Key Points
This is the first report to describe the influence of BAMBI on both hemostasis and thrombus stability.
BAMBI present in the blood vessel wall (most likely the endothelium) rather than platelet BAMBI is required for thrombus stability.
Abstract
Bone morphogenetic protein and activin membrane-bound inhibitor (BAMBI) is a transmembrane protein related to the transforming growth factor-β superfamily, and is highly expressed in platelets and endothelial cells. We previously demonstrated its positive role in thrombus formation using a zebrafish thrombosis model. In the present study, we used Bambi-deficient mice and radiation chimeras to evaluate the function of this receptor in the regulation of both hemostasis and thrombosis. We show that Bambi−/− and Bambi+/− mice exhibit mildly prolonged bleeding times compared with Bambi+/+ littermates. In addition, using 2 in vivo thrombosis models in mesenterium or cremaster muscle arterioles, we demonstrate that Bambi-deficient mice form unstable thrombi compared with Bambi+/+ mice. No defects in thrombin generation in Bambi−/− mouse plasma could be detected ex vivo. Moreover, the absence of BAMBI had no effect on platelet counts, platelet activation, aggregation, or platelet procoagulant function. Similar to Bambi−/− mice, Bambi−/− transplanted with Bambi+/+ bone marrow formed unstable thrombi in the laser-induced thrombosis model that receded more rapidly than thrombi that formed in Bambi+/+ mice receiving Bambi−/− bone marrow transplants. Taken together, these results provide strong evidence for an important role of endothelium rather than platelet BAMBI as a positive regulator of both thrombus formation and stability.
Introduction
Recent transcriptomic and proteomic studies of platelets and their precursor cells, megakaryocytes (MKs), have greatly expanded our understanding of platelet biology and thrombus formation.1-5 As part of this effort, we previously identified a panel of platelet membrane proteins that were expressed more highly in MKs and resting endothelial cells (ECs) than in other blood cell lineages, but with hitherto unknown roles in platelet function or thrombus formation.6 Of the 279 genes identified, 4 (including bone morphogenetic protein and activin membrane-bound inhibitor [BAMBI]) were selected for functional analysis in zebrafish.7 Morpholino-mediated knockdown of Bambi in zebrafish larvae resulted in decreased thrombus formation following laser injury in vivo,7 suggesting that BAMBI supports thrombus formation.
BAMBI is a 260-aa transmembrane protein that is very highly conserved in vertebrates from humans to zebrafish.8-11 BAMBI has been assigned to the transforming growth factor-β (TGFβ) superfamily due to the high homology of its extracellular domain to the TGFβ type I receptors (TGFβRI). Similar to TGFβRI, BAMBI can dimerize with itself, as well as associate stably with all type I (except ALK2) and type II TGFβ superfamily receptors, in a ligand-independent manner.10,12,13 It has, therefore, been postulated that BAMBI can inhibit, or modulate, TGFβ/bone morphogenetic protein (BMP)/activin-mediated signaling by preventing the homo- and heterodimerization of type I/II receptors that is required for transducing TGFβ/BMP/activin-dependent signals.10,12 Interestingly, and in contrast to TGFβRI, BAMBI is devoid of an intracellular kinase domain. Indeed, the amino acid sequence of its intracellular domain does not resemble any known domains, making it difficult to predict the function or mode of action of this domain. In various cell lines, BAMBI expression can be upregulated by TGFβ,14 BMP4,15 β-catenin,16 and downregulated by lipopolysaccharide.13 For the latter, it is interesting to note that this effect was not observed in ECs17 suggesting that the modulation of BAMBI expression (and its potential effect on TGFβ/BMP/activin-mediated signaling) is cell-type dependent.
Several studies have implied a role of BAMBI in human diseases,13,18-21 particularly in certain cancers.18,22-24 Members of the TGFβ superfamily play an important role in cardiac development and angiogenesis, which is highlighted by the phenotypes exhibited by knockout mice. Tgfb1−/−,25 Tgfb2−/−,26 Tgfb3−/−,27 Bmp2−/−,28 Bmp4−/−,29 Bmp10−/−,30 Alk1−/−,31 Alk3−/−,32,33 and Smad4−/−34 knockout mice all have cardiac defects and have an embryonic lethal phenotype. It was perhaps, therefore, unexpected that Bambi-deficient mice are both viable and fertile.35,36 A recent study highlighted differences in the endothelial composition of the myocardium and glomeruli in Bambi−/− mice compared with wild-type animals.37 An enhanced angiogenic response in vivo using Matrigel implants was also observed.37 A role for BAMBI in some of the remodeling processes following heart pressure overload38 and mechanical injury of the femoral artery has also been reported,39 although how these phenotypes are conferred is still unclear.
In light of our initial findings in the zebrafish, the present study was initiated to further understand the role of BAMBI in hemostasis and its contribution to thrombus formation in mammals. Given its high expression in both platelets and ECs, we hypothesized that BAMBI plays an important hemostatic role by influencing platelet and/or endothelial function/phenotype. Using 2 in vivo models of thrombosis, we confirm the role of BAMBI as a positive regulator of thrombus formation. Our data further suggest that BAMBI derived from the vessel wall, in particular the endothelium, is key to its influence on hemostasis and thrombus stability.
Materials and methods
Generation of Bambi−/− mice (breeding, genotyping)
All animal work was performed in compliance with animal ethics guidelines at Imperial College London according to the UK Home Office’s Animals (Scientific Procedures) Act 1986. The Bambiflox mice in a C57BL/6 background have been described previously.35 Bambiflox embryos (The Jackson Laboratory) were rederived by standard techniques. Bambi+/− mice were generated by crossing Bambiflox/flox mice with βactin-Cre transgenic mice (kind gift from Dr T. Rodriguez, Imperial College London) and subsequently backcrossed onto a C57BL/6 background (Charles River Laboratories) to delete the Cre allele. Bambi+/− mice were intercrossed for the generation and maintenance of Bambi−/− mice and to provide Bambi+/− and Bambi+/+ control littermates.
Genotyping was performed by polymerase chain reaction (PCR) amplification of total genomic DNA (gDNA) prepared from ear-punch samples. The wild-type Bambi (215 bp) and deleted Bambi (316 bp) alleles were detected by PCR using previously described F2R2 and F2R4 primers, respectively35 (for supplemental Methods, see supplemental Data available on the Blood Web site).
Reverse transcription PCR
To detect Bambi messenger RNA (mRNA), total RNA was extracted from mouse lung and bone marrow tissues using Trizol reagent (Invitrogen). Total RNA (2 µg) was reverse-transcribed to generate complementary DNA using the One Taq PCR kit (New England Biolabs). Three different primer pairs were used to detect Bambi as well as a Gapdh control (see supplemental Methods for cycling conditions and primers).
Materials
See supplemental Data for more information on materials.
Platelet preparation
Mice were anesthetized intraperitoneally with Hypnorm/Midazolam (10 µL/g) and bled from the retro-orbital plexus into tubes containing 3.8% citrate anticoagulant. Platelets were prepared according to standard procedures with few modifications to increase the yield of platelet recovery (see supplemental Methods).
Hematologic analysis and bleeding time
Complete blood counts were obtained using an automated cell counter (Sysmex XE2100). Platelet counts were also evaluated using calibrated beads (Saxon Europe) and flow cytometry was performed according to the manufacturer’s instructions. Tail-bleeding assays were performed in anesthetized animals (age- and litter-matched, ∼6 weeks old) by cutting 2 mm of the tail tip. Tails were immediately immersed in phosphate-buffered saline; time taken to arrest bleeding was recorded up to 20 minutes. In each case, the volume of blood loss after 10 minutes was measured.
