Abstract
Aim:
To examine whether selective serotonin reuptake inhibitor citalopram interacts with Kv1.5, one of the cardiovascular-specific Kv channel isoforms.
Methods:
The interaction between citalopram and Kv1.5 expressed in Chinese hamster ovary cells was studied using the whole-cell patch-clamp technique.
Results:
Citalopram reduced Kv1.5 whole-cell currents in a reversible concentration-dependent manner, with an IC50 value and a Hill coefficient of 2.8±1.1 μmol/L and 0.8±0.3, respectively. Citalopram-induced inhibition of Kv1.5 is associated with time-dependent development of block without modifying the kinetics of current activation. The inhibition increased steeply between −30 and 0 mV, which corresponded with the voltage range for channel opening. In the voltage range positive to 0 mV, inhibition displayed an additional voltage dependence, consistent with an electrical distance δ of 0.19. Citalopram slowed the deactivation time course, resulting in a tail crossover phenomenon when the tail currents, recorded in the presence and absence of citalopram, were superimposed. Inhibition of Kv1.5 by citalopram was use-dependent.
Conclusion:
The present results suggest that citalopram acts on Kv1.5 currents as an open-channel blocker, and much caution about arrhythmogenic risk is required when using citalopram in the treatment with depressed patients.
Keywords: citalopram, serotonin reuptake inhibitors, Kv1.5, Shaker-type K+ channels, open channel block
Introduction
Selective serotonin reuptake inhibitor (SSRI) is thought to be safer than tricyclic antidepressant (TCA) in the risk of cardiovascular causes, because TCA is known to induce QT prolongation of the electrocardiogram and the associated arrhythmogenic risk in vivo1, 2, 3, 4. Citalopram is a SSRI and the drug exerts its antidepressant activity by inhibiting the reuptake of 5-hydroxytryptamine (5-HT, serotonin) in the central nervous system, without critical effects on other neurotransmitter reuptake systems5, 6, 7, 8. Therefore, citalopram may be an attractive drug for treatment of patients suffering from the cardiovascular adverse effects of TCA. However, accumulating evidence indicates that citalopram may lead to cardiovascular risk within the clinical range9, 10, 11. Furthermore, citalopram inhibits voltage-gated Ca2+ channels, Na+ channels and K+ channels in ventricular myocytes or cardiovascular smooth muscle cells12, 13, 14, 15, 16. Pharmacological blockade of voltage-gated K+ channels (Kv channels) in cardiac muscle has been associated with adverse cardiac arrhythmias, suggesting that citalopram may interact with cardiac Kv channels. However, the effects of citalopram on cardiac Kv channels remain to be elucidated.
Kv1.5 is one of the cardiovascular-specific Kv channel isoforms. Kv1.5 channels play an important role in determining the length of cardiac action potentials and, therefore, have been the targets of antiarrhythmic drugs17, 18. Because of its rapid activation and little inactivation, Kv1.5 can contribute to repolarization of atrial action potentials. Dysfunction of Kv1.5 results in a prolongation of cardiac action potentials, which eventually leads to cardiac arrhythmias with serious morbidity18, 19, 20.
In this study, we investigated whether citalopram interacts with Kv1.5 cloned from the rat brain, and determined the mechanisms of actions of citalopram on the Kv1.5.
Materials and methods
Cell culture and transfection
Chinese hamster ovary (CHO) cells expressing Kv1.5 channels derived from rat brain were used for electrophysiological recordings21. Kv1.5 cDNA22 was transferred into the plasmid expression vector pCR3.1 (Invitrogen Corporation, San Diego, CA, USA). CHO cells were transfected with Kv1.5 cDNA using FuGENE™6 reagent (Boehringer Mannheim, Indianapolis, IN, USA). The transfected cells were cultured in Iscove's modified Dulbecco's medium (Invitrogen Corporation) supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 0.1 mmol/L hypoxanthine, 0.01 mmol/L thymidine, and 300 μg/ml G418 (AG Scientific, San Diego, CA, USA), in 95% humidified air-5% CO2 at 37 °C. The cultures were passaged every 4−5 d with a brief trypsin-EDTA treatment followed by seeding onto glass coverslips (diameter: 12 mm, Fisher Scientific, Pittsburgh, PA, USA) in a Petri dish. After 12−24 h, the cell-attached coverslips were used for electrophysiological recordings.
