Abstract
Aim:
To investigate the protective effects of prostaglandin E1 (PGE1) against H2O2-induced oxidative damage on human umbilical vein endothelial cells (HUVECs).
Methods:
HUVECs were pretreated with PGE1 (0.25, 0.50, and 1.00 μmol/L) for 24 h and exposed to H2O2 (200 μmol/L) for 12 h, and cell viability was measured by the MTT assay. LDH, NO, SOD, GSH-Px, MDA, ROS, and apoptotic percentage were determined. eNOS expression was measured by Western blotting and real-time PCR.
Results:
PGE1 (0.25−1.00 μmol/L) was able to markedly restore the viability of HUVECs under oxidative stress, and scavenged intracellular reactive oxygen species induced by H2O2. PGE1 also suppressed the production of lipid peroxides, such as MDA, restored the activities of endogenous antioxidants including SOD and GSH-Px, and inhibited cell apoptosis. In addition, PGE1 significantly increased NO content, eNOS protein, and mRNA expression.
Conclusion:
PGE1 effectively protected endothelial cells against oxidative stress induced by H2O2, an activity that might depend on the up-regulation of NO expression.
Keywords: prostaglandin E1, human umbilical vein endothelial cells, hydrogen peroxide, apoptosis, nitric oxide
Introduction
The endothelium is located in a strategic anatomical position within the blood vessel wall and acts as a barrier between the blood and vascular smooth muscle. Therefore, the functional integrity of the endothelium is essential to prevent vascular leakage and the formation of vascular diseases1. Generation of reactive oxygen species (ROS) by vascular endothelial cells is involved in several clinical conditions such as atherosclerosis, hypercholesteremia and disseminated intravascular coagulation, among others2, 3. The up-regulation of ROS in vascular lesions will exert detrimental effects on the peroxidation of membrane lipids, endothelium-derived enzyme inactivation, apoptotic occurrences, etc4. In contrast, antioxidants that react preferentially with ROS to inactivate them might have therapeutic applications in treating ROS-induced endothelial injury.
Prostaglandin E1 (Alprostadil, PGE1), which is an important member of the prostaglandin family, is a product of arachidonic acid metabolism by cyclooxygenase. PGE1 has vasodilator effects on the systemic and pulmonary circulation5 and cardio-protective effects during ischemia and reoxygenation6. In addition, beneficial effects on angiogenesis, platelet aggregation, blood viscosity and fibrinolysis have been observed7, 8, 9, 10, 11, 12, 13. These observations may be partly mediated by nitric oxide (NO) and vascular endothelial growth factor (VEGF)14, 15, 16. Moreover, increasing attention has now been paid to the cytoprotective action and anti-oxidant activity of PGE1. PGE1 can protect human retinal pigment epithelial cells against oxidative injury17 and prevent the production of ROS in the intestinal mucosa of methotrexate-treated rats18. However, no direct evidence has been provided about the protective effects of PGE1 on oxidative stress-induced vascular endothelium dysfunction, which is an important contributor to the development of cardiovascular diseases.
In the present study, we used human umbilical vein endothelial cells (HUVECs) to examine the protective effects of PGE1 under oxidative stress, as well as the underlying mechanisms involved in this protection.
Materials and methods
Chemicals and reagents
PGE1 was purchased from the National Institute for the Control of Pharmaceuticals and Biological Products (Beijing, China). Hydrogen peroxide (H2O2), dimethylsulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), vitamin C (Vc), Hoechst 33258, and NG-Nitro-L-arginine methylester (L-NAME) were obtained from Sigma (St Louis, MO, USA). The reagent kits for the measurement of the levels of LDH, MDA, SOD, NO, and GSH-Px were purchased from Nanjing Institute of Jiancheng Bioengineering (Nanjing, China). The annexin V-FITC apoptosis detection kit was obtained from Jingmei (Shenzhen, China). Rabbit anti-eNOS antibody, mouse anti-β-actin antibody, goat-anti-rabbit-IgG-HRP, goat-anti-mouse-IgG-HRP and Western blotting Luminol reagent were obtained from Santa Cruz (Santa Cruz, CA, USA). 2′,7′-dichlorofluorescein diacetate (DCFH-DA), TRIzol reagent and M-MLV reverse transcriptase were obtained from Invitrogen (Carlsbad, CA, USA), and Green Real-time PCR Master Mix was from Toyobo (Osaka, Japan). All other reagents used were of analytical grade.
Cell culture and treatment
HUVECs were maintained in Endothelial Cell Medium (ECM) supplemented with 5% fetal bovine serum and 1% Endothelial Cell Growth Supplement (ECGS) in Poly-L-Lysine (PLL) pretreated flasks at 37 °C in a 5% CO2 incubator. HUVECs, ECM, fetal bovine serum, ECGS and PLL were all purchased from Sciencell Research Laboratories (Carlsbad, CA, USA).
HUVECs were pretreated with ECM containing PGE1 (0.25, 0.50, and 1.00 μmol/L) or Vc (1.5 mmol/L). Then, the culture supernatant was removed and the cells were exposed to H2O2 (200 μmol/L) diluted in ECM for 12 h at 37 °C until further assay.
Cell viability measurement
The viability of HUVECs was measured by the MTT assay19. The absorbance (A) at 570 nm in each well was determined with a microplate autoreader (Bio-Rad, Hercules, CA, USA). The viability of HUVECs in each well was presented as a percentage of the control group.
Measurement of intracellular ROS
Measurement of intracellular ROS was based on ROS-mediated conversion of nonfluorescent 2′,7′-DCFH-DA into DCFH20. Following the same procedure as the incubation studies, cells were harvested and incubated with DCFH-DA (10 μmol/L) diluted in ECM at 37 °C for 30 min. Subsequently, cells were washed with PBS three times and analyzed by flow cytometry performed on a FACScalibur cell analyzer using Cell Quest software (Becton Dickinson, Mountain View, CA, USA).