Aggregometry
Light transmission was measured on a 2-channel aggregometer (Chronolog 700) with continuous stirring at 1200 rpm at 37°C (>5 minutes). Platelet aggregation was induced in washed platelets (3 × 108/mL) supplemented with 1mM CaCl2 and fibrinogen (70 µg/mL) and by addition of either adenosine 5′-diphosphate (ADP) (0-10μM), U46619 (0-1μM), thrombin (0-0.2 U/mL), or collagen (0-5 μg/mL).
Flow cytometry
To quantify platelet surface glycoprotein expression, whole blood was diluted 1/20 with modified Tyrode buffer and platelets were stained with appropriate fluorophore-conjugated antibodies (Abs) (αIIbβ3 [Leo.H4], GPVI [JAQ1], GPIbβ [×488]) for 15 minutes at room temperature. For activation studies, washed platelets were incubated with various agonists (see “Aggregometry”) and stained for 15 minutes at room temperature with JON/A and Wug.E9 Abs, or Annexin V (BD Biosciences) in the presence of 1 mM or 2.5 mM CaCl2, respectively. Samples were analyzed using a FACSCalibur flow cytometer.
Flow chamber assay
Vena8 Fluoro+ biochips (Cellix) were coated overnight with 0.1 mg/mL fibrillar type I collagen (Nycomed) and blocked with 1% bovine serum albumin. Mouse blood was collected into 3.8% citrate and 1 U/mL Heparin (Sanofi) and incubated with 2.5µM DIOC6 (Molecular Probes) for 5 to 10 minutes at 37°C. Blood was recalcified prior to perfusion at 1500 s−1 using Mirus pump (Cellix) and aggregate formation was visualized with a Vert.A1 inverted microscope (Zeiss; 20× objective) equipped with ExiBlue camera (Q Imaging). Fluorescence pictures were recorded and analyzed offline using Slidebook software.
Intravital microscopy
Experiments were conducted using a VIVO platform (3i) with a large platform stage plate and an Axio Examiner Z1 microscope (Zeiss), with simultaneous capture of brightfield and fluorescent images through a high-sensitivity CCD camera (Rolera em-c2; Q-Imaging). The same settings (light intensities, binning, intensifier gain, and exposure times) were used in each set of experiments. Genotypes of the mice were blinded to the operator during both data acquisition and analysis.
FeCl3 thrombosis model in mesenteric arterioles.
Bambi−/− mice and littermates (4-5.5 week old) were anesthetized as described in “Platelet preparation” and injected IV with DIOC6 (1.6 nmol/g). The mesentery was exposed and the injury was induced by applying a 10% FeCl3-saturated filter paper on the vessels for 2 minutes. The filter paper was removed and thrombus formation in arterioles was monitored for 40 minutes or until complete occlusion of the vessel (blood flow arrest for >1 minutes).
Laser-induced thrombosis model in the cremaster muscle.
Male Bambi−/− mice and littermates (20-31 g) were anesthetized and injected IV with DIOC6 (1.8 nmol/g). The cremaster muscle was exteriorized, connective tissue was removed, and the muscle was spread on a glass coverslip that was continuously superfused with prewarmed sterile saline buffer (Macoflex). Mice were maintained at 37°C on a thermo-controlled rodent blanket (Harvard Apparatus). The injury was induced by a pulse laser (Ablate!, 3i) focused through a 63× water-immersion objective. Typically, the laser beam was adjusted to 65% to 75% of maximal intensity and 2 to 4 pulses were applied for all injuries in arterioles of similar diameter. Up to 7 thrombi were formed per mouse with new thrombi always formed upstream of earlier ones and at least 4 mice per group were studied. Data were collected over 4 minutes and analyzed using Slidebook software to determine the median integrated fluorescence intensities (IFIs) over time.40 Thrombus stability was calculated by measuring the time required after each thrombus reached maximal size to decrease by 50%.
Thrombin generation
Thrombin generation in citrated mouse platelet-rich plasma (PRP) or in platelet-poor plasma (PPP) was assessed by calibrated automated thrombography (CAT) using a Fluoroskan Ascent FL plate reader (Thermo) in combination with Thrombinoscope software (Synapse BV) as described previously41,42 (see supplemental Methods).
Radiation chimeras
Bone marrow cells (BMCs) were harvested under sterile conditions from the tibiae and femur of adult female Bambi+/+ and Bambi−/− mice, washed, and resuspended in Dulbecco modified Eagle medium supplemented with 20% fetal bovine serum. All male recipient mice received a total body irradiation of 8.0 Gy and were transplanted with 107 donor BMCs within 4 hours postirradiation. Mice were maintained in sterile cages containing autoclaved food and water supplemented with antibiotics for 6 weeks posttransplantation. BMC uptake by recipient male mice was determined by PCR from gDNA isolated from 106 BMCs using the Pure Link Genomic DNA kit (Invitrogen). The wild-type and deleted Bambi alleles were amplified as described in “Generation of Bambi−/− mice.” Survival rate was 100% and levels of chimerism in each mouse were estimated to be >80% using mixtures of Bambi+/+ and Bambi−/− gDNA as standards.
Statistical analysis
Unless otherwise indicated, results are presented as mean ± SEM from n ≥3 mice per experimental group and analyzed using GraphPad Prism (version 4.02). Statistical analysis between Bambi+/+ and Bambi−/− mice was assessed by the unpaired Student t test or the Mann-Whitney test. All experiments comparing 3 groups of animals were analyzed by analysis of variance (ANOVA) (Kruskal-Wallis). Bleeding times were analyzed by the χ2 linear trend test with SPSS software (version 20).
Results
Bambi−/− mice exhibit a mild hemostatic defect
Bambiflox/flox mice were crossed with βactin-Cre mice to delete loxP-flanked sequences in germ cells. Successful ablation of the Bambi allele was confirmed at the DNA level (Figure 1A). Western blot detection of BAMBI was not possible as no commercial Abs are currently available that specifically recognize murine BAMBI. Therefore, reverse transcription PCR (RT-PCR) analysis was performed, which revealed the presence of the Bambi mRNA in BMCs and lungs of wild-type mice but not in Bambi−/− mice (Figure 1B and supplemental Figure 1). Bambi−/− mice were born at the expected Mendelian frequencies with female Bambi−/− mice displaying a significant reduction in body weight (supplemental Figure 2), consistent with a previous report.35 However, we also observed that 11 of 84 female Bambi−/− mice died of yet unexplained cause between 3.5 and 5 weeks after birth; no mortality was observed for Bambi+/+ littermates at the same age. No other overt phenotype (eg, spontaneous bleeding) other than reduced body weight has been observed in Bambi−/− mice.
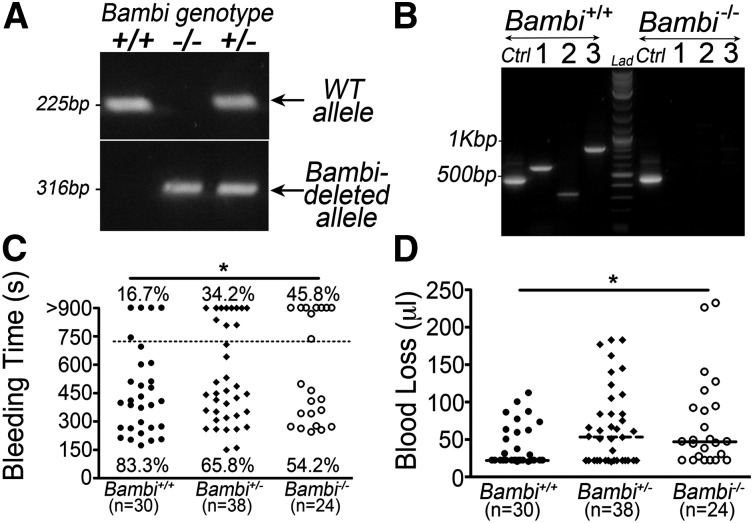
Figure 1.