Electrophysiology
Kv1.5 currents were recorded from CHO cells, with a whole-cell patch-clamp technique23 at room temperature (22−23 °C). The micropipettes fabricated from glass capillary tubing (PG10165-4; World Precision Instruments, Sarasota, FL, USA) with a double-stage vertical puller (PC-10; Narishige, Tokyo) had a tip resistance of 2−3 MΩ when filled with the pipette solution. Whole-cell currents were amplified with MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA), digitized with Digidata 1440A (Molecular Devices) at 5 kHz and low-pass filtered with four-pole Bessel filter at 2 kHz. Capacitive currents were canceled and series resistance was compensated at 80% with the amplifier, while leak subtraction was not used. The generation of voltage commands and acquisition of data were controlled with pClamp 10.1 software (Molecular Devices) running on an IBM-compatible Pentium computer. Recording chamber (RC-13, Warner Instrument Corporation, Hamden, CT, USA) was continuously perfused with bath solution (see below for composition) at a rate of 1 mL/min.
Solutions and drugs
The intracellular pipette solution for whole-cell recordings contained 140 mmol/L KCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L HEPES, and 10 mmol/L EGTA, and was adjusted to pH 7.3 with KOH. The bath solution for whole-cell recordings contained 140 mmol/L NaCl, 5 mmol/L KCl, 1.3 mmol/L CaCl2, 1 mmol/L MgCl2, 20 mmol/L HEPES and 10 mmol/L glucose, and was adjusted to pH 7.3 with NaOH. Citalopram (Sigma Chemical Co, Saint Louis, MO, USA) was dissolved in ethanol at 30 mmol/L and further diluted into the bath solution. Final concentration of ethanol in the bath solution, which was less than 0.1%, had no effect on Kv1.5 currents.
Data analysis
Data were analyzed with Origin 7.0 (OriginLab Corp, Northampton, MA, USA) and Clampfit 10.1 software (Molecular Devices). IC50 and Hill coefficient (n) were obtained from fitting concentration dependence data to the following equation:
I (%)=1/{1+(IC50/[D])n} (1)
in which I (%) is the percent inhibition of current (I (%)=[1− Idrug/Icontrol]×100) at test potential and [D] represents various drug concentrations. The steady-state activation curves were fitted with the Boltzmann equation:
y=1/{1+exp(−(V − V1/2)/k)} (2)
where k represents the slope factor, V the test potential, and V1/2 the potential at which the conductance was half-maximal. An activation time constant was calculated by fitting the latter 50% of activation (ie, rise from 50% to 100% of peak amplitude) with a single exponential function. The drug-induced time constant and deactivation time constant were determined by fitting with the sum of the exponentials:
y=B+A1exp(−t/τ1 )+A2exp(−t/τ2 )+...+Anexp(−t/τn) (3)
in which τ1, τ2, and τn are the time constants; A1, A2, and An are the amplitudes of each component of the exponential; and B is the baseline value.
To investigate the voltage dependence of Kv1.5 inhibition by the drug, the relative current was plotted as a function of the membrane potential. The resultant percent inhibition data between 0 and +50 mV were fitted with a Woodhull equation24:
I(%)/100 = [D]/{[D]+KD(0)×exp(−zδFV/RT)} (4)
where KD(0) represents the apparent affinity at 0 mV, z the charge valence of the drug, δ the fractional electrical distance, F Faraday's constant, R the gas constant, and T the absolute temperature. A value of 25.4 mV was used for RT/F at 22 °C in the present study. Equation 6 can be modified to get a linear transformation as follows:
ln{(Icontrol – Idrug)/Idrug} = ln{[D]/KD(0)}+δzFV/RT (5)
Results were expressed as mean±SEM. Student's t-test and analysis of variance (ANOVA) were used for statistical analysis. A value of P<0.05 was considered statistically significant.