Preparation of cell lysate
The cells were treated as described above, and the culture supernatant was collected for analysis of LDH and NO release. The cells were scraped from the plates into ice-cold RIPA lysis buffer and the protein concentration was determined by the bicinchoninic acid (BCA) method, using BSA as a reference standard. Cell lysate was stored at -80 °C until used for the detection of MDA, SOD, and GSH-Px.
Measurement of LDH and NO release, as well as intracellular SOD, GSH-Px and MDA
LDH, an indicator of cell injury, was detected after exposure to H2O2 with an assay kit according to the manufacturer's protocol. Enzyme activity was expressed as units per liter and the absorbance was read at 440 nm.
The concentration of nitrites (NO2−) and nitrates (NO3−), stable end products of nitric oxide (NO), was determined by the Griess reaction. NO production was determined by measuring the optical density at 550 nm and was expressed in units per liter.
The concentration of MDA and the activities of SOD and GSH-Px were determined using commercially available kits. All procedures complied with the manufacturer's instructions. The results of the MDA, SOD, and GSH-Px assays were expressed as units per milligram of protein. The assay for SOD activity was based on its ability to inhibit the oxidation of hydroxylamine by O2 −· produced from the xanthine–xanthineoxiase system. One unit of SOD activity was defined as the amount that reduced the absorbance at 550 nm by 50%. The assay for GSH-Px activity was assayed by quantifying the rate of oxidation of reduced glutathione to oxidized glutathione by H2O2, as catalyzed by GSH-Px. One unit of GSH-Px was defined as the amount that reduced the level of GSH at 412 nm by 1 μmol/L in 1 min/mg protein. MDA was measured at a wavelength of 532 nm by reacting with thiobarbituric acid (TBA) to form a stable chromophoric product. Values for the MDA level were expressed in units per milligram of protein.
Flow cytometric evaluation of apoptosis
Cells grown in 6-well plates were harvested and washed. Samples were double-stained by Annexin V-FITC and propidium iodide (PI) at room temperature for 30 min in the dark and quantitatively analyzed by flow cytometry performed on a FACScalibur cell analyzer using CellQuest software (Becton Dickinson, Mountain View, CA, USA).
Nuclear morphology
Cell death was determined by Hoechst 33258 and PI double fluorescent staining. Cells were cultured on cover slides and treated as described previously. Samples were stained with PI (10 μg/mL) and Hoechst 33258 (10 μg/mL) and then fixed with 4% paraformaldehyde. For each cover slide, 1000−1500 cells were examined under a fluorescence microscope (Olympus Corp, Tokyo, Japan) and photographed with a digital camera (Olympus DP70, Japan).
Western blot analysis
Samples (40 μg protein) from the cell lysate were applied to 10% SDS-PAGE, followed by transfer to nitrocellulose membranes. Blots were then blocked with 5% skim milk in TBST for 1 h and incubated with rabbit anti-eNOS and mouse anti-β-actin antibodies at dilutions recommended by the supplier. Then, the membrane was washed, and primary antibodies were detected with goat-anti-rabbit-IgG-HRP or goat-anti-mouse-IgG-HRP. Protein expression levels were determined by analyzing the signals captured on the nitrocellulose membranes using Western blotting Luminol reagent and Konica X-ray film (Konica, Tokyo, Japan).
Quantitative real-time PCR assays
Total RNA was isolated using TRIzol reagent according to the manufacturer's instructions. The cDNA was synthesized from 2 μg RNA using M-MLV reverse transcriptase. Real-time quantitative PCR analysis was carried out in a MyiQ single color real time PCR detection instrument (Bio-Rad, Hercules, CA, USA). Specific sense and antisense primers used were as follows: eNOS sense: 5′-CCTTCTGTGTGGGAGAGGAT-3′, and antisense: 5′-TTGTAGCCTGGAACATCTTCC-3′ β-actin sense: 5′-CACTGTGTTGGCGTACAGGT-3′, and anti-sense 5′-TCATCACCATTGGCAATGAG-3′. The reaction was conducted with an initial denaturing step at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 55 °C for 10 s and 72 °C for 15 s. The reaction was terminated by a cooling step at 4 °C. Each experiment was performed in duplicate and all data were analyzed using Bio-Rad iQ5 Software.
Statistical analysis
All values are expressed as the mean±SD. The data were analyzed by a one-way ANOVA and a two-tailed Student's t-test. P<0.05 was considered statistically significant.
