Abstract
The microsporidium Pseudoloma neurophilia was initially reported to infect the central nervous system of zebrafish causing lordosis and eventually death. Subsequently, muscle tissue infections were also identified. To understand the infection process, development, and ultrastructural pathology of this microsporidium, larval and adult zebrafish were fed P. neurophilia spores. Spores were detected in the larval fish digestive tract 3 h post-exposure (PE). By 4.5 days (d) PE, developing parasite stages were identified in muscle tissue. Wet preparations of larvae collected at 8 d PE showed aggregates of spores in the spinal cord adjacent to the notochord. All parasite stages, including spores, were present in the musculature of larval fish 8 d PE. Adult zebrafish sacrificed 45 d PE had fully developed infections in nerves. Ultrastructural study of the developmental cycle of P. neurophilia revealed that proliferative stages undergo karyokinesis, producing tetranucleate stages that then divide into uninucleate cells. The plasmalemma of proliferative cells has a previously unreported glycocalyx-like coat that interfaces with the host cell cytoplasm. Sporogonic stages form sporophorous vacuoles (SPOV) derived from the plasmalemmal dense surface coat, which “blisters” off sporonts. Uninucleate sporoblasts and spores develop in the SPOV. The developmental cycle is identical in both nerve and muscle. The SPOV surface is relatively thick and is the outermost parasite surface entity; thus xenomas are not formed. Based on the new information provided by this study, the taxonomic description of the genus Pseudoloma and its type species, P. neurophilia is modified and its life cycle described.
Keywords: Danio rerio, life cycle, Microsporidia, pathology, Pseudoloma neurophilia, taxonomy, ultrastructure
The microsporidia are a protistan phylum of eukaryotic obligate intracellular parasites, most closely related to the Fungi. They infect every major group of animals but they are most commonly known from infections in insects, fish, and mammals, including man. In fish there are over 100 species distributed between approximately 20 genera (Cali and Takvorian 2011; Canning and Lom 1986). Pseudoloma neurophilia was first reported as a microsporidium causing mortality in the zebrafish Danio rerio (De Kinkelin et al. 1980). Subsequently, it was studied and the genus and species named by Matthews et al. (2001), who reported the infection from the University of Oregon Zebrafish facility and other laboratory colonies as well as from pet stores. The parasite was placed in a new genus, based on both molecular and morphological data presented (Matthews et al. 2001). Since the infection was observed and studied in nervous tissue, the species was named neurophilia. However, the infection is also found elsewhere, particularly somatic muscle (Kent and Bishop-Steward 2003). The current study presents the developmental cycle in skeletal muscle as well as in nervous tissue along with a unique host-parasite interface in the host cells. A change in the interpretation of the pathology associated with this infection, required a taxonomic revision of the genus, which is also presented.
Methods and Materials
Larval fish
Five days post fertilization, zebrafish larvae were exposed to the microsporidium Pseudoloma neurophilia at a dosage of approximately10,000 spores/larva. Larval fish were held in 250-ml beakers in 100 ml of autoclaved sterilized dechlorinated city water at 28 °C and about 90% of the water was changed daily. The larval fish were derived from a parasite-free stock of AB, a wild-type zebrafish maintained in the Oregon State University laboratory. The inoculum was prepared by harvesting brains and spinal cords from a stock of known infected AB fish also from our laboratory. Infected tissue was mixed with sterilized water, macerated, and passed through a 40-μm Nitex filter (Becton-Dickinson, Franklin Lakes, NJ) and quantified using a hemocytometer. Fish larvae were collected at 3 h, 4.5 days (d) and 8 d post-exposure (PE) for fixation.
Adult fish
Three adult zebrafish (i.e. 47-mm male, 40- and 37-mm females) were obtained from a stock of AB zebrafish held at the Sinnhuber Aquatic Research Laboratory, Oregon State University. This laboratory is Specific Pathogen Free for P. neurophilia (Kent et al. 2011). Fish were held in a flow-through aquarium receiving dechlorinated city water at 28 °C and fed twice daily with an artificial diet. Donor fish from our infected stock were euthanized and the three fish were exposed as described above. At 45 d PE, the fish were sacrificed and their brains and spinal cords were preserved. The 37-mm female exhibited severe spinal deformity (i.e. scoliosis, kyphosis) demonstrating frank neurological infection.
Light microscopy
Wet mounts of hind brain and spinal cords were examined and photographed by bright field or Nomarski phase interference. Thick Epon sections (i.e. 0.5 μm) were stained with toluidine blue and examined/photographed with a Zeiss Axiophot 200 microscope (Zeiss, Thornwood, NY).
Transmission electron microscopy (TEM)
Larval fish and brain tissue from adult fish were fixed in 0.1 M cacodylate buffered 2.5% (v/v) glutaraldehyde, post-fixed in 1% (w/v) OsO4, and processed for TEM. Thin sections were stained with uranyl acetate and lead citrate. The TEM samples were observed and photographed with a FEI (Phillips) Tecnai 12 TEM. All samples were prepared, observed, and photographed at the Rutgers -Newark microscopy facility.
Results
Light microscopy
Macerated fresh infected brain tissue examined in wet mounts contained spores that consistently occurred in aggregates of up to 16 spores, but numerous individual free spores were also observed (Fig. 1--3). The fresh spores were ovoid to pyriform, with a mean length of 5.4 (4.8—5.9, SD = 2.0) μm and a mean width of 2.7 (2.5—3.1, SD = 2.0) μm.