Results
Concentration-dependent inhibition of Kv1.5 by citalopram
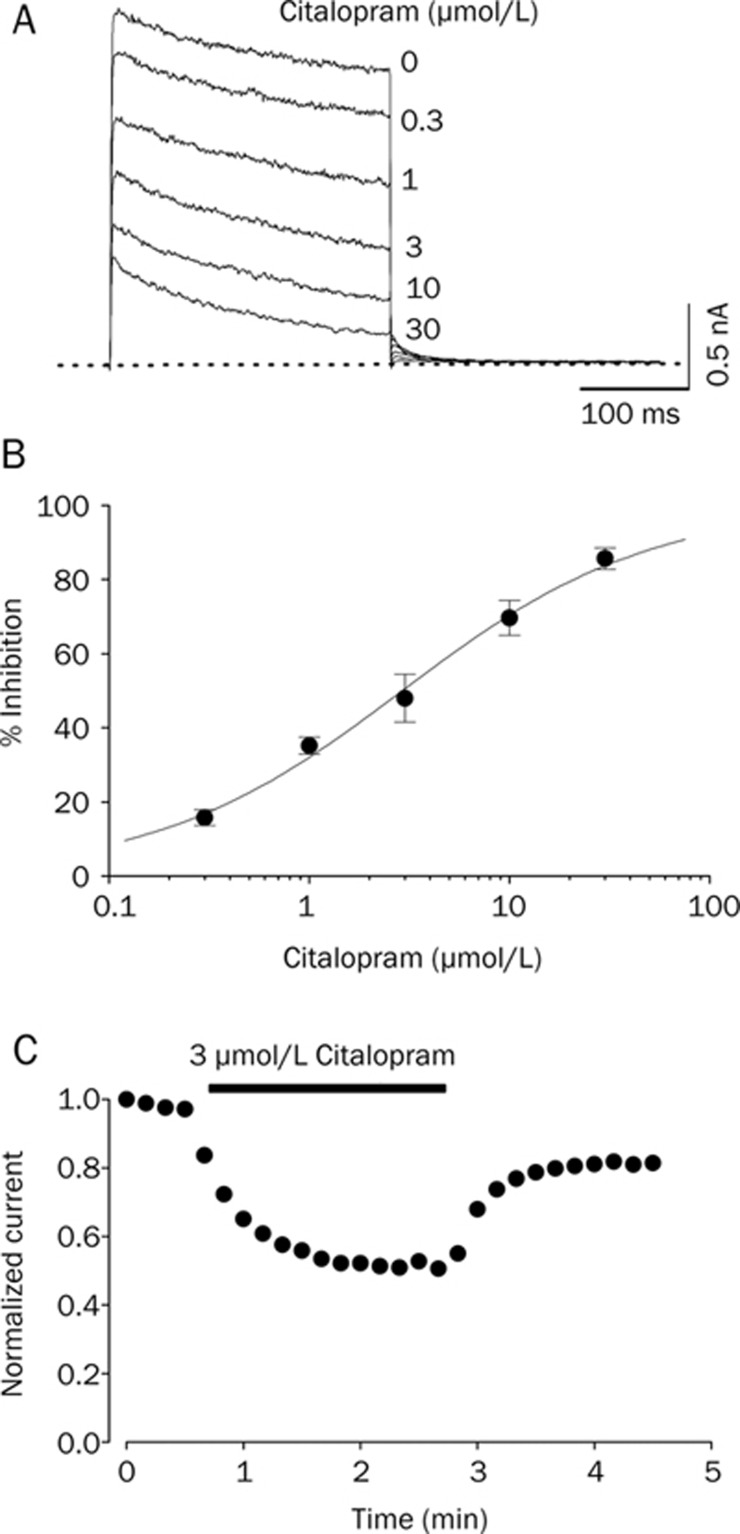
As shown in Figure 1A, Kv1.5 currents were activated under control conditions with a fast rising to a peak and then slowly inactivated, as reported previously21. The dominant time constant of activation under control conditions was 1.51±0.21 ms (n=4) with a 250-ms depolarizing pulse from −80 to +50 mV. In the presence of 3 μM citalopram, the time constant of activation was 1.46±0.18 ms (n=4), which indicates that the activation kinetics were not significantly modified by citalopram. When exposed to different concentrations of citalopram (0.3, 1, 3, 10, 30 μmol/L), the Kv1.5 currents were inhibited in a concentration-dependent manner. Figure 1B shows the concentration dependence of Kv1.5 current inhibition by citalopram. Inhibition of the current was measured at the end of a 250-ms pulse of +50 mV. A nonlinear least-squares fit of the Hill equation to the individual data points yielded an IC50 value and a Hill coefficient of 2.8±1.1 μmol/L and 0.8±0.3 (n=6), respectively. As shown in Figure 1C, when switched to solutions containing citalopram, inhibition of Kv1.5 reached a steady state within 2 min, resulting in 52.1%±6.5% control value measured at the end of a depolarizing pulse of +50 mV (n=4). The washout by perfusion of drug-free solution was also reached within 2 min, and currents recovered to 81.4%±7.5% of control value (n=4), indicating that effects of citalopram were largely reversible upon washout.