Results
Effects of PGE1 on the viability of H2O2-induced HUVECs
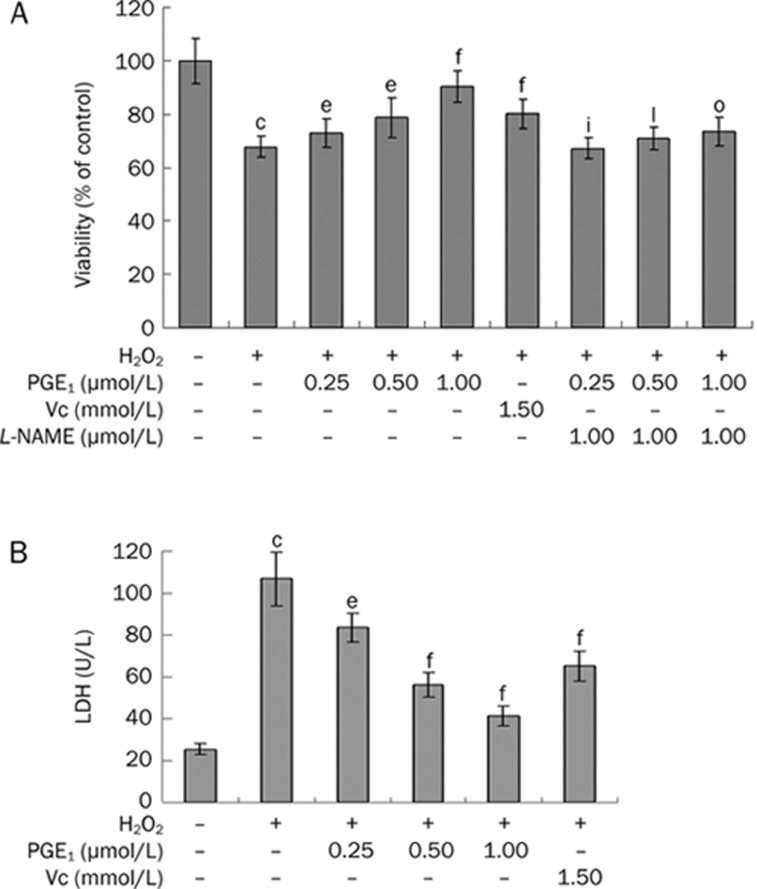
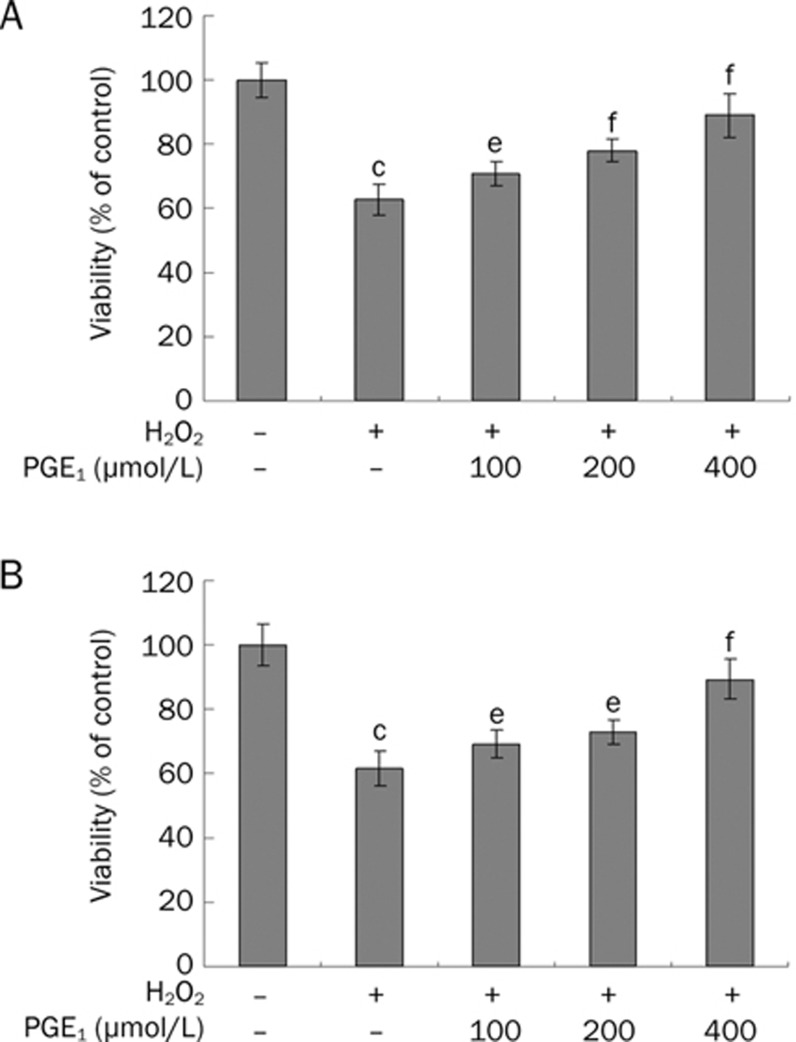
The effects of PGE1 on the viability of H2O2-induced HUVECs were evaluated by MTT analysis. The survival rate of HUVECs was 65.92%±3.48% after exposure to 200 μmol/L of H2O2 for 12 h (Figure 1A). However, pretreatment with PGE1 (0.25, 0.50, and 1.00 μmol/L) dose-dependently increased the viability of H2O2-induced HUVECs. This effect of PGE1 was significantly attenuated by the NO-inhibitor L-NAME. When incubated with 0.0625−2.00 μmol/L PGE1 alone for 24 h, cell viability did not show marked changes compared with the control group, but significant cytotoxicity was observed with the 4.00 μmol/L PGE1 treatment for 24 h (data not shown). Therefore, a range of 0.25−1.00 μmol/L PGE1 concentrations was used throughout subsequent experiments. Furthermore, addition of PGE1 (0.25, 0.50, and 1.00 μmol/L) simultaneously with or immediately after H2O2 treatment also produced protective outcomes (Figure 2).
Figure 1.
Effects of PGE1 on cell viability of H2O2-induced HUVECs as evaluated by MTT analysis (A) and LDH release (B). Values are expressed as mean±SD. n=6. cP<0.01 vs control group; eP<0.05, fP<0.01 vs the H2O2-alone group; iP<0.01 vs the H2O2+0.25 μmol/L PGE1 group; lP<0.01 vs the H2O2+0.50 μmol/L PGE1 group; oP<0.01 vs the H2O2+1.00 μmol/L PGE1 group.
Figure 2.