Genetic ablation of Bambi leads to increased tail-bleeding time. (A) PCR was performed on DNA isolated from ear biopsies using previously described primers35 leading to the amplification of the wild-type allele (top; 225bp), and the Bambi-deleted allele (bottom; 316bp). (B) RT-PCR analysis of BMCs isolated from wild-type and Bambi−/− mice using primers specific to Bambi transcript (1:587 bp; 2:329 bp; 3:812 bp) or Gapdh (Ctrl; 452bp). (C-D) Analysis of tail-bleeding experiments on Bambi+/+ Bambi+/− and Bambi−/− mice. (C) Graph showing the time to cessation in bleeding for all animals according to genotype. The percentage of mice that were still bleeding after 12 minutes (dotted line) was significantly higher in Bambi-deficient mice compared with wild-type littermates. Statistical analysis was performed using the χ2 test for linear trend (P < .02). Each symbol represents 1 animal. (D) Bambi-deficient mice exhibited a significant increase in blood loss (determined after 10 minutes). Each symbol represents 1 animal. Horizontal lines intersecting data sets represent the median. Statistical analysis was performed using ANOVA (Kruskal-Wallis). *P < .05.
Despite normal platelet counts and other hematologic parameters (Table 1), Bambi-deficient mice displayed a mild but significant increase in bleeding times compared with wild-type littermates (Figure 1C). Whereas bleeding stopped within 12 minutes in 25 of 30 (83%) wild-type mice (mean bleeding time 7.5 ± 0.7 minutes), 13 of 38 (34%) of Bambi+/−, and 11 of 24 (46%) Bambi−/− mice were still bleeding (mean 9.2 ± 0.7 minutes, 9.8 ± 1 minutes, respectively). This increase in bleeding time was accompanied by an increase in blood loss in Bambi+/− and Bambi−/− mice (67 ± 8 µL, 70 ± 12 µL, respectively) compared with Bambi+/+ mice (41 ± 5 µL) (Figure 1D). These results suggest that Bambi deficiency elicits a hemostatic defect.
Table 1.
Hematologic values in Bambi-deficient mice
| Mouse genotype | |||
|---|---|---|---|
| Bambi+/+ | Bambi+/− | Bambi−/− | |
| RBC, 106/µL | 9.7 ± 0.2 | 10 ± 0.2 | 9.7 ± 0.4 |
| WBC, 103/µL | 6.9 ± 0.6 | 8.1 ± 0.5 | 8.8 ± 1.2 |
| Hg, g/dL | 15 ± 0.3 | 15.1 ± 0.3 | 14.7 ± 0.7 |
| Ht, % | 48.9 ± 1.3 | 49.8 ± 0.9 | 48.2 ± 1.7 |
| PLT, 103/µL | 1025 ± 42.1 | 1085 ± 27.0 | 1045 ± 85.1 |
| MPV, fL | 6.3 ± 0.1 | 6.4 ± 0.02 | 6.4 ± 0.1 |
Values given are mean ± SEM of n >4 males for each genotype.
Hg, hemoglobin; Ht, hematocrit; MPV, mean platelet volume; PLT, platelet; RBC, red blood cell; WBC, white blood cell.
BAMBI plays a crucial role in the formation of stable thrombi in vivo
To investigate the effect of BAMBI on thrombus formation in vivo, we performed the FeCl3-induced thrombosis assay in mesenteric arterioles. Although initial platelet aggregate formation was comparable between Bambi-deficient mice and wild-type littermates (Figure 2A, 2 minutes), the time for vessels to occlude was significantly prolonged in both Bambi−/− and Bambi+/− animals (Figure 2). Complete occlusion was observed after 14 ± 2 minutes in Bambi+/+ mice, whereas no vessel occlusion was observed in arterioles of Bambi−/− mice during this time period (mean 28 ± 3 minutes) (Figure 2 and supplemental Videos 1-2). Interestingly, Bambi+/− mice displayed an increased time to occlusion (26 ± 2 minutes) compared with Bambi+/+ mice and numerous embolization events during thrombus formation, similar to Bambi−/− littermates (supplemental Video 3).
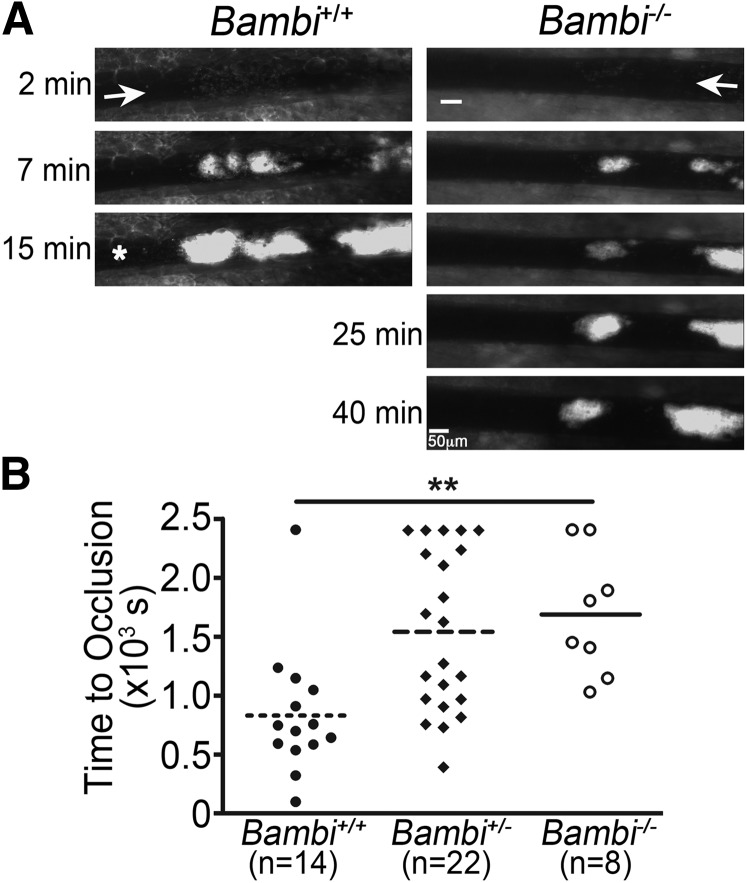
Figure 2.
Impaired thrombus formation in Bambi-deficient mice after FeCl3 injury in vivo. (A-B) Mesenteric arterioles were injured with FeCl3 and adhesion and thrombus formation of fluorescently labeled platelets was followed by intravital microscopy. (A) Representative images of thrombus formation at indicated times after removal of the filter paper. Scale bar represents 50 µm. A white asterisk indicates occlusion of the vessel for the wild-type mouse. (B) Bambi+/− and Bambi−/− mice displayed a delayed vascular occlusion compared with Bambi+/+ littermates. Each symbol represents 1 animal. Horizontal lines intersecting data sets represent the mean. Statistical analysis was performed using ANOVA (Kruskal-Wallis). **P < .01.