Figure 1.
Concentration dependence of citalopram-induced inhibition of Kv1.5 currents. (A) Superimposed current traces were produced by applying 250-ms depolarizing pulses from a holding potential of −80 to +50 mV followed by a 250-ms repolarizing pulse to −40 mV every 10 s in the absence and presence of 0.3, 1, 3, 10, and 30 μmol/L citalopram, as indicated. The dotted line represents zero current. (B) Concentration-dependent curve of inhibition by citalopram. Current amplitudes of Kv1.5 measured at the end of the depolarizing pulses were used as an index of steady-state inhibition, and percentage inhibition was plotted against various concentrations of citalopram. The solid line is fitted to the data points by the Hill equation. (C) Time course for inhibition in the presence of 3 μmol/L citalopram. The current amplitudes were measured at the end of a 250-ms depolarizing pulses from a holding potential of −80 to +40 mV every 10 s in the presence of 3 μmol/L citalopram and normalized to the first current amplitude and the normalized data were plotted as a function of time. Data are expressed as mean±SEM.
Voltage-dependent inhibition of Kv1.5 by citalopram
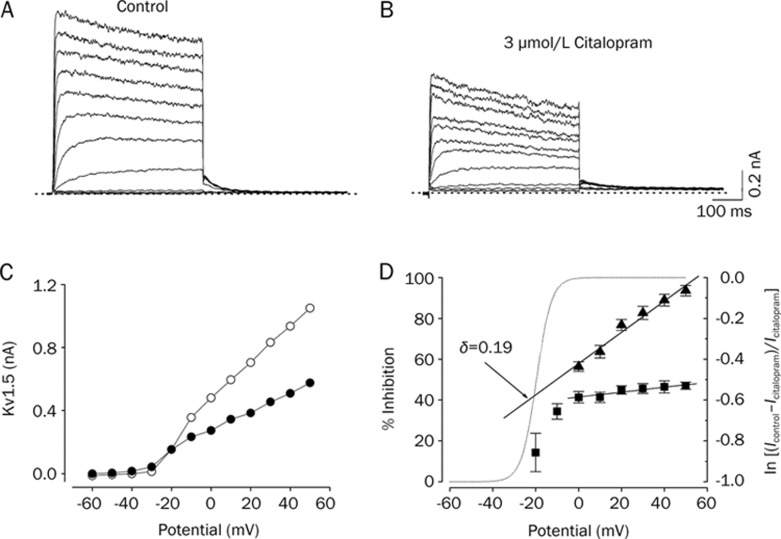
Figure 2 shows the effect of 3 μmol/L citalopram on current-voltage (I–V) relations. Under control conditions, the Kv1.5 current activated at pulses greater than −30 mV, and the steady-state I–V relationship showed a sigmoidal shape at potentials between −30 and 0 mV (Figure 2A and 2C). The inhibition of Kv1.5 currents by 3 μmol/L citalopram was observed in the whole voltage range over which Kv1.5 was activated (Figure 2B and 2C). By plotting percent inhibition (see Materials and methods) versus potential, a high degree of inhibition with a strong voltage dependence was observed between −30 and 0 mV, which involved the voltage range of channel opening (Figure 2D). Between 0 and +50 mV, despite Kv1.5 being fully activated at this voltage range, inhibition continued to increase with a shallow voltage dependence: 41.3%±2.7% inhibition at 0 mV, and 47.0%±1.8% at +50 mV (n=4, P<0.05). Given the assumption that citalopram interacts intracellularly with Kv1.5, we investigated this effect by a linear curve fitting of the data at potentials positive to 0 mV, using a linear transformation of Woodhull equation (see Materials and methods). The solid lines in Figure 2D represents a fitted curve which yielded δ=0.19±0.02 (n=4).