Effects of PGE1 on cell viability. (A) HUVECs were co-incubated with H2O2 and PGE1 for 24 h, and then cell viability was evaluated by MTT analysis. (B) HUVECs were pre-incubated with H2O2 for 1 h and then exposed to PGE1 for 24 h. The viability of cells was evaluated by MTT analysis. Values are expressed as mean±SD. n=6. cP<0.01 vs control group; eP<0.05, fP<0.01 vs the H2O2-alone group.
To further investigate the protective effects of PGE1, the LDH assay, another indicator of cell toxicity, was performed. LDH release was minimal in the vehicle treated control group (25.40±2.80 U/L) and a dramatic increase (106.82±12.71 U/L) was observed after 12 h exposure to 200 μmol/L H2O2. However, pre-treatment with PGE1 (0.25, 0.50, and 1.00 μmol/L) attenuated the H2O2-induced increase in LDH release to 83.49±7.05, 56.32±5.84, and 41.11±4.73 U/L, respectively (Figure 1B).
Intracellular radical scavenging ability of PGE1
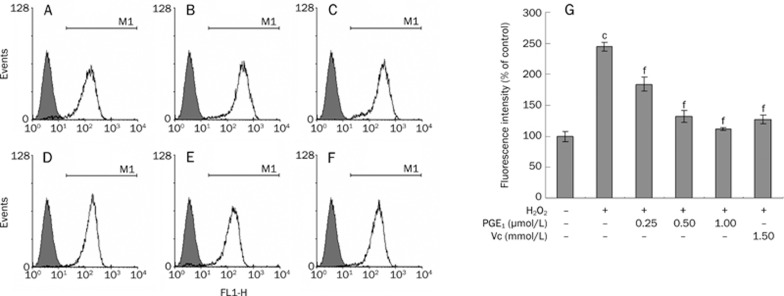
The intracellular ROS concentration was determined by measuring the intensity of DCFH fluorescence, as the intensity of fluorescence reflects enhanced oxidative stress.
The DCFH fluorescence intensity in the H2O2-treated control group increased to 244.82%±7.24%, whereas pre-incubation with PGE1 (0.25, 0.50, and 1.00 μmol/L) significantly reduced the increase in fluorescence induced by H2O2 in a concentration-dependent manner (Figure 3).
Figure 3.
Effects of PGE1 on the production of intracellular ROS was determined by measuring the intensity of DCFH fluorescence. (A) Control group; (B) H2O2-alone group; (C) H2O2+0.25 μmol/L PGE1 group; (D) H2O2+0.50 μmol/L PGE1 group; (E) H2O2+1.00 μmol/L PGE1 group; (F) H2O2+1.5 mmol/L Vc group; (G) Effects of PGE1 on the production of intracellular ROS. The values are expressed as percentages of the fluorescence intensity. Mean±SD. n=4. cP<0.01 vs control group; fP<0.01 vs the H2O2-alone group.
Effects of PGE1 on SOD and GSH-Px activities and MDA content
The effects of PGE1 on lipid peroxidation and endogenous antioxidant preservation were also measured. Treatment with H2O2 (200 μmol/L, 12 h) decreased the activities of SOD and GSH-Px to 59.6%±6.42% and 54.3%±4.49%, respectively, compared to no treatment. However, pre-incubation with PGE1 (0.25, 0.50, and 1.00 μmol/L) or Vc (1.5 mmol/L) significantly attenuated the changes in SOD and GSH-Px activity (Table 1).
Table 1. Effects of PGE1 on SOD and GSH-Px activities as well as MDA content in H2O2-induced HUVECs. Values are mean±SD. n=6. cP<0.01 vs control group; eP<0.05, fP<0.01 vs the H2O2-alone group.
| Parameters | SOD (U/mg protein) | GSH-Px (U/mg protein) | MDA (U/mg protein) |
|---|---|---|---|
| Control | 21.39±0.46 | 124.01±12.20 | 1.27±0.08 |
| H2O2 | 12.76±1.37c | 67.28±5.56c | 2.27±0.05c |
| H2O2+0.25 μmol/L PGE1 | 14.54±0.52e | 76.69±3.80f | 1.98±0.07f |
| H2O2+0.50 μmol/L PGE1 | 17.21±0.89f | 101.10±4.33f | 1.76±0.11f |
| H2O2+1.00 μmol/L PGE1 | 20.13±1.17f | 113.47±4.25f | 1.53±0.13f |
| H2O2+1.5 mmol/L Vc | 17.46±0.78f | 82.59±2.54f | 1.91±0.13f |
Additionally, treatment with H2O2 (200 μmol/L, 12 h) increased intracellular MDA levels by 79.1%±4.32%, while pre-incubation with PGE1 (0.25, 0.50, and 1.00 μmol/L) or Vc (1.5 mmol/L) markedly attenuated the increase to 151.2%, 139.2%, 120.7%, and 151.1% of the control group, respectively (Table 1).
Effects of PGE1 on H2O2-induced apoptosis
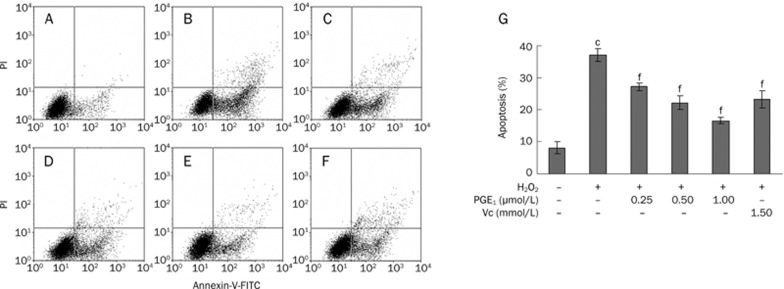
The effects of PGE1 against H2O2-induced apoptosis were measured using a FACScalibur cell analyzer and fluorescence microscope. Using Annexin V-FITC and PI staining, the apoptotic percentage of untreated cells was determined to be 8.05%±1.91% (Figure 4A) in the present experiment. After treatment with H2O2 for 12 h, the percentage of apoptotic cells increased to 37.0%±1.94% (Figure 4B). Upon pretreatment with PGE1 (0.25, 0.50, and 1.00 μmol/L), the apoptotic rate induced by H2O2 decreased to 27.15%±1.22%, 22.20%±2.02%, and 16.60%±0.93%, respectively (Figure 4C, 4D, 4E).
