation and cytoprotective role of hexokinase III. PLoS One. 2010;5(11):e13823. doi: 10.1371/journal.pone.0013823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medina RA, Southworth R, Fuller W, Garlick PB. Lactate-induced translocation of GLUT1 and GLUT4 is not mediated by the phosphatidyl-inositol-3-kinase pathway in the rat heart. Basic Res Cardiol. 2002;97(2):168–176. doi: 10.1007/s003950200008. [DOI] [PubMed] [Google Scholar]
- 48.Kim MS, Lee J, Ha J, et al. ATP stimulates glucose transport through activation of P2 purinergic receptors in C(2)C(12) skeletal muscle cells. Arch Biochem Biophys. 2002;401(2):205–214. doi: 10.1016/S0003-9861(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 49.Visser G, Rake JP, Labrune P, et al. Granulocyte colony-stimulating factor in glycogen storage disease type 1b. Results of the European Study on Glycogen Storage Disease Type 1. Eur J Pediatr. 2002;161(suppl 1):S83–S87. doi: 10.1007/s00431-002-1010-0. [DOI] [PubMed] [Google Scholar]