Figure 2.
Voltage dependence of citalopram-induced inhibition of Kv1.5 currents. The Kv1.5 currents were produced by applying 250-ms pulses between −60 and +50 mV in 10-mV increments followed by a 250-ms repolarizing pulse to −40 mV every 10 s, from a holding potential of −80 mV under control conditions (A), and after the addition of 3 μmol/L citalopram (B). The dotted lines in (A) and (B) represent zero current. (C) Resultant I-V relationships taken at the end of the test pulses in the absence (open circle) and presence (closed circle) of 3 μmol/L citalopram. (D) Percentage current inhibition (closed square) from data in (C) was plotted against the membrane potential. For potentials positive to 0 mV, the data of percentage current inhibition was recalculated by using ln{(Icontrol–Icitalopram)/Icitalopram} (closed triangle) and replotted against membrane potential. The voltage dependence was linear fitted with equation 5 (see Materials and methods), shown by the solid line with the indicated values for the equivalent electrical distance (δ=0.19±0.02, n=4). The dotted line represents the activation curve of Kv1.5 under control conditions, which was obtained from a deactivating tail current amplitude at −40 mV after 250-ms depolarizing pulses to potentials between −60 to +50 mV in steps of 10 mV from a holding potentials of −80 mV and thereafter normalization using equation 2 (see Materials and methods). Data are expressed as mean±SEM.
Time-dependent inhibition of Kv1.5 by citalopram
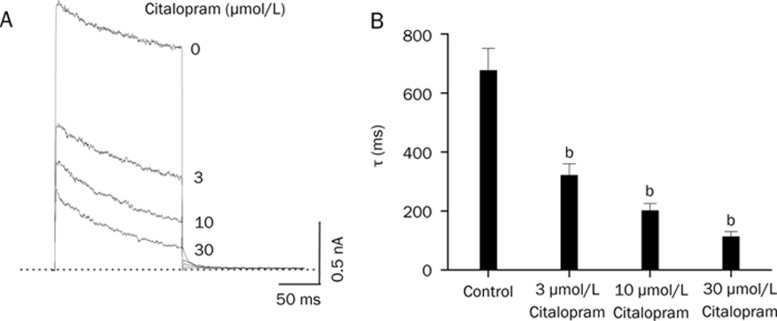
Figure 3A shows the effect of citalopram on the time course of channel current during the 250-ms depolarizing pulse at +50 mV. With a single exponential fitting to the traces of current decay at each concentration of citalopram, time constants were obtained. To avoid contamination by the time constant of the intrinsic slow and partial inactivation current of Kv1.5 under control conditions, a time constant value obtained at low concentrations (0.3 and 1 μmol/L) of citalopram was omitted. Figure 3B shows a summary of the time constants at various concentrations of citalopram. The values of time constant decreased as the concentration of citalopram increased, suggesting that the concentration-dependent citalopram-induced inhibition of Kv1.5 is associated with time-dependent development of block.
Figure 3.
Concentration-dependent kinetics of Kv1.5 inhibition by citalopram. (A) Superimposed Kv1.5 current traces were elicited by applying 250-ms depolarizing pulses from a holding potential of −80 to +50 mV every 10 s in the presence of citalopram (3, 10, and 30 μmol/L). The drug-induced time constants were obtained by a single exponential fitting to the decaying traces of Kv1.5 currents. The dotted line represents zero current. (B) Summary data obtained form (A). The time constants (τ) were plotted versus various concentrations of citalopram (n=4; bP<0.05 vs control data). Data are expressed as mean±SEM.