Figure 4.
Effects of PGE1 against H2O2-induced apoptosis in cultured HUVECs by flow cytometric analysis. (A) Control group; (B) H2O2-alone group; (C) H2O2+0.25 μmol/L PGE1 group; (D) H2O2+0.50 μmol/L PGE1 group; (E) H2O2+1.00 μmol/L PGE1 group; (F) H2O2+1.5 mmol/L Vc group; (G) Protective effects of PGE1 against H2O2-induced apoptosis in H2O2-induced HUVECs. Values are expressed as mean±SD. n=4. cP<0.01 vs control group; fP<0.01 vs the H2O2-alone group.
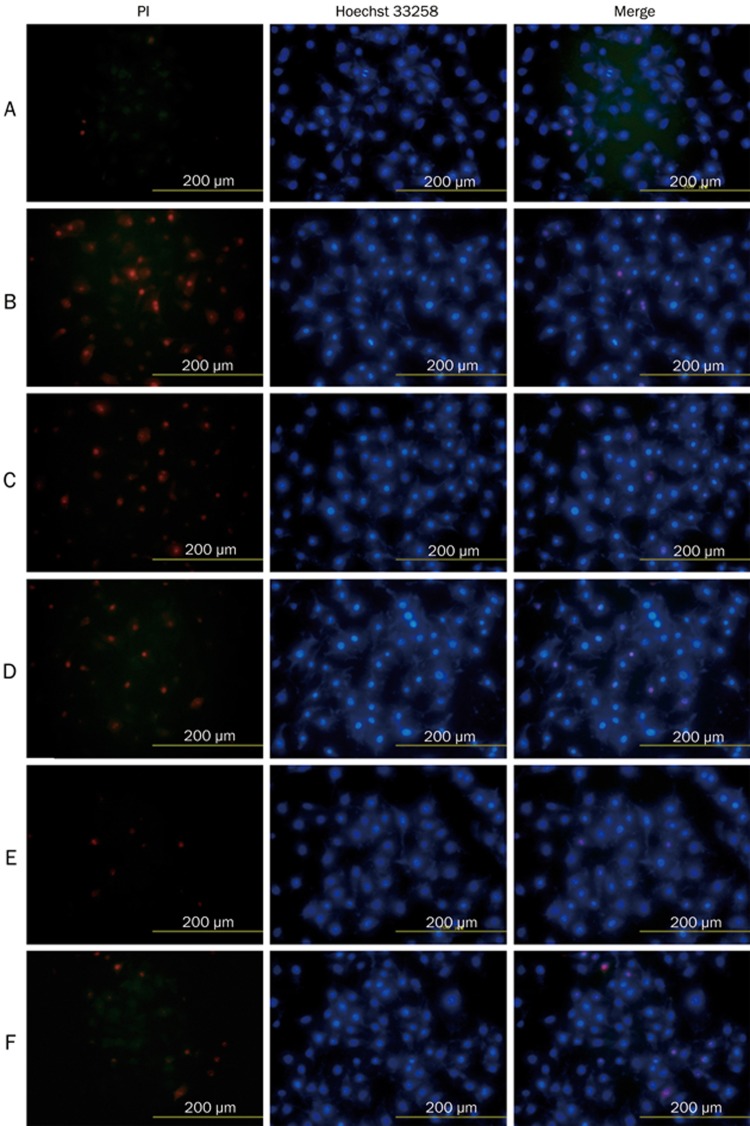
In the control group, little morphological sign of nuclear damage or chromatin condensation was observed (Figure 5A). Once injured by H2O2, cells exhibited condensed nuclei, apoptotic bodies and detachment indicative of cell death (Figure 5B). However, pre-incubation with PGE1 (0.25, 0.50, and 1.00 μmol/L) before H2O2-injury significantly attenuated cell damage (Figure 5C, 5D, 5E).
Figure 5.
Protective effects of PGE1 on H2O2-induced HUVEC death as visualized by Hoechst 33258/PI staining. (A) Control group; (B) H2O2-alone group; (C) H2O2+0.25 μmol/L PGE1 group; (D) H2O2+0.50 μmol/L PGE1 group; (E) H2O2+1.00 μmol/L PGE1 group; (F) H2O2+1.5 mmol/L Vc group.
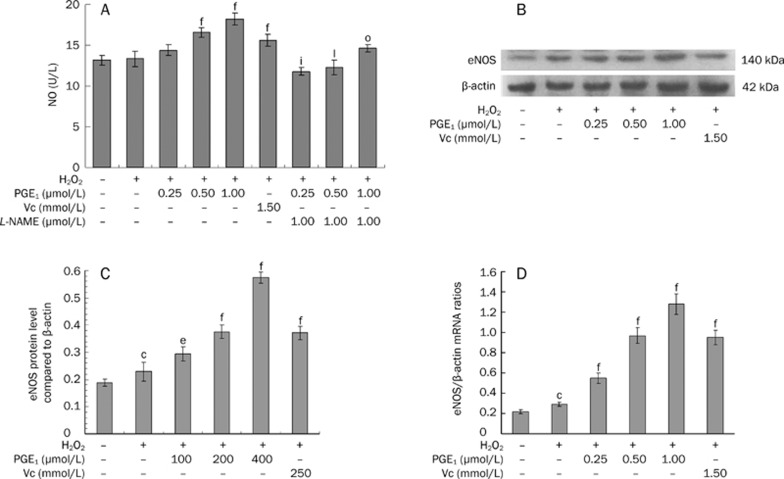
Detection of NO release in culture supernatant and expression of eNOS protein and mRNA in HUVECs
To investigate the mechanisms that mediate the antioxidative effect of PGE1, the NO release and eNOS expression were measured. Pre-incubation with PGE1 (0.25, 0.50, and 1.00 μmol/L) was observed to significantly increase NO content in a dose-dependent manner (Figure 6A). However, co-incubation with 1 μmol/L L-NAME and PGE1 for 24 h before H2O2 treatment significantly reduced NO release, as well as cell viability, compared to the PGE1 treatment group (Figures 1A and 6A).