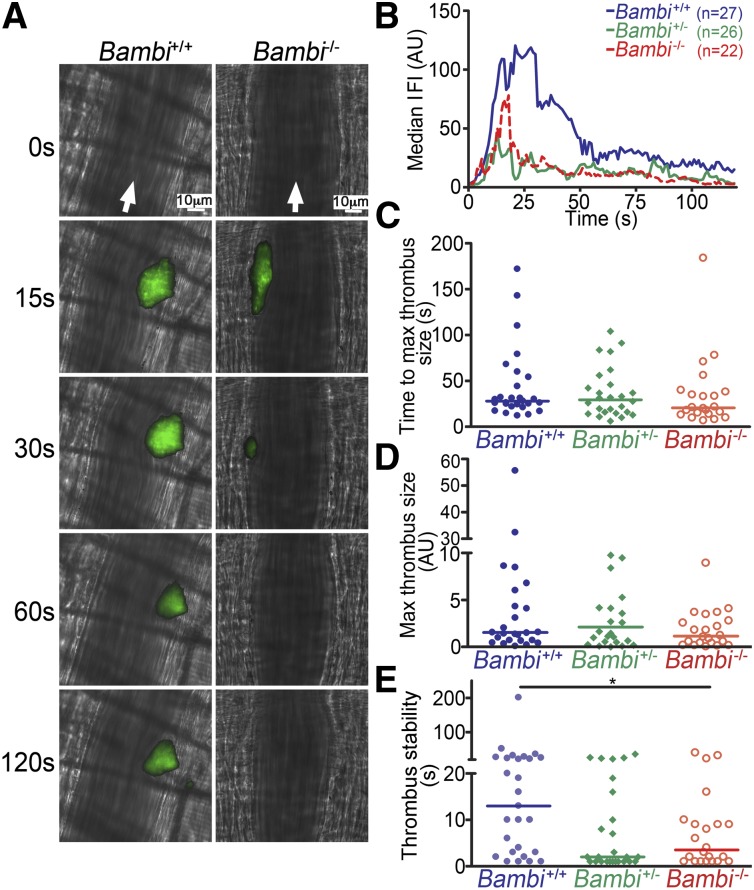
To further explore the role of BAMBI in thrombus formation, a second in vivo model of thrombosis was initiated by laser injury of cremaster muscle arterioles. In this model, both Bambi+/− and Bambi−/− mice displayed impaired thrombus stability compared with wild-type littermates (Figure 3). Initial kinetic profiles were similar in all mice (Figure 3A-B) as we observed no differences that reached significance for the time to peak thrombus size (Figure 3C) or the maximal thrombus size (Figure 3D). Interestingly, thrombi receded more rapidly in Bambi-deficient mice compared with wild-type littermates as illustrated in Figure 3B (>30 seconds postinjury). Indeed, many more embolization events could be observed in Bambi+/− and Bambi−/− mice when compared with Bambi+/+ mice (supplemental Videos 4-6). This effect was quantified by measuring the time taken for each thrombus after reaching maximal size to decrease by 50%. Using this parameter, we demonstrated a significant decrease in thrombus stability for Bambi-deficient mice compared with wild-type animals (Figure 3E).
Figure 3.
Decreased thrombus stability in Bambi-deficient mice after laser-induced thrombosis model in vivo. (A) Representative composite fluorescence and brightfield images of laser-induced thrombus formation in arterioles of Bambi+/+ and Bambi−/− mice. Scale bar represents 10 µm. (B) Median IFI is depicted (AU) as a function of time after the injury. Distribution of the time to maximal thrombus size (C) and maximal thrombus size expressed in IFI AU (D) in wild-type and Bambi-deficient mice. (E) Thrombus stability was assessed by determining the time taken for each thrombus at maximal size to fall by 50% of maximal IFI. See supplemental Videos 4-6 for better visualization of the differences in thrombus stability between the different animal groups. Each symbol represents 1 thrombus: Bambi+/+ (n = 27), Bambi+/− (n = 26), and Bambi−/− mice (n = 22). Horizontal lines intersecting data sets represent the median. Statistical analysis was performed using ANOVA (Kruskal-Wallis). *P < .05. AU, arbitrary unit.
Taken together, these data indicate that BAMBI plays a role in thrombus formation/stabilization, irrespective of the vascular bed or type of injury.
BAMBI deficiency does not affect platelet function
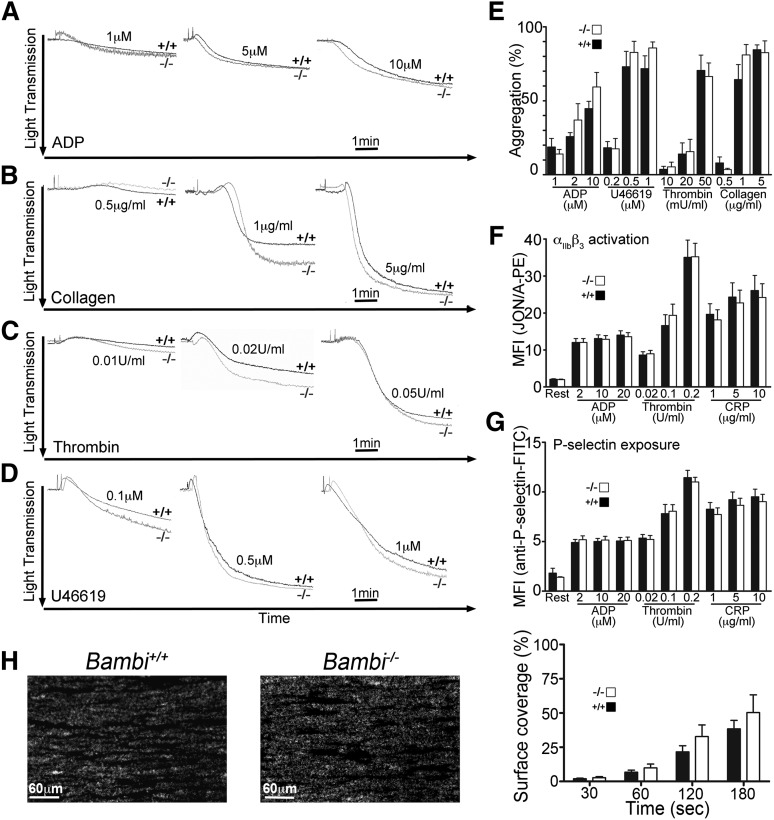
We initially hypothesized that the mechanism underlying the hemostatic defect and altered thrombus formation/stability observed in Bambi-deficient mice might be due to a platelet defect. However, platelets from Bambi−/− mice aggregated normally compared with Bambi+/+ mice in response to all agonists (ADP, U46619, thrombin, collagen) used at low, intermediate, or high concentration (Figure 4A-E). Consistent with this, there was no difference observed in the activation profile of the platelet integrin αIIbβ3 nor in the expression of P-selectin following stimulation with ADP, thrombin, or collagen-related peptide (CRP) in Bambi−/− platelets compared with Bambi+/+ platelets (Figure 4F-G). In addition, surface expression levels of 3 major platelet glycoproteins (αIIbβ3, GPVI, and GPIb) on resting platelets were similar in Bambi−/− mice compared with Bambi+/+ mice (supplemental Figure 3). There was also no difference in platelet adhesion and aggregate formation over collagen-coated surfaces at 1500 s−1 shear rate (Figure 4H). Based on these findings, we believe it unlikely that the altered hemostasis and thrombus formation observed in Bambi-deficient mice is due to a defect in platelet function.
Figure 4.