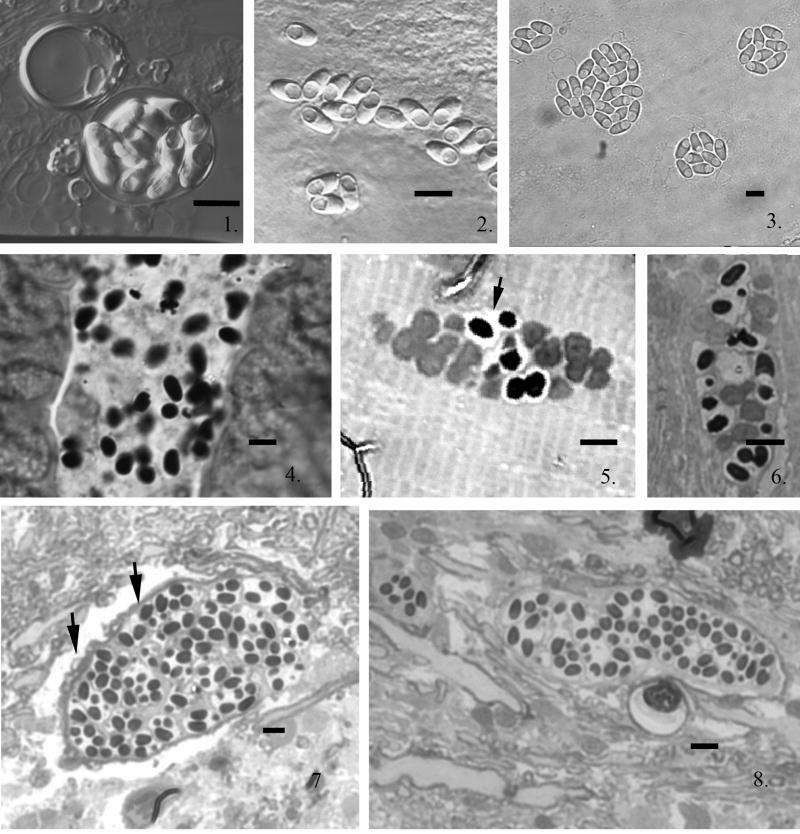
Fig. 1--3.
Light micrographs of Pseudoloma neurophilia spores from wet mount preparation of infected zebrafish neural tissue. Fig. 1. Ten spores within a persistent sporophorous vesicle (SPOV). Fig. 2. Fresh spores with prominent posterior vacuole. Fig. 3. Typical SPOVs containing eight spores all with prominent posterior vacuoles. Scale bars = 5 μm. Fig. 4--8. Light micrographs of thick epon sections of zebrafish infected with Pseudoloma neurophilia. Fig. 4. Longitudinal section of larval zebrafish intestine 3 h post exposure (PE) containing numerous spores in the lumen. Fig. 5. Section of larval zebrafish muscle tissue containing proliferative and some sporogonic stages of infection by108 h PE. The proliferative developmental stages appear less dense and are in direct contact with the host muscle fibers and the sporogonic stages are in SPOVs (short arrow). Fig. 6. Section of larval zebrafish muscle tissue containing mainly sporogonic stages with a few proliferative stages of development by 8 days PE. The proliferative development continues resulting in increased infection by asynchronous development. Fig. 7. Section from adult zebrafish nervous tissue. The infection is present within the axoplasm of a mylenated nerve fiber (short arrows). There are many SPOVs containing spores present. Fig. 8. Sagital section through a nerve containing spores. The infection appears to develop along the nerve fiber. Scale bars = 5 μm.
Larval fish
Examination of wet mounts of larval fish at 8 d PE revealed spores in both the somatic muscle and spinal cord adjacent to the notochord. Additionally, the location of infection and presence of developmental stage(s) were observed in thick Epon sections. At 3 h post-exposure (PE), spores were observed in the gut lumen of the zebrafish larvae (Fig. 4). By 108 h PE clusters of both proliferative and sporogonic stages of infection were detected in muscle tissue. These stages were recognized by their relationship to the host tissue: the proliferative stages were in direct contact with the muscle cell cytoplasm while sporonts were in vesicles that separated them from the surrounding host material. Additionally, the proliferative stages found in direct contact with the host cell cytoplasm appeared less dense than the parasite stages in the sporogonic vesicles (Fig. 5). These isolation vesicles are formed by the parasite and thus are called sporophorous vesicles (SPOV's). At 8 d PE all stages of parasite development were present (Fig. 6).
Although we did not detect parasites in the brain or spinal cord in Epon sections, wet preparations of larvae collected at 8 d PE showed aggregates of spores in tissue presumed to be spinal cord, which was adjacent to the notochord.
Adult fish
In 0.5-μm Epon sections of the adult fish nervous tissue, infection was observed in the myelinated nerves (Fig 7). The nerve infection appeared to follow nerve fibers as illustrated by the elongated infected segment filled with SPOV's containing spores (Fig. 8). Several degenerate nerve fibers with microsporidial SPOV's containing spores were also observed.