Figure 6.
Effects of PGE1 on NO release (A), eNOS protein (B, C) and mRNA expression (D). Values are expressed as mean±SD. n=6. (A) fP<0.01 vs the H2O2-alone group; iP<0.01 vs the H2O2+0.25 μmol/L PGE1 group; lP<0.01 vs the H2O2+0.50 μmol/L PGE1 group; oP<0.01 vs the H2O2+1.00 μmol/L PGE1 group. (C, D) cP<0.01 vs the empty control group; eP<0.05, fP<0.01 vs the H2O2-alone group.
In parallel, the eNOS protein (Figure 6B) and mRNA (Figure 6D) levels in HUVECs were also assayed. Pre-incubation with PGE1 (0.25, 0.50, and 1.00 μmol/L) also triggered eNOS protein and mRNA expression markedly in a dose-dependent manner. These results were consistent with the observed change in NO content over the course of the experiments.
Discussion
The results presented here indicated that PGE1 protected endothelial cells from H2O2-induced injury. It decreased the amount of ROS, LDH, and MDA, decreased the percentage of apoptotic cells and increased SOD and GSH-Px activity. Furthermore, PGE1 increased NO release and upregulated eNOS protein and mRNA expression.
Oxidative stress plays a critical role in endothelial dysfunction21 and is a critical pathogenic factor in the development of cardiovascular diseases, such as atherosclerosis, hypercholesteremia and disseminated intravascular coagulation22. Here, we demonstrated that H2O2, a precursor of other ROS, can markedly increase cell permeability, damage cellular antioxidant defenses and induce endothelial cell apoptosis at 200 μmol/L.
Agents that inhibit production of ROS or enhance cellular antioxidant defenses can protect cells from the damaging effects of oxygen radicals23. Our study first proved that PGE1 not only strikingly scavenged intracellular ROS induced by H2O2 (Figure 3), but also effectively increased the viability of H2O2-induced endothelial cells (Figure 1). Miho et al. suggest that PGE1 protects human retinal pigment epithelial cells from oxidative injury17 and prevents the production of ROS in the intestinal mucosa of methotrexate-treated rats18. These results indicate that the protective effect is associated with PGE1 scavenging intracellular ROS.
Lipid peroxidation is one of the primary events in free radical-mediated cell injury. MDA is a by-product of lipid peroxidation induced by excessive ROS and is widely used as a biomarker of oxidative stress24. Antioxidant enzymes, such as SOD and GSH-Px, however, are thought to be effective for the augmentation of antioxidant defenses in endothelial cells25. In our study, when endothelial cells were incubated with PGE1, these H2O2-induced cellular events were almost completely blocked (Table 1). These results jointly suggest that the scavenging of ROS might be related to the increased activities of antioxidant enzymes.
Hydrogen peroxide not only decreased the viability of cells, but also induced cell apoptosis21. Previous studies have shown that PGE1 protects human liver sinusoidal endothelial cells and endothelial progenitor cells from apoptosis26, 27. In the present study, we demonstrate that 200 μmol/L H2O2 induces massive cell apoptosis, while PGE1 (0.25−1.00 μmol/L) prevents the H2O2-induced apoptosis in a concentration-dependent manner (Figures 4 and 5). Thus, we presumed that PGE1 prevented cell apoptosis due to decreased intracellular ROS and increased antioxidant enzymatic activities.
Nitric oxide (NO) is a soluble gas continuously synthesized by the endothelium and has a wide range of biological properties that maintain vascular homeostasis, including modulation of vascular dilator tone, regulation of local cell growth, and protection of the vessel from injury as a consequence of platelets and cells circulating in blood28. Endothelial dysfunction, defined as impaired endothelium-dependent vasorelaxation, is usually attributed to a deficiency of nitric oxide29. Our studies show that pretreatment with PGE1 significantly increases NO and eNOS expression compared to H2O2-alone (Figure 6). The increasing NO content is paralleled by a reduction of cell injury induced by H2O2 (Figures 1A and 6A). NO terminates chain reactions during lipid peroxidation, as observed in model lipid systems of low-density lipoprotein oxidation and in cells14, 30. These results suggest that the NO pathway plays an important role in the protective activity of prostaglandin E1 against H2O2-induced oxidative injury, but the mechanisms are still unclear.