Bambi−/− mice display normal platelet function. (A-D) Representative aggregation traces of washed platelets isolated from Bambi+/+ and Bambi−/− mice and stimulated with the indicated concentrations of agonists. Light transmission was recorded on a Chrono-log 2 channel aggregometer over 5 minutes. (E) Bar graphs of results obtained by aggregometry. Results are given as the mean percentage of aggregation ± SEM (n ≥ 3 mice for each condition). Statistical analysis was performed using the unpaired Student t test (P > .05). (F-G) Flow cytometry analysis of αIIbβ3 integrin activation (F) and P-selectin expression (G) in response to the indicated concentrations of agonists in platelets from Bambi+/+ (n = 8) and Bambi−/− mice (n = 11). Data represent mean fluorescence intensities ± SEM. Statistical analysis was performed using the unpaired Student t test (P > .05). (H) Whole blood from Bambi+/+ and Bambi−/− mice was perfused over a collagen-coated surface (0.1 mg/mL) at a shear rate of 1500 s−1. (Left) Representative fluorescence images of aggregate formation on collagen after 3 minutes of perfusion time. Scale bar equals 60 µm. (Right) Surface covered by fluorescently labeled platelets expressed as the percentage of the total surface observed was calculated at the indicated perfusion times. Data represent mean ± SEM (n > 6 for each group). Statistical analysis was performed using the unpaired Student t test (P > .05).
Unchanged procoagulant function in Bambi−/− mice
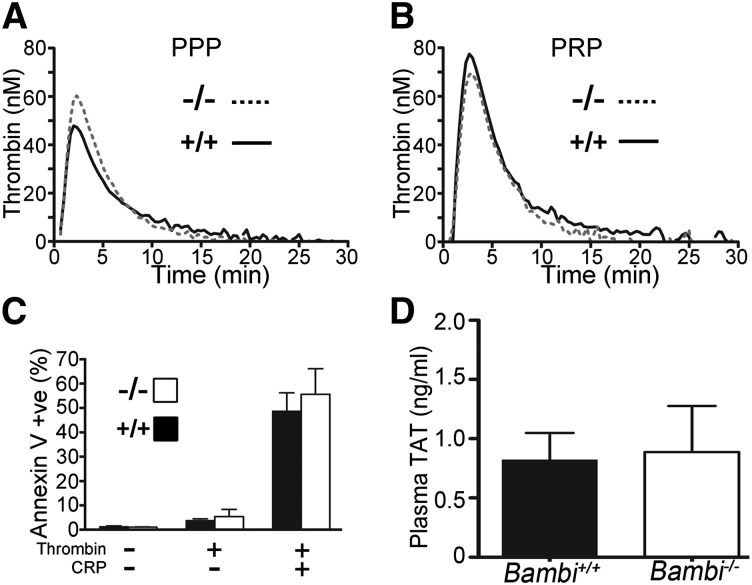
Due to the normal function of Bambi−/− platelets, we next tested whether Bambi deficiency influenced thrombin generation using the CAT assay. In the presence of 1pM tissue factor (TF), no difference in the lag time or the thrombin peak height could be observed in Bambi−/− mouse PPP compared with control Bambi+/+ littermates (Figure 5A and supplemental Table 1). Moreover, the ability of Bambi−/− platelets to contribute to thrombin generation was assayed using PRP, and was again, indistinguishable from Bambi+/+ PRP (Figure 5B and supplemental Table 1). This was supported by the very similar levels of phosphatidylserine exposure (measured by Annexin V binding) after stimulation of platelets with thrombin and CRP compared with Bambi+/+ platelets (Figure 5C). Finally, levels of thrombin/antithrombin complexes (TATs) were also similar in plasma from Bambi−/− compared with littermates (Figure 5D).
Figure 5.
Bambi−/− platelets express PS normally and retain their procoagulant ability. Thrombin generation was measured in mouse citrate-anticoagulated plasma supplemented with 1pM TF, 16.6mM CaCl2, fluorogenic substrate Z-GGR-AMC, and 4µM phospholipids (A) or PRP (108/mL final concentration) (B) in the presence of corn trypsin inhibitor. Representative traces of thrombin generation in PPP (A) or PRP (B) from Bambi+/+ (n = 4) and Bambi−/− mice (n = 3). Thrombin generation was determined from the accumulation of fluorescent product and calculated relative to a thrombin calibrator. Each PPP and PRP sample was run in duplicate. For peak height values, see supplemental Table 3. (C) Exposure of PS was detected with Annexin V–FITC by flow cytometry using Bambi+/+ and Bambi−/− washed platelets stimulated with thrombin (1 U/mL), or a combination of thrombin and CRP (10 µg/mL) for 15 minutes. Data represent mean ± SEM (n = 3). Statistical analysis was performed using the unpaired Student t test (P > .05). (D) Levels of TAT complexes in the plasma of Bambi+/+ and Bambi−/− mice. Values are given as mean ± SEM of n = 5 animals for each group with technical replicates >2 for each animal. Statistical analysis was performed using the unpaired Student t test (P > .05). FITC, fluorescein isothiocyanate; PS, phosphatidylserine.
Endothelial BAMBI plays an important role in thrombus stability
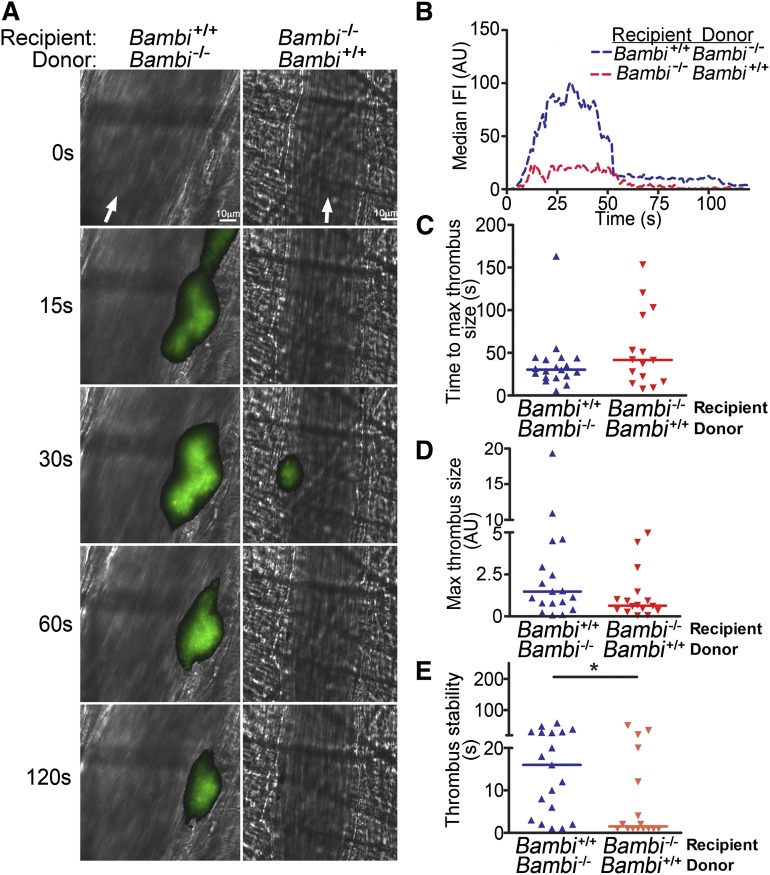
Collectively, our results suggested that BAMBI deficiency did not lead to a platelet or plasma coagulation defect that could be responsible for the in vivo phenotype observed in thrombosis models. As BAMBI was found to be highly expressed in ECs and in platelets,6,7,17,37 we generated radiation chimeric mice to evaluate the contribution of endothelium BAMBI in thrombus formation. BMCs were isolated from Bambi−/− and Bambi+/+ mice and injected into lethally irradiated male Bambi+/+ and Bambi−/− mice, respectively. Bambi−/− mice receiving Bambi+/+ BMC (Bambi−/−/Bambi+/+) lack endothelial BAMBI but express BAMBI in hematopoietic cells, including platelets. Conversely, Bambi+/+ receiving Bambi−/− BMCs (Bambi+/+/Bambi−/−) express endothelial BAMBI but lack BAMBI in their hematopoietic cells. Successful bone marrow transplantation was confirmed by PCR on bone marrow samples (supplemental Figure 3). In addition, all mice achieved successful hematopoietic reconstitution with values in the normal range 7 weeks posttransplantation (supplemental Table 2) when they were subjected to the laser thrombosis model. Similar to the global Bambi knockout, the median IFI over time was reduced in Bambi−/− mice receiving Bambi+/+ BMCs compared with Bambi+/+ mice receiving Bambi−/− BMCs (Figure 6A-B and supplemental Videos 7-8). Although no significant difference in the time to maximal thrombus, or the maximal thrombus size, was observed between chimeric mice (Figure 6C-D), Bambi−/−/Bambi+/+ mice however, displayed a significant decrease in thrombus stability (Figure 6E) very similar to the defect observed in the Bambi−/− mice. These results strongly suggest that BAMBI in the endothelium or underlying vessel wall plays an important role in hemostasis and thrombosis, and that BAMBI lacking from the endothelium is most likely to account for the phenotype in the Bambi−/− mice.