Electron microscopy of larval fish
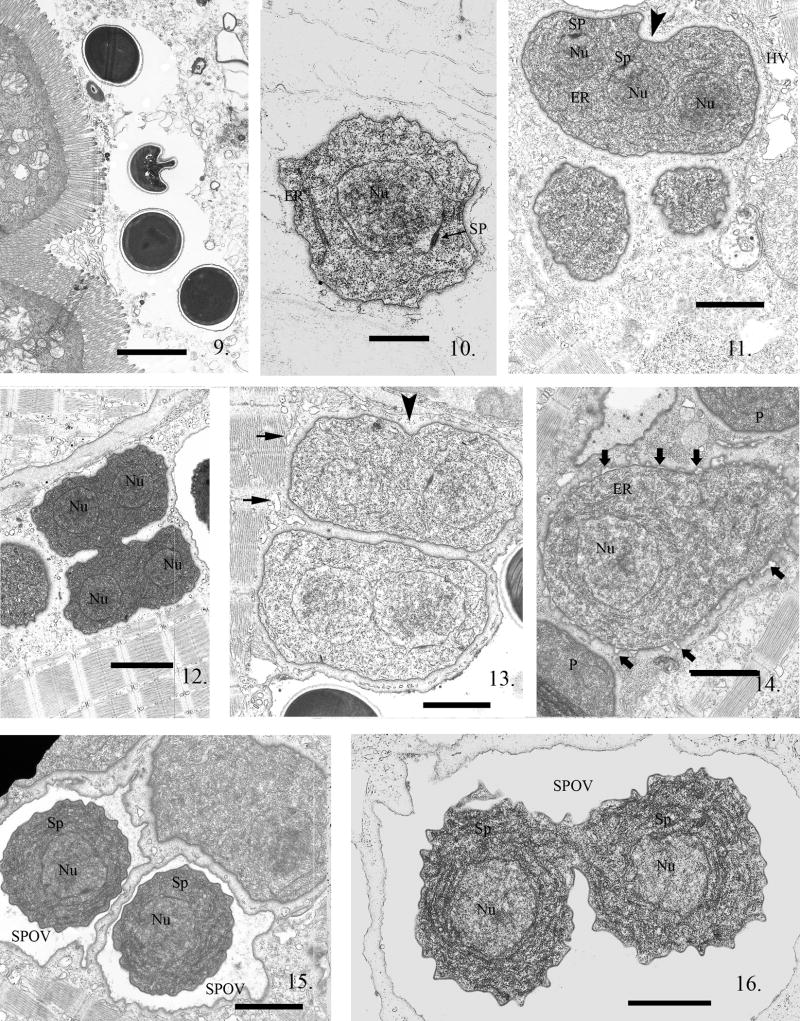
All developmental stages of the P. neurophilia infection were observed in larval zebrafish. The first fish larvae sacrificed were examined at 3 h PE, and processed for electron microscopy (EM). Within the gut lumen spores were observed (Fig. 9). The sporoplasm, within a host cell, is single nucleated, and in direct contact with the host cell cytoplasm (Fig. 10). Subsequent development from the earliest proliferative stages through the beginning of sporogony was observed in direct contact with the host muscle cell cytoplasm. The parasite plasmalemmal surface presents a somewhat homogeneous zone around the developing proliferative parasite cells, preventing direct contact of host organelles with the parasite plasmalemma. This was always observed in infected skeletal muscle cells (Fig. 11). Parasite nuclear division occurred without cytokinesis, resulting in the formation of multinucleate proliferative cells, which generally contained four nuclei before cytokinesis commenced (Fig. 11, 12). Parasite organelles, such as ER, ribosomes, and nuclear/spindle plaques that indicated nuclear division in the microsporidia, were present (Fig. 10, 11).
Fig. 9--16.
Electron micrographs (EM) of zebrafish infected with Pseudoloma neurophilia. Fig. 9. Four spores in the intestinal lumen of a larval zebrafish 3 h PE. Scale bar = 2.3 μm. Fig. 10. A uninucleate sporoplasm in the host cell cytoplasm. The nuclear membrane has a dense spindle plaque (SP) indicating this parasite cell is about to undergo karyokinesis. Note the prominent nucleus (Nu), the presence of endoplasmic reticulum (ER) and ribosomes in the sporoplasm. Scale bar = 800 nm. Fig. 11. Proliferative stages in direct contact with the host cell cytoplasm, one containing three nuclei (Nu). Two of the nuclei have prominent SP on their nuclear membranes. The parasite plasmalemma appears to have a glycocalyx-like surface, giving the cells the appearance of a halo surrounding them. Note that karyokinesis is not linked to cytokinesis (arrowhead), resulting in production of multinucleated parasite cells. The host cytoplasm has sarcoplasmic reticulum vacuoles (HV) in the region of the infection. Scale bar = 1.7 μm. Fig. 12. A proliferative cell with four visible nuclei (Nu) in the process of dividing into two multinucleate cells. They will divide repeatedly, resulting in the production of several uninucleate cells. These dividing cells maintain their glycocalyx- like coat, outlining the parasite plasmalemma, during division. Note the presence of small vesicles of the sarcoplasmic reticulum outlining the parasite cells but absent in the glycocalyx-like halo. Scale bar = 2.3 μm. Fig. 13. Two recently divided proliferative cells, each containing two nuclei. The start of a cytoplasmic invagination (arrowhead) indicates this cell is beginning another cytokinesis. Note the presence of host vesicles (short arrows) of sarcoplasmic reticulum abutting the contractile fibers but limited from parasite plasmalemmal contact by the glycocalyx-like coating. Scale bar = 1.4 μm. Fig. 14. Parasite cell starting the transition from proliferative to sporogonic development. Note the difference between it and the abutting proliferative cell (P) membrane which is still covered with its glycocalyx-like surface coat. The plasmalemma has numerous “blisters” starting to emerge (bold arrows) from it and the new plasmalemma is now “thickened” in the areas under the blisters. The blistering continues until it forms an isolation chamber, an SPOV indicating the commencement of sporogony. Scale bar = 1.7 μm. Fig. 15. Two uninucleate (Nu) sporonts (Sp) each in an SPOV. The outer edge of the SPOVs are in contact with the host muscle cell cytoplasm while the sporonts inside them now have a thickened plasmalemma and are isolated from the host cytoplasm. Scale bar = 1.4 μm. Fig. 16. SPOV containing uninucleate (Nu) sporonts (Sp) undergoing cytokinesis. These sporont cells are still narrowly connected and have a thickened plasmalemma and a marked increase in endoplasmic reticulum. Scale bar = 1.2 μm.