PGE1 activates adenylate cyclase and increases intracellular cAMP via binding to prostanoid receptor subtypes EP2, EP3, EP4, and IP31. The cAMP-responsive elements (CRE) within the human eNOS promoter play an important role in the inducible expression of the human eNOS gene32, 33. Progressive 5′-deletion and site-specific mutation analyses defined the beraprost-responsive sequences as CRE, located at -733 and -603 within the human eNOS promoter32, 33, and the hypoxia-responsive sequences located at -924 to -92134, while the PGE1-responsive sequences are still unclear. PGE1 increases eNOS expression through PKA pathway activation and cAMP-responsive element binding protein (CREB) phosphorylation. Phosphorylated CREB predominantly binds to CRE, which leads to increased promoter activity and upregulation of the eNOS protein and mRNA expression32, 33, 34. Previous studies have also reported that PKA signaling increased phosphorylation of Ser-1177 and dephosphorylation of Thr-495 to activate eNOS, which led to the upregulation of NO35. Activation of perinuclear EP3 receptors induced eNOS expression, which depends on nuclear envelope K (Ca) channels, protein kinases, and NF-kappaB36. Studies also showed that MAPK is a central pathway to transmit the actions of PGE1 on eNOS14.The mechanism by which PGE1 upregulates NO expression requires further research before it is well understood.
As our study was limited to the cell level, further studies are needed to confirm the cardiovascular protective effect of PGE1 in organs or humans. As stated above, we concluded that pretreatment of HUVECs with PGE1 significantly protected those cells from H2O2-induced cell death, which was mediated, at least in part, by a mechanism linked to the up-regulation of NO expression. It is noteworthy that the finding of the present study may shed light on the pharmacological basis for the clinical application of PGE1 for the treatment of atherosclerosis and acute coronary syndrome, which are relevant to endothelial cell death.
Author contribution
Wen-tong FANG designed and performed the research, analyzed and interpreted data, and drafted the paper; Hong-jian LI and Liao-sheng ZHOU designed the research, analyzed data, critically evaluated the manuscript for its important intellectual content, and corrected the manuscript.
Acknowledgments
We thank Drs Zhi-gang TIAN and Jian ZHANG from the Institute of Immunopharmacology and Immunotherapy, School of Pharmaceutical Sciences, Shandong University, Ji-nan, China, for their generous support of this work.
References
- Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 2001;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- Pandian RP, Kutala VK, Liaugminas A, Parinandi NL, Kuppusamy P. Lipopolysaccharide-induced alterations in oxygen consumption and radical generation in endothelial cells. Mol Cell Biochem. 2005;278:119–27. doi: 10.1007/s11010-005-6936-x. [DOI] [PubMed] [Google Scholar]
- Fasanaro P, Magenta A, Zaccagnini G, Cicchillitti L, Fucile S, Eusebi F, et al. Cyclin D1 degradation enhances endothelial cell survival upon oxidative stress. FASEB J. 2006;20:1242–4. doi: 10.1096/fj.05-4695fje. [DOI] [PubMed] [Google Scholar]
- Xu HB, Huang ZQ. Icariin enhances endothelial nitric-oxide synthase expression on human endothelial cells in vitro. Vascul Pharmacol. 2007;47:18–24. doi: 10.1016/j.vph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Gottfried JL, Michael G, Heidrun L, Brigitte S, Julia K, Sylvia K, et al. Prostaglandin E1 does not influence plasmatic coagulation, hepatic synthesis, or postoperative blood loss in patients after coronary-artery bypass grafting. J Clin Anesth. 2000;12:363–70. doi: 10.1016/s0952-8180(00)00170-7. [DOI] [PubMed] [Google Scholar]
- Tauber S, Menger M, Lehr H. Microvascular in vivo assessment of reperfusion injury: significance of prostaglandin E1 and I2 in postischemic “No-Reflow” and “Reflow-Paradox”. J Surg Res. 2004;120:1–11. doi: 10.1016/S0022-4804(03)00332-9. [DOI] [PubMed] [Google Scholar]
- Sinha AK, Colman RW. Prostaglandin E1 inhibits platelet aggregation by a pathway independent of adenosine 3′,5′-monophosphate. Science. 1978;200:202–3. doi: 10.1126/science.204997. [DOI] [PubMed] [Google Scholar]
- Marchesi S, Pasqualini L, Lombardini R, Vaudo G, Lupattelli G, Pirro M, et al. Prostaglandin E1 improves endothelial function in critical limb ischemia. J Cardiovasc Pharmacol. 2003;41:249–53. doi: 10.1097/00005344-200302000-00014. [DOI] [PubMed] [Google Scholar]
- Schror K, Hohlfeld T. Mechanisms of anti-ischemic action of prostaglandin E1 in peripheral arterial occlusive disease. Vasa. 2004;33:119–24. doi: 10.1024/0301-1526.33.3.119. [DOI] [PubMed] [Google Scholar]
- Mehrabi MR, Serbecic N, Tamaddon F, Kaun C, Huber K, Pacher R, et al. Clinical and experimental evidence of prostaglandin E1-induced angiogenesis in the myocardium of patients with ischemic heart disease. Cardiovasc Res. 2002;56:214–24. doi: 10.1016/s0008-6363(02)00591-6. [DOI] [PubMed] [Google Scholar]
- Kuss M, Heidrich H, Koettgen E. Hemostatic and fibrinolytic effects of systemic prostaglandin E1 therapy in patients with peripheral arterial disease. Vasa. 2003;32:145–8. doi: 10.1024/0301-1526.32.3.145. [DOI] [PubMed] [Google Scholar]
- Weiss C, Regele S, Velich T, Bartsch P, Weiss T. Hemostasis and fibrinolysis in patients with intermittent claudication: effects of prostaglandin E1. Prostaglandins Leukot Essent Fatty Acids. 2000;63:271–7. doi: 10.1054/plef.2000.0214. [DOI] [PubMed] [Google Scholar]