Figure 6.
Endothelial BAMBI is responsible for the defect in thrombus stability in vivo. (A) Representative composite fluorescence and brightfield images of laser-induced thrombus formation in arterioles of Bambi+/+ and Bambi−/− mice receiving BMCs from Bambi−/− and Bambi+/+ mice, respectively. Scale bar represents 10 µm. (B) IFI is depicted (AU) as a function of time after the injury. Distribution of the time to maximal thrombus size (C) and maximal thrombus size expressed in IFI AU (D) in Bambi+/+/Bambi−/− and Bambi−/−/Bambi+/+ mice. (E) Thrombus stability was assessed by determining the time for each thrombus after reaching maximal size to reduce by 50% of maximal IFI. See supplemental Videos 7-8 for better visualization of the differences in thrombus stability between the different animal groups. Each symbol represents 1 thrombus: Bambi+/+/Bambi−/− (n = 19) and Bambi−/−/Bambi+/+ mice (n = 16). Horizontal lines intersecting data sets represent the median. Statistical analysis was performed using the unpaired Mann-Whitney test. *P < .05.
Discussion
We used a gene deletion approach to investigate the effect of BAMBI deficiency on hemostasis and thrombus formation. Our study demonstrates that Bambi-deficient mice exhibit altered hemostasis and impaired thrombus stability using 3 different in vivo models. We also highlight the likely contribution of endothelial BAMBI in these processes, as opposed to cells derived from the hematopoietic compartments, including platelets.
We investigated the role of BAMBI in thrombus formation using 2 well-characterized in vivo models. Using the FeCl3 arterial injury model in the mesenterium, both Bambi+/− and Bambi−/− mice displayed a significant delay in the time to vessel occlusion. Initial rates of thrombus growth (to 20 µm2) were similar in all mice (data not shown), suggesting that BAMBI does not play a crucial role during, or influence components of, the initial phases of thrombus formation. However, numerous embolization events were evident in both homo- and heterozygous Bambi-deficient mice. It was this phenomenon that appeared to delay or preclude vessel occlusion, and suggested a contribution of BAMBI to thrombus stability (Figure 2). In the laser injury model, the kinetics of thrombus formation over 4 minutes confirmed the reduced thrombus stability in Bambi-deficient mice. Indeed, Bambi-deficient mice were able to form thrombi of similar sizes and at a similar rate as their wild-type littermates. However, once again, thrombi were unstable and embolized rapidly from the vessel walls (Figure 3). These results support the concept that BAMBI acts as a positive regulator of stable thrombus formation in both small cremaster arterioles and larger mesenteric arterioles, irrespective of the injury model used. This is consistent with our previously published work using a zebrafish laser-injury model of thrombosis.7
BAMBI is highly expressed in platelets6,7 and ECs.17,37 Lack of thrombus stability observed in Bambi-deficient mice could be due to a lack of BAMBI in one or both of these cell types. To address this, laser-induced thrombus formation was examined in radiation chimeric mice generated through bone marrow transplantation. Wild-type mice receiving Bambi−/− BMC displayed similar kinetics of thrombus formation and stability than wild-type mice, indicating that BAMBI in the hematopoietic compartment does not influence these processes. This is also compatible with the ex vivo functional tests performed in Bambi−/− platelets demonstrating no defect in platelet activation or aggregation using agonists stimulating tyrosine kinase–linked, or G protein–coupled, receptors and flow chamber assays (Figure 4). In addition, Bambi−/− platelets also retained their procoagulant function, which is important for enhancing coagulation (Figure 5). However, in contrast to this, and similar to the full knockout mice, Bambi−/− mice receiving wild-type BMC transplants were unable to produce stable thrombi and exhibited increased embolization (Figure 6). These results indicate that platelet BAMBI does not contribute appreciably to thrombus stabilization in vivo and strongly implicate the absence of BAMBI from the vessel wall as the cause of reduced thrombus stability. Due to the high expression of BAMBI in ECs, we believe that BAMBI in this compartment is important for these processes; however, involvement of BAMBI in other cells of the vessel wall cannot be discounted at this time.
Thrombus formation and its stabilization is complex and tightly coordinated by cells and components of the vessel wall, platelets, and other blood cells in conjunction with plasma proteins.43,44 Many factors can influence thrombus stability including platelet-ligand interactions, outside-in signaling events via αIIbβ3, presence of soluble molecules such as Gas6, or secondary wave mediators ADP and thromboxane A2. Both nitric oxide and prostacyclin (PGI2) released from the endothelium as well as adenosine triphosphate (ATP) diphosphohydrolase (CD39) and ectonucleotidase (CD73) can influence thrombus formation, as recently shown for Kindlin+/− mice.45 No difference in basal levels of PGI2 (data not shown) was observed in plasma from Bambi−/− mice compared with Bambi+/+ mice, suggesting that PGI2 may not be involved; however, this pathway could be altered when the vessel wall is challenged. Gas6 produced by ECs was recently shown to influence venous thrombus formation through downregulation of TF expression.46 Like BAMBI, other transmembrane proteins expressed in platelets and the endothelium can modulate thrombus growth and/or stability including platelet endothelial cell adhesion molecule (PECAM-1),47 endothelial cell-selective adhesion molecule (ESAM),48 junction adhesion molecule A (JAM-A),49 and tetraspanin 151,50 although for all of these, the attributed phenotype is associated with the platelet, rather than endothelial, pool of these receptors. In that regard, BAMBI appears to differ by influencing this process through its role in the endothelium. At this time, one can only speculate how this might be mediated. For example, it is possible that in the absence of BAMBI, levels of P-selectin and/or immunoglobulin superfamily cellular adhesion molecules such as intercellular adhesion molecule (ICAM)-1 and ICAM-2, PECAM-1, or JAM-A in the endothelium could be altered, in turn impairing anchoring of the thrombi to the injured vessel wall. We are currently exploring some of these possibilities in combination with dissecting the composition of the thrombi (eg, P-selectin and fibrin generated) in the core and shell of the thrombus, as elegantly described in a recent study.51
In addition to its role in thrombus formation, we evaluated the role of BAMBI in hemostasis. Bambi−/− mice displayed mildly prolonged bleeding time in the tail-bleeding assay, and this was ascertained by an increase in blood loss. Interestingly, Bambi+/− mice also had a tendency to bleed, consistent with the outcomes observed in the in vivo thrombosis models used in this study. The bleeding tendency in Bambi-deficient mice is perhaps compatible with a recent study using a mechanical injury of the femoral artery that demonstrated that despite a more rapid re-endothelialization in Bambi−/− mice compared with wild-type animals, Bambi-deficient mice exhibited larger neointima due to important red blood cell extravasion.39 Although the mechanisms underlying these bleeding phenotypes are distinct, and remain to be fully defined, our results suggest that BAMBI in the endothelium is likely to be largely responsible.