The homogenous surface coat remained present as the parasite cells multiplied in the proliferative phase of development (Fig. 11, 12). Small vesicles of host sarcoplasmic reticulum were sometimes present between the parasite cells and the contractile fibers of the infected host muscle cell (Fig. 11, 13).
While several karyokineses occurred before cytokinesis, forming multinucleate cells, the nuclei were not in diplokaryotic arrangement (Fig. 11--13). Cell division to uninucleate cells occurred in a step-wise progression (i.e. four to two then two to one) (Fig. 12). At some point, after multiple divisions, these parasite cells committed to sporogony, morphologically discernable by the appearance of a “blistering” or separation of the surface coat that had been an integral part of the plasmalemmal surface (Fig. 14).
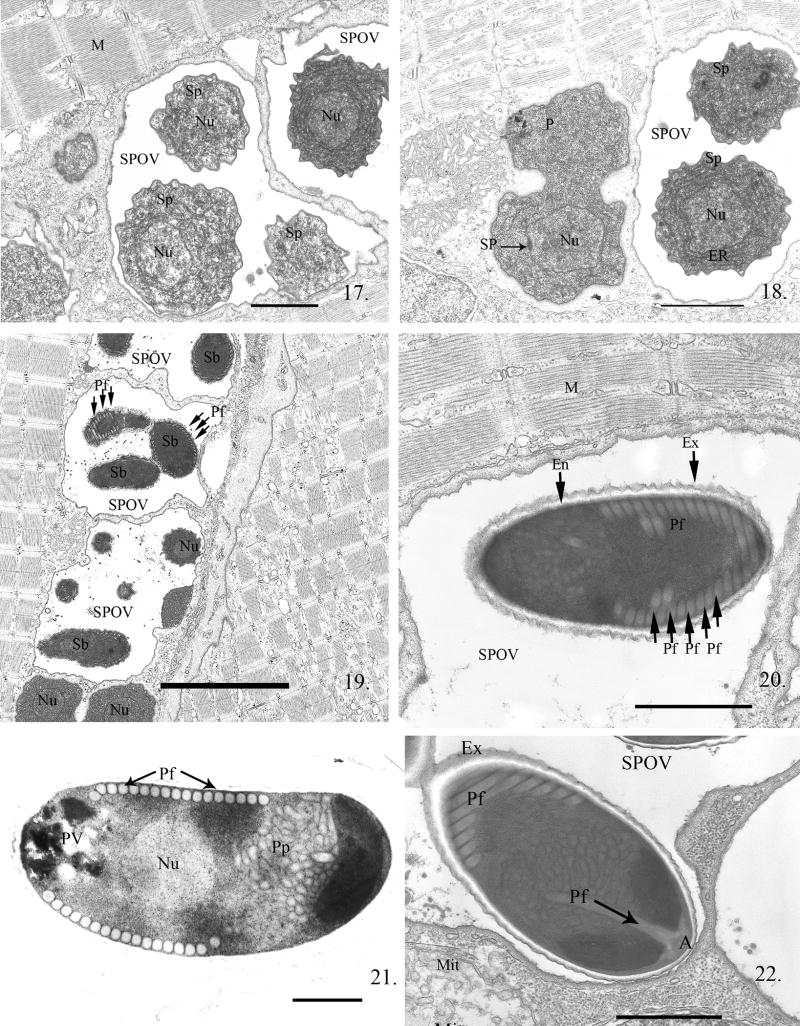
The “blistering” continued, eventually completely separating from the parasite plasmalemmal surface layer and resulted in the formation of a SPOV in which the parasite cell was suspended (Fig. 15). Within the SPOV, the cell underwent numerous nuclear and cytoplasmic divisions (Fig. 16, 17), resulting in the formation of several parasites, now called sporonts. These sporonts were characterized by the presence of a dense “thickened” plasmalemma (Fig. 16, 18) and greatly increased ER. After the last cell division, the resulting cells, sporoblasts, within the SPOV became very dense and crenated as they developed the spore organelles. Thin sections of SPOVs were observed to contain four to six sporoblasts (Fig. 19).
Fig. 17--22.
Electron micrographs (EM) of zebrafish infected with Pseudoloma neurophilia undergoing sporogonic development. Fig. 17. Multiple SPOVs containing uninucleate (Nu) sporonts (Sp). The center one contains three Sp in this section. Note the normal appearing host muscle cell (M) cytoplasm immediately outside the SPOVs. Scale bar = 1.7 μm. Fig. 18. Progression of the developmental cycle from proliferative cell (P) to sporogonic cell (Sp). The sporogonic cell cytoplasm illustrates the increase in ER and general cytoplasmic density in the sporonts. Note the proliferative cell (P) is undergoing cytokinesis and has a spindle plaque (SP). Scale bar = 1.7 μm. Fig. 19. Three SPOVs, one containing 6 sporoblasts (Sb). After the last cytokinetic division, the sporonts begin metamorphosis to spores and are called sporoblasts (Sb). Polar filament (Pf) development is visible in sporoblasts. Scale bar = 5 μm. Fig. 20. A spore in an SPOV. Spores can be differentiated from sporoblasts by the presence of a thick lucent endospore coat (En) that develops beneath the dense exospore (Ex). Sections of the polar filament (Pf) coils are visible. Scale bar = 1.9 μm. Fig. 21. Longitudinal section through a mature spore containing 17 polar filament coil cross sections arranged in a single row. The single nucleated (Nu) sporoplasm and posterior vacuole (PV) are visible. The anterior polaroplast (Pp) is composed of both a series of tightly stacked elongated membranes and a tubular complex filling the anterior third of the spore. Scale bar = 600 nm. Fig. 22. SPOV containing a mature spore. In this section, the anterior attachment complex (A) of the polar filament (Pf) is visible abutting the narrowed portion of the anterior endospore. Immediately posterior to it is the straight portion of the polar filament. The polar filament then curves toward the periphery of the spore and forms several coils. A host mitochondrion (Mit) is present in the cytoplasm close to the SPOV. Scale bar = 900 nm.
Within the sporoblasts, the development of the polar filament and its anterior attachment complex proceeded before the lucent endospore coat was formed (Fig. 19). Cross-sections of the forming polar filament were visible in the late sporoblasts. The mature spores contained the extrusion apparatus and a single nucleated sporoplasm within a resistant spore coat consisting of a thick lucent endospore surrounded by the dense exospore (Fig. 20). All sporogonic stages were suspended in the SPOVs, which were persistent throughout the infection.
The spore is ∼ 5 μm long in thin sections and contains 14--17 cross sections of the polar filament, arranged in a single row (Fig. 20, 21). The filament coil appears to be of uniform diameter (i.e. isofilar). The anterior third of the spore contains an elaborate lamellar and tubular-vesicular polaroplast immediately below the anterior attachment complex and above the single nucleus (Fig. 22).
By 108 h or 4.5 d PE, all developmental stages were observed. Multiple SPOVs containing spores were located directly within the host cell cytoplasm, abutting the remaining contractile fibers within the muscle cells. No xenoma formation was observed (Fig. 19).
Electron microscopy of adult fish
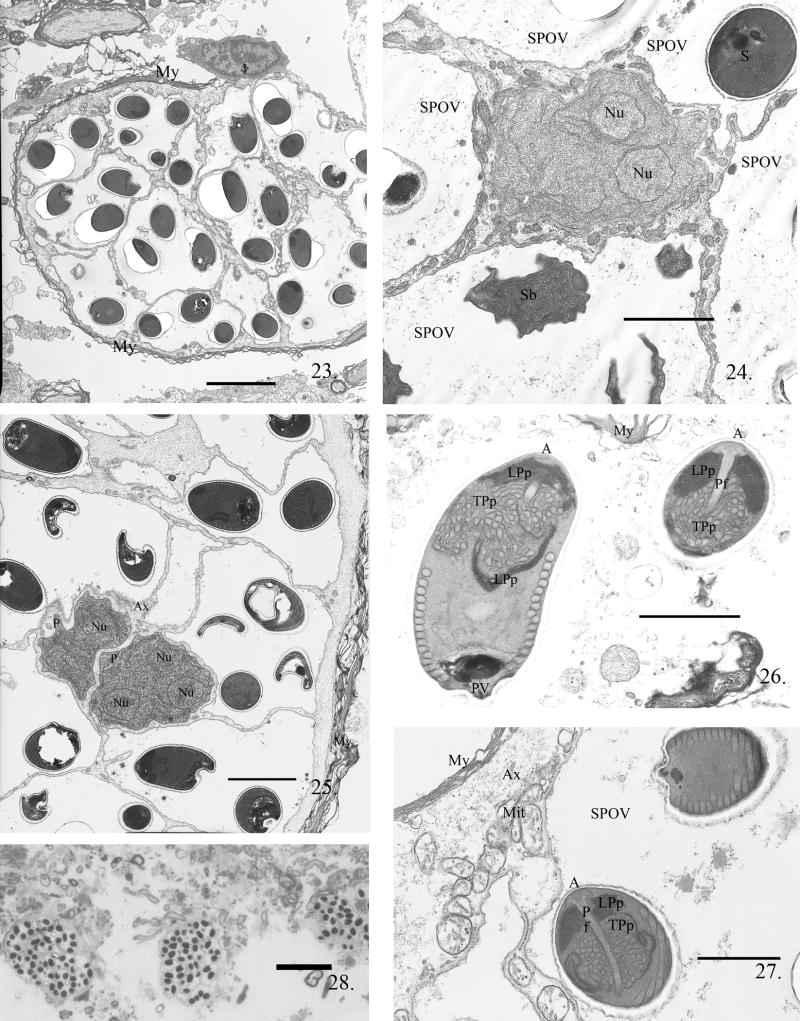
Nervous tissue infection was observed in myelinated nerve fibers of adult fish (Fig. 23). These infections were observed in adult fish at 45 d PE. They contained primarily spores and sporoblasts within SPOVs with a few multinucleate proliferative stages in direct contact with the remaining nerve fiber cytoplasm. Higher magnification of the infected nerves (Fig. 24) demonstrated the presence of the host cytoplasm containing mitochondria surrounding a proliferative stage, all surrounded by SPOVs. With minimal host cytoplasm present, these multinucleate proliferative stages underwent cytokinesis, and continued the developmental cycle (Fig. 25). The parasite development observed in the brain tissue was within the cytoplasm of the nerve fibers that were surrounded by myelin (Fig. 23--25). As in muscle, no xenoma formation was observed.
Fig. 23--28.
The myelinated nerves of adult zebrafish infected with P. neurophilia. Fig. 23. Low power image of a myelinated nerve with a long-term infection. The axoplasm filled with 13 SPOVs containing spores; note the presence of a scant amount of axoplasm just inside the myelin (My) layers. Scale bar = 5 μm. The two proliferated cells (P) surround by five SPOVs containing spores (S) and sporoblast (Sb). Fig. 24. Multinucleated proliferative cell in direct contact with remaining axoplasm and surrounded by numerous SPOVs containing spores and one SPOV containing sporoblasts (Sb). Scale bar = 2.3 μm. Fig. 25. Two multinucleate dividing proliferative cells (P) in nerve fiber axoplasm surrounded by SPOVs containing multiple spores. This section is through the edge of the nerve fiber and both the remaining axoplasm (Ax) and myelin (My) are visible. Scale bar = 3 μm. Fig. 26. Two spores in an SPOV. The anterior structure of the spore is well-illustrated in the spores in this section. In the longitudinally sectioned spore, the lamellar polaroplast (LPp) surrounds the tubular portion (TPp) and clearly extends about half the length of the spore to the nucleus. The polar filament coil cross-sections are visible as 16 sections in a single row and are near the posterior vacuole (PV). In the anterior sagital section of a spore, the anchoring disk (A) with the anterior most portion of the attached polar filament (Pf) is visible. The lamellar and tubular polaroplast tightly abut the anterior end of the polar filament. Scale bar = 1.2 μm. Fig. 27. Two fully matured spores in an SPOV. The surrounding mylenation (My) and axoplasm (Ax) containing mitochondria (Mit) of the nerve fiber are still intact. Note the complex nature of the anchoring disc-polarfilament-polaroplast complex in the anterior portion or this spore. Scale bar = 1.0 μm. Fig. 28. Low-power light image of the remnants of degenerated myelinated nerves encompass over 13 SPOVs each SPOV contains several spores. Scale bar = 25.0 μm
Mature spores from the adult fish also contained the elaborate polaroplast. The anterior-most portion surrounded the attachment complex of the polar filament and was lamellar, with a portion of it wrapping around the tubular-vesicular polaroplast (Fig. 26, 27). Longitudinal sections through the spore consistently demonstrated that the polar filament formed a single row of 14--17 coils (Fig. 21, 26). The posterior of the spore contained amorphous dense material in the area of the posterior vacuole (Fig. 21, 26). The single nucleus of the spore was located below the polaroplast complex. The mature spores (Fig. 20, 21) and the developmental stages (Fig. 15--19) observed in muscle tissue appeared to be identical to spores (Fig. 26, 27) and developing stages (Fig. 24, 25) in nervous tissue. The heavily infected nerves eventually degenerated (Fig. 28), releasing the spore-filled SPOVs and free spores into the host, possibly initiating additional infections.
Discussion
De Kinkelin (1980) first reported the presence of a microsporidium in zebrafish from pet stores. He described lordosis with clusters of microsporidial spores associated with the ventral side of the nerve cord. De Kinkelin (1980) attributed the morbidity and mortality of the zebrafish to this parasite and reported that the spores were 3–4 μm long × 1.5–2 μm wide. Twenty years later, in a large research facility, Matthews et al. (2001) observed a microsporidium in the central nervous system of zebrafish that they considered the same as described by De Kinkelin (1980). Matthews et al. (2001) provided molecular and morphological information resulting in the establishment of a new genus, Pseudoloma, and its type species neurophilia as a consequence of its location in the host. They concluded that the aggregates of spores represented xenomas in the nervous tissue containing multiple SPOVs filled with up to 16 single nucleated spores that measured 5.4 × 2.7 μm as fresh spores, containing 13--16 cross-sections of the polar filament coil. Kent and Bishop-Stewart (2003) described experimental transmission of P. neurophilia in zebrafish by water-borne exposure. Histological examination revealed infection in several other tissues in addition to the nervous system, including the muscle and ovaries.
Most microsporidial infections are established by ingestion of infective spores but the precise sequence of events leading to infection, in vertebrates, is poorly understood (Cali and Takvorian 1999). Regarding fish, Sanchez et al. (2001) developed in situ probes and showed that Loma salmonae was first detected in the intestinal epithelium. Presporogonic stages were then found in the heart endothelium, and sporogony was ultimately observed in the gills (Kent and Speare 2005). We fed macerated infected fish tissue to zebrafish larvae and adults presuming that, in nature, an infected fish will eventually die to release spores into the environment.
The first larvae, sacrificed at 3 h PE, had spores in their intestines, clearly in close proximity to the villar surface. Within the host tissue, single nucleated sporoplasm cells were observed. These cells were in direct contact with the host cell cytoplasm and underwent several karyokineses prior to cytoplasmic division, resulting in the formation of multinucleate plasmodial cells, which underwent a stepwise division.
The plasmalemma, from the earliest proliferative stages through the beginning of sporogony, appears to have a unique molecular surface layer. It is similar to the glycocalyx of a gut epithelial cell surface; however, the parasite cell surface coat is intracellular, in direct contact with the host cell cytoplasm. This surface coat presents a more or less homogeneous zone around the developing proliferative parasite cells, preventing direct contact of host organelles with the parasite plasmalemma. Additionally, this “zone” was observed on dividing proliferative cells, as the new plasmalemma is formed during cytokinesis.
It is not until the beginning of sporogony that this surface material “blisters” off an apparently newly formed dense plasmalemma. The “blistering” continues, eventually completely separating from the parasite plasmalemmal surface layer, resulting in the formation of a true or parasite-derived SPOV (Cali and Takvorian 1999). The plasmalemma of each sporont cell is now a “dense membrane”. These sporonts undergo nuclear divisions resulting in the formation of plasmodial stages with multiple nuclei in each SPOV. Cytoplasmic divisions result in the production of multiple uninucleate cells. After the last cell division of the sporonts, these uninucleate cells begin the metamorphosis into spores and are thus sporoblasts.
The sporoblasts appear much denser and crenated as they develop the polar filament and related organelles. The last structure to develop is the thick, lucent endospore coat. It forms directly below the dense sporont surface secretions (i.e. exospore coat) and immediately above the plasmalemma. These changes signify the completion of the metamorphosis into a spore. No autoinfective (i.e. germinated) spores were observed. On average, four to six sporoblasts were observed in sectioned material; however, in fresh tissue preparations 8 to 16 spores were observed per SPOV.
The mature unfixed spores averaged 5.4 μm in length, as previously described (Matthews et al. 2001). Ultrastructurally, the spores contained a single row of polar filament coils that are uniform in diameter and number 14--17. They surround a single nucleus and abut an elaborate polaroplast. The anterior-most portion of the polaroplast is lamellar transitioning into a vesicular mass, which is further surrounded by a ring of the lamellar region. The polaroplast fills approximately one-third to one-half of the spore and surrounds the straight or manubroid portion of the polar filament. While the posterior vacuole is obvious in fresh spores, it appears as an irregular amorphous mass when fixed.
At 8 d PE all stages of parasite development are present in the larval fish, corresponding to the rapid development that was observed in cell culture (Monaghan et al. 2009). The presence of proliferative stages in the same cluster that contains spores in SPOVs demonstrates asynchronous development. The proliferative stages in the remnants of muscle cell cytoplasm and surrounded by SPOVs demonstrated the relationship of the parasite within the host muscle cells. Intact actin/myosin contractile fiber bundles can be seen surrounding the SPOVs within the muscle fibers with no further observable pathological change.
In the nervous tissue, the infection was observed in myelinated nerve fibers in which the SPOVs are suspended in the nerve fiber axoplasm. Clearly, the SPOV is the outermost parasite surface that interfaces with the host cytoplasm, and P. neurophilia does not form xenomas.
A true xenoma is a hypertrophied host cell surrounded by host generated collagenous fiber material (Cali and Takvorian 1999). It is actually an intimately close union of the host and parasite in which the host cell grows in size or hypertrophies, its nucleus undergoes amitotic division into numerous nuclei, and host organelles (e.g. mitochondria) increase dramatically producing a parasite-supportive environment. As a consequence, the description of the genus Pseudoloma should be modified to reflect the lack of xenoma formation. Because Loma induces xenoma formation and Pseudoloma does not, this further separates Loma from Pseudoloma.
Approximately six spores were observed in EM sections of SPOVs and 8--16 within SPOVs liberated from tissues in wet preparations, demonstrating the polysporous nature of this organism. In contrast, the genus Loma is disporous (Morrison and Sprague 1981) while the genetically more closely related genus Ichthyosporidium (Matthews et al. 2001) is tetrasporous and diplokaryotic (Sprague, Becnel, and Hazard 1992).
The molecular characterization using small subunit rDNA sequences indicated that P. neurophilia clustered with Ichthyosporidium, but showed only a 12% similarity to this microsporidium (Matthews et al. 2001). Additionally, Ichthyosporidium is a diplokaryotic genus while Pseudoloma is not. The justification for not placing it in the genus Loma, a common xenoma-forming genus infecting fishes, was that while appearing similar in morphology to Loma, it did not cluster with the Loma clad (Matthews et al. 2001). The original description of the genus Pseudoloma included the formation of xenomas. However, our results clearly demonstrate the lack of xenoma formation in both muscle and nervous tissue. Lom (2002) questioned the validity of describing the parasitized cells as xenomas, and his skepticism is validated by our results. Additionally, the entire developmental cycle is presented here and thus is added to the redescription of the genus.
In conclusion, we describe here the developmental cycle of the parasite as well as the ultrastructural, intracellular pathology. The nature of the host-parasite interface during early development (glycocalyx-like material) has not been previously reported in other microsporidia and should be investigated further in this system. If observed in other organisms, it should be included in the taxonomic description for comparison. It should be noted that in Kent and Bishop-Stewart (2003) the zebrafish had no clinical manifestations at 20 weeks PE yet all were histologically positive for this parasite. Ramsay et al. (2009) found that fish remain infected for up to a year after exposure. This presents problems for scientists utilizing these fish for research, and recently, Oregon State University established specific pathogen-free (SPF) zebrafish, which they are now providing to the research community (Kent et al. 2011). A study of the long-term host-parasite relationship in these fish would provide valuable information about how this microsporidium adapts and develops in different tissues, serving as a possible model for microsporidian dissemination in its host.
Taxonomic Summary
Phylum Microsporidia
Class Microsporea
Order Microsporida
Family Ichthyosporidiidae
Emended description of the genus Pseudoloma Matthews et al., 2001
Polysporous, sporophorous vesicles with uninucleate spores in aggregates of 8 to 16. Elaborate polaroplast, combining both lamellar and tubular-vesicular regions occupies approximately one-third to one-half of the spore. Some stages are multinucleated, none diplokaryotic. Early development, proliferative stages (meronts), in direct contact with the host cell cytoplasm, produces a unique molecular surface layer, reminiscent of the glycocalyx of a gut epithelial cell, excluding the direct contact of host organelles with the parasite plasmalemma. Sporogonic development proceeds in intracellular sporophorous vesicles, which are the outermost structure interfacing the host cytoplasm. Xenoma formation absent.
Emended description of Pseudoloma neurophilia Matthews et al., 2001
Spores uninucleate ovoid to pyriform, length 5.4 (4.8–5.9) μm, width 2.7 (2.3–3.1) μm; contain large posterior vacuole without an inclusion body, elaborate polaroplast, an isofilar polar filament with 14 to 17 coils. Persistent sporophorous vesicles contain 8--16 spores in aggregates up to 200 μm in diameter; developmental stages undergo karyokinesis producing tetranucleate cells which then undergo step-wise cytokinesis, forming uninucleate cells; development is asynchronous.
Type and only known host
Zebrafish Danio rerio Hamilton-Buchanan, 1822
Site of infection
Hind brain and spinal cord (usually ventral), nerve root ganglia, muscle, ovaries, occasionally eggs.
DNA sequences
Signature sequences in small subunit rDNA at position 168–174, TTTTGTT and at position 1271–1276, TTATTT. The gene sequence is deposited with GenBank accession number AF322654.
Acknowledgments
This work was supported by NIH grant 5RO1AI031788-19 from the NIAID and National Center for Research Resources 5R24RR017386-02.
Literature Cited
- Cali A, Takvorian PM. Microsporidia. In: Margulis L, editor. Handbook of Protoctista. 2nd. Jones and Bartlett; Boston, MA: 2011. [Google Scholar]
- Cali A, Takvorian PM. Developmental morphology and life cycles of the Microsporidia. In: Wittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. ASM Press; Washington D. C.: 1999. pp. 85–128. [Google Scholar]
- Canning EU, Lom J. The Microsporidia of Vertebrates. Academic Press; London, England: 1986. p. 289. [Google Scholar]
- De Kinkelin P. Occurrence of a microporidian infection in zebra danio Brachydanio rerio (Hamilton-Buchanan) J Fish Dis. 1980;3:71–73. [Google Scholar]
- Kent ML. Development and maintenance of a specific pathogen-free (SPF) zebrafish facility for Pseudoloma neurophilia. Dis Aquat Organ. 2011 doi: 10.3354/dao02333. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Bishop-Stewart JK. Transmission and tissue distribution of Pseudoloma neurophilia (Microsporidia) of zebrafish, Danio rerio (Hamilton) J Fish Dis. 2003;26:423–426. doi: 10.1046/j.1365-2761.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Kent ML, Speare DJ. Review of the sequential development of Loma salmonae (Microsporidia) based on experimental infections of rainbow trout (Oncorhynchus mykiss) and Chinook salmon (O. tshawytscha) Folia Parasitol. 2005;52:63–67. doi: 10.14411/fp.2005.009. [DOI] [PubMed] [Google Scholar]
- Lom J. A catalogue of described genera and species of microsporidians parasitic in fish. Syst Parasitol. 2002;53:81–99. doi: 10.1023/a:1020422209539. [DOI] [PubMed] [Google Scholar]
- Matthews JL, Brown A, Larison K, Bishop-Stewart JK, Rogers P, Kent ML. Pseudoloma neurophilia n g, n sp, a new microsporidium from the central nervous system of the zebrafish (Danio rerio) J Eukaryot Microbiol. 2001;48:227–233. doi: 10.1111/j.1550-7408.2001.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Monaghan S, Kent M, Watral V, Kaufman R, Lee L, Bols N. Animal cell cultures in microsporidial research: their general roles and their specific use for fish microsporidia. Vitro Cell Dev Biol– Anim. 2009;45:135–147. doi: 10.1007/s11626-008-9172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CM, Sprague V. Electron microscopical study of a new genus and new species of microsporida in the gills of Atlantic cod Gadus morhua L*. JFish Dis. 1981;4:15–32. [Google Scholar]
- Ramsay JM, Watral V, Schreck CB, Kent ML. Pseudoloma neurophilia infections in zebrafish Danio rerio: effects of stress on survival, growth, and reproduction. Dis Aquat Organ. 2009;88:69–84. doi: 10.3354/dao02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JG, Speare DJ, Markham R, Jones S. Isolation of a Loma salmonae variant: biological characteristics and host range. J Fish Biol. 2001;59:427–441. [Google Scholar]
- Sprague V, Becnel JJ, Hazard EI. Taxonomy of phylum Microspora. Crit Rev Microbiol. 1992;18:285–395. doi: 10.3109/10408419209113519. [DOI] [PubMed] [Google Scholar]