- Vaughan DE, Plavin SR, Schafer AI, Loscalzo J. PGE1 accelerates thrombolysis by tissue plasminogen activator. Blood. 1989;73:1213–7. [PubMed] [Google Scholar]
- Haider DG, Bucek RA, Giurgea AG, Maurer G, Glogar H, Minar E, et al. PGE1 analog alprostadil induces VEGF and eNOS expression in endothelial cells. Am J Physiol Heart Circ Physiol. 2005;289:H2066–72. doi: 10.1152/ajpheart.00147.2005. [DOI] [PubMed] [Google Scholar]
- Weiss TW, Mehrabi MR, Kaun C, Zorn G, Kastl SP, Speidl WS, et al. Prostaglandin E1 induces vascular endothelial growth factor-1 in human adult cardiac myocytes but not in human adult cardiac fibroblasts via a cAMP-dependent mechanism. J Mol Cell Cardiol. 2004;36:539–46. doi: 10.1016/j.yjmcc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–44. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miho Y, Norihito S, Hisao T, Keizo F, Akihiro O, Yoshihito H, et al. Induction of human thioredoxin in cultured human retinal pigment epithelial cells through cyclic amp-dependent pathway; involvement in the cytoprotective activity of prostaglandin E1. Exp Eye Res. 1997;65:645–52. doi: 10.1006/exer.1997.0370. [DOI] [PubMed] [Google Scholar]
- Feng G, Toshiharu H. A synthetic analog of prostaglandin E1 prevents the production of reactive oxygen species in the intestinal mucosa of methotrexate-treated rats. Life Sci. 2002;71:1091–9. doi: 10.1016/s0024-3205(02)01795-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mendis E, Kim MM, Rajapakse N, Kim SK. An in vitro cellular analysis of the radical scavenging efficacy of chitooligosaccharides. Life Sci. 2007;80:2118–27. doi: 10.1016/j.lfs.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Malek AM, Izumo S. Molecular aspects of signal transduction of shear stress in the endothelial cell. J Hypertens. 1994;12:989–99. [PubMed] [Google Scholar]
- Wang YK, Huang ZQ. Protective effects of icariin on human umbilical vein endothelial cell injury induced by H2O2in vitro. Pharmacol Res. 2005;52:174–82. doi: 10.1016/j.phrs.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Cini M, Fariello RG, Bianchetti A, Moretti A. Studies on lipid peroxidation in the rat brain. Neurochem Res. 1994;19:283–8. doi: 10.1007/BF00971576. [DOI] [PubMed] [Google Scholar]
- Luo T, Xia Z. A small dose of hydrogen peroxide enhances tumor necrosis factor-alpha toxicity in inducing human vascular endothelial cell apoptosis: reversal with propofol. Anesth Analg. 2006;103:110–6. doi: 10.1213/01.ane.0000221183.02244.80. [DOI] [PubMed] [Google Scholar]
- Hong Y, Majno P, Morel P, Toso C, Triponez F, Oberholzer J, et al. Prostaglandin E1 protects human liver sinusoidal endothelial cell from apoptosis induced by hypoxia reoxygenation. Microvascul Res. 2002;64:94–103. doi: 10.1006/mvre.2002.2404. [DOI] [PubMed] [Google Scholar]
- Christoph G, Yvonne C, Christian W, Milad H, Michael B, Ulrich L. Regulation of endothelial progenitor cells by prostaglandin E1 via inhibition of apoptosis. J Mol Cell Cardiol. 2007;42:670–7. doi: 10.1016/j.yjmcc.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Cannon R. 3rd. Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin Chem. 1998;44:1809–19. [PubMed] [Google Scholar]
- Bełtowski J, Wójcicka G, Jamroz-Wiśniewska A. Role of nitric oxide and endothelium-derived hyperpolarizing factor (EDHF) in the regulation of blood pressure by leptin in lean and obese rats. Life Sci. 2006;79:63–71. doi: 10.1016/j.lfs.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Stephen GH, Anthony JF, Sean MM, Freya QS, Garry RB. Nitric oxide as a cellular antioxidant: a little goes a long way. Free Radic Biol Med. 2006;40:501–6. doi: 10.1016/j.freeradbiomed.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi K, Sugimoto Y, Ichikawa A. Prostanoid receptor subtypes. Prostaglandins Other Lipid Mediat. 2002;68–69:535–56. doi: 10.1016/s0090-6980(02)00054-0. [DOI] [PubMed] [Google Scholar]
- Niwano K, Arai M, Tomaru K, Uchiyama T, Ohyama Y, Kurabayashi M. Transcriptional stimulation of the eNOS gene by the stable prostacyclin analogue beraprost is mediated through cAMP-responsive element in vascular endothelial cells: close link between PGI2 signal and NO pathways. Circ Res. 2003;93:523–30. doi: 10.1161/01.RES.0000091336.55487.F7. [DOI] [PubMed] [Google Scholar]
- Niwano K, Arai M, Koitabashi N, Hara S, Watanabe A, Sekiguchi K, et al. Competitive binding of CREB and ATF2 to cAMP/ATF responsive element regulates eNOS gene expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1036–42. doi: 10.1161/01.ATV.0000215179.76144.39. [DOI] [PubMed] [Google Scholar]
- Min J, Jin YM, Moon JS, Sung MS, Jo SA, Jo I. Hypoxia-induced endothelial no synthase gene transcriptional activation is mediated through the tax-responsive element in endothelial cells. Hypertension. 2006;47:1189–96. doi: 10.1161/01.HYP.0000222892.37375.4d. [DOI] [PubMed] [Google Scholar]
- Michell BJ, Chen ZP, Tiganis T, Stapleton D, Katsis F, Power DA, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–8. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- Gobeil FJ, Dumont I, Marrache AM, Vazquez TA, Bernier SG, Abran D, et al. Regulation of eNOS expression in brain endothelial cells by perinuclear EP(3) receptors. Circ Res. 2002;90:682–9. doi: 10.1161/01.res.0000013303.17964.7a. [DOI] [PubMed] [Google Scholar]