At a molecular level, BAMBI has been shown to be involved in Wnt/β-catenin signaling and to negatively influence TGFβ signaling.10,12,13,36-38,52 Of particular interest, Bambi−/− mice have an apparent increased angiogenic potential,37 and exacerbated remodeling in response to heart pressure overload.38 Both of these studies implicated alteration of the TGFβ signaling pathway (canonical and noncanonical) in EC and primary cardiac fibroblasts, respectively. Platelets contain large amounts of latent TGFβ1 in their α-granules (∼40-100× more than other cell types)53 that is released upon platelet activation.54,55 It is possible that altered TGFβ signaling via the endothelium is at least partly responsible for the thrombus instability phenotype observed in vivo. However, Meyer and colleagues recently showed that platelet-deleted TGFβ mice (with 85% reduction of TGFβ levels) occluded normally in a FeCl3-induced carotid thrombosis model,56 which may suggest that our results demonstrating that BAMBI influences thrombus stability may be independent of TGFβ. Indeed, although much has been inferred for the cellular function of BAMBI based on the homology of its extracellular domain to TGFβRI, its unrelated cytoplasmic domain suggests that there is much yet to learn about its actual physiological role and its mode of action.
In summary, we have demonstrated for the first time that BAMBI influences hemostasis and is essential for thrombus stability using 3 complementary in vivo assays. Our findings point to an important role of BAMBI in the endothelium. The mechanism by which BAMBI exerts its effects warrants further investigation. It is perhaps reasonable to speculate that BAMBI could also play an important role in other types of thrombosis and atherosclerosis.
Supplementary Material
Acknowledgments
The authors thank Prof M. Botto (Imperial College) and her staff for their help with animal procedures. They also thank Dr R. Szydlo (Imperial College) for his assistance in statistical analyses, Dr Richard Manning (Haematology Laboratory-Hammersmith Hospital) for the use of the aggregometer, and staff of Imperial College Central Biomedical Services.
This work was supported by the British Heart Foundation: Intermediate Research Fellow grant (I.I.S.-C.; FS/10/004/28165).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: I.I.S.-C. designed and performed experiments, analyzed data, and wrote the paper; J.H.M. and J.A. performed experiments and analyzed data; D.A.L. designed the research, analyzed data, and revised the manuscript; and J.T.B.C. designed the research, performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Isabelle I. Salles-Crawley, Centre for Haematology, Imperial College London, 5th Floor Commonwealth Building, Hammersmith Hospital Campus, Du Cane Rd, London, W12 0NN, United Kingdom; e-mail: i.salles@imperial.ac.uk.
References
- 1.Soranzo N, Rendon A, Gieger C, et al. A novel variant on chromosome 7q22.3 associated with mean platelet volume, counts, and function. Blood. 2009;113(16):3831–3837. doi: 10.1182/blood-2008-10-184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodall AH, Burns P, Salles I, et al. Bloodomics Consortium. Transcription profiling in human platelets reveals LRRFIP1 as a novel protein regulating platelet function. Blood. 2010;116(22):4646–4656. doi: 10.1182/blood-2010-04-280925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouwehand WH Bloodomics and Cardiogenics Consortia. The discovery of genes implicated in myocardial infarction. J Thromb Haemost. 2009;7(suppl 1):305–307. doi: 10.1111/j.1538-7836.2009.03441.x. [DOI] [PubMed] [Google Scholar]
- 4.Gieger C, Radhakrishnan A, Cvejic A, et al. New gene functions in megakaryopoiesis and platelet formation. Nature. 2011;480(7376):201–208. doi: 10.1038/nature10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senis YA, Tomlinson MG, García A, et al. A comprehensive proteomics and genomics analysis reveals novel transmembrane proteins in human platelets and mouse megakaryocytes including G6b-B, a novel immunoreceptor tyrosine-based inhibitory motif protein. Mol Cell Proteomics. 2007;6(3):548–564. doi: 10.1074/mcp.D600007-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins NA, Gusnanto A, de Bono B, et al. Bloodomics Consortium. A HaemAtlas: characterizing gene expression in differentiated human blood cells. Blood. 2009;113(19):e1–e9. doi: 10.1182/blood-2008-06-162958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor MN, Salles II, Cvejic A, et al. Bloodomics Consortium. Functional genomics in zebrafish permits rapid characterization of novel platelet membrane proteins. Blood. 2009;113(19):4754–4762. doi: 10.1182/blood-2008-06-162693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degen WG, Weterman MA, van Groningen JJ, et al. Expression of nma, a novel gene, inversely correlates with the metastatic potential of human melanoma cell lines and xenografts. Int J Cancer. 1996;65(4):460–465. doi: 10.1002/(SICI)1097-0215(19960208)65:4<460::AID-IJC12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Grotewold L, Plum M, Dildrop R, Peters T, Rüther U. Bambi is coexpressed with Bmp-4 during mouse embryogenesis. Mech Dev. 2001;100(2):327–330. doi: 10.1016/s0925-4773(00)00524-4. [DOI] [PubMed] [Google Scholar]
- 10.Onichtchouk D, Chen YG, Dosch R, et al. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401(6752):480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 11.Tsang M, Kim R, de Caestecker MP, Kudoh T, Roberts AB, Dawid IB. Zebrafish nma is involved in TGFbeta family signaling. Genesis. 2000;28(2):47–57. doi: 10.1002/1526-968x(200010)28:2<47::aid-gene20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Yan X, Lin Z, Chen F, et al. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling. J Biol Chem. 2009;284(44):30097–30104. doi: 10.1074/jbc.M109.049304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 14.Sekiya T, Oda T, Matsuura K, Akiyama T. Transcriptional regulation of the TGF-beta pseudoreceptor BAMBI by TGF-beta signaling. Biochem Biophys Res Commun. 2004;320(3):680–684. doi: 10.1016/j.bbrc.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Karaulanov E, Knöchel W, Niehrs C. Transcriptional regulation of BMP4 synexpression in transgenic Xenopus. EMBO J. 2004;23(4):844–856. doi: 10.1038/sj.emboj.7600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekiya T, Adachi S, Kohu K, et al. Identification of BMP and activin membrane-bound inhibitor (BAMBI), an inhibitor of transforming growth factor-beta signaling, as a target of the beta-catenin pathway in colorectal tumor cells. J Biol Chem. 2004;279(8):6840–6846. doi: 10.1074/jbc.M310876200. [DOI] [PubMed] [Google Scholar]
- 17.Xavier S, Gilbert V, Rastaldi MP, et al. BAMBI is expressed in endothelial cells and is regulated by lysosomal/autolysosomal degradation. PLoS ONE. 2010;5(9):e12995. doi: 10.1371/journal.pone.0012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khin SS, Kitazawa R, Win N, et al. BAMBI gene is epigenetically silenced in subset of high-grade bladder cancer. Int J Cancer. 2009;125(2):328–338. doi: 10.1002/ijc.24318. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto N, Hayashi S, Masui A, et al. Deletion at chromosome 10p11.23-p12.1 defines characteristic phenotypes with marked midface retrusion. J Hum Genet. 2012;57(3):191–196. doi: 10.1038/jhg.2011.154. [DOI] [PubMed] [Google Scholar]
- 20.Pils D, Wittinger M, Petz M, et al. BAMBI is overexpressed in ovarian cancer and co-translocates with Smads into the nucleus upon TGF-beta treatment. Gynecol Oncol. 2010;117(2):189–197. doi: 10.1016/j.ygyno.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Teratani T, Tomita K, Suzuki T, et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142(1):152-164.e110. [DOI] [PubMed]
- 22.Fritzmann J, Morkel M, Besser D, et al. A colorectal cancer expression profile that includes transforming growth factor beta inhibitor BAMBI predicts metastatic potential. Gastroenterology. 2009;137(1):165–175. doi: 10.1053/j.gastro.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 23.Gómez del Pulgar T, Bandrés E, Espina C, et al. Differential expression of Rac1 identifies its target genes and its contribution to progression of colorectal cancer. Int J Biochem Cell Biol. 2007;39(12):2289–2302. doi: 10.1016/j.biocel.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Togo N, Ohwada S, Sakurai S, et al. Prognostic significance of BMP and activin membrane-bound inhibitor in colorectal cancer. World J Gastroenterol. 2008;14(31):4880–4888. doi: 10.3748/wjg.14.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni AB, Karlsson S. Transforming growth factor-beta 1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol. 1993;143(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Sanford LP, Ormsby I, Gittenberger-de Groot AC, et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124(13):2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenvers KL, Tursky ML, Harder KW, et al. Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol Cell Biol. 2003;23(12):4371–4385. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122(10):2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 29.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9(17):2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Shi S, Acosta L, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131(9):2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh SP, Seki T, Goss KA, et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97(6):2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaussin V, Morley GE, Cox L, et al. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ Res. 2005;97(3):219–226. doi: 10.1161/01.RES.0000177862.85474.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Bizri N, Guignabert C, Wang L, et al. SM22alpha-targeted deletion of bone morphogenetic protein receptor 1A in mice impairs cardiac and vascular development, and influences organogenesis. Development. 2008;135(17):2981–2991. doi: 10.1242/dev.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song L, Yan W, Chen X, Deng CX, Wang Q, Jiao K. Myocardial smad4 is essential for cardiogenesis in mouse embryos. Circ Res. 2007;101(3):277–285. doi: 10.1161/CIRCRESAHA.107.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Bush JO, Ovitt CE, Lan Y, Jiang R. The TGF-beta pseudoreceptor gene Bambi is dispensable for mouse embryonic development and postnatal survival. Genesis. 2007;45(8):482–486. doi: 10.1002/dvg.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tramullas M, Lantero A, Diaz A, et al. BAMBI (bone morphogenetic protein and activin membrane-bound inhibitor) reveals the involvement of the transforming growth factor-beta family in pain modulation. J Neurosci. 2010;30(4):1502-1511. [DOI] [PMC free article] [PubMed]
- 37.Guillot N, Kollins D, Gilbert V, et al. BAMBI regulates angiogenesis and endothelial homeostasis through modulation of alternative TGFβ signaling. PLoS ONE. 2012;7(6):e39406. doi: 10.1371/journal.pone.0039406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villar AV, García R, Llano M, et al. BAMBI (BMP and activin membrane-bound inhibitor) protects the murine heart from pressure-overload biomechanical stress by restraining TGF-β signaling. Biochim Biophys Acta. 2013;1832(2):323–335. doi: 10.1016/j.bbadis.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Guillot N, Kollins D, Badimon JJ, Schlondorff D, Hutter R. Accelerated reendothelialization, increased neovascularization and erythrocyte extravasation after arterial injury in BAMBI-/- mice. PLoS ONE. 2013;8(3):e58550. doi: 10.1371/journal.pone.0058550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubois C, Atkinson B, Furie BC, Furie B. Real-time in vivo imaging of platelets during thrombus formation. In: Michelson AD, editor. Platelets. 2nd ed. Oxford, United Kingdom: Elsevier Inc; 2007. pp. 611–626. [Google Scholar]
- 41.Ahnström J, Andersson HM, Canis K, et al. Activated protein C cofactor function of protein S: a novel role for a γ-carboxyglutamic acid residue. Blood. 2011;117(24):6685–6693. doi: 10.1182/blood-2010-11-317099. [DOI] [PubMed] [Google Scholar]
- 42.van der Meijden PE, Munnix IC, Auger JM, et al. Dual role of collagen in factor XII-dependent thrombus formation. Blood. 2009;114(4):881–890. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
- 43.Brass LF, Wannemacher KM, Ma P, Stalker TJ. Regulating thrombus growth and stability to achieve an optimal response to injury. J Thromb Haemost. 2011;9(suppl 1):66–75. doi: 10.1111/j.1538-7836.2011.04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cosemans JM, Angelillo-Scherrer A, Mattheij NJ, Heemskerk JW. The effects of arterial flow on platelet activation, thrombus growth, and stabilization. Cardiovasc Res. 2013;99(2):342–352. doi: 10.1093/cvr/cvt110. [DOI] [PubMed] [Google Scholar]
- 45.Pluskota E, Ma Y, Bledzka KM, et al. Kindlin-2 regulates hemostasis by controlling endothelial cell-surface expression of ADP/AMP catabolic enzymes via a clathrin-dependent mechanism. Blood. 2013;122(14):2491–2499. doi: 10.1182/blood-2013-04-497669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robins RS, Lemarié CA, Laurance S, Aghourian MN, Wu J, Blostein MD. Vascular Gas6 contributes to thrombogenesis and promotes tissue factor up-regulation after vessel injury in mice. Blood. 2013;121(4):692–699. doi: 10.1182/blood-2012-05-433730. [DOI] [PubMed] [Google Scholar]
- 47.Falati S, Patil S, Gross PL, et al. Platelet PECAM-1 inhibits thrombus formation in vivo. Blood. 2006;107(2):535–541. doi: 10.1182/blood-2005-04-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stalker TJ, Wu J, Morgans A, et al. Endothelial cell specific adhesion molecule (ESAM) localizes to platelet-platelet contacts and regulates thrombus formation in vivo. J Thromb Haemost. 2009;7(11):1886–1896. doi: 10.1111/j.1538-7836.2009.03606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naik MU, Stalker TJ, Brass LF, Naik UP. JAM-A protects from thrombosis by suppressing integrin αIIbβ3-dependent outside-in signaling in platelets. Blood. 2012;119(14):3352–3360. doi: 10.1182/blood-2011-12-397398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orlowski E, Chand R, Yip J, et al. A platelet tetraspanin superfamily member, CD151, is required for regulation of thrombus growth and stability in vivo. J Thromb Haemost. 2009;7(12):2074–2084. doi: 10.1111/j.1538-7836.2009.03612.x. [DOI] [PubMed] [Google Scholar]
- 51.Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wanninger J, Neumeier M, Bauer S, et al. Adiponectin induces the transforming growth factor decoy receptor BAMBI in human hepatocytes. FEBS Lett. 2011;585(9):1338–1344. doi: 10.1016/j.febslet.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258(11):7155–7160. [PubMed] [Google Scholar]
- 54.Assoian RK, Sporn MB. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grainger DJ, Wakefield L, Bethell HW, Farndale RW, Metcalfe JC. Release and activation of platelet latent TGF-beta in blood clots during dissolution with plasmin. Nat Med. 1995;1(9):932–937. doi: 10.1038/nm0995-932. [DOI] [PubMed] [Google Scholar]
- 56.Meyer A, Wang W, Qu J, et al. Platelet TGF-β1 contributions to plasma TGF-β1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood. 2012;119(4):1064–1074. doi: 10.1182/blood-2011-09-377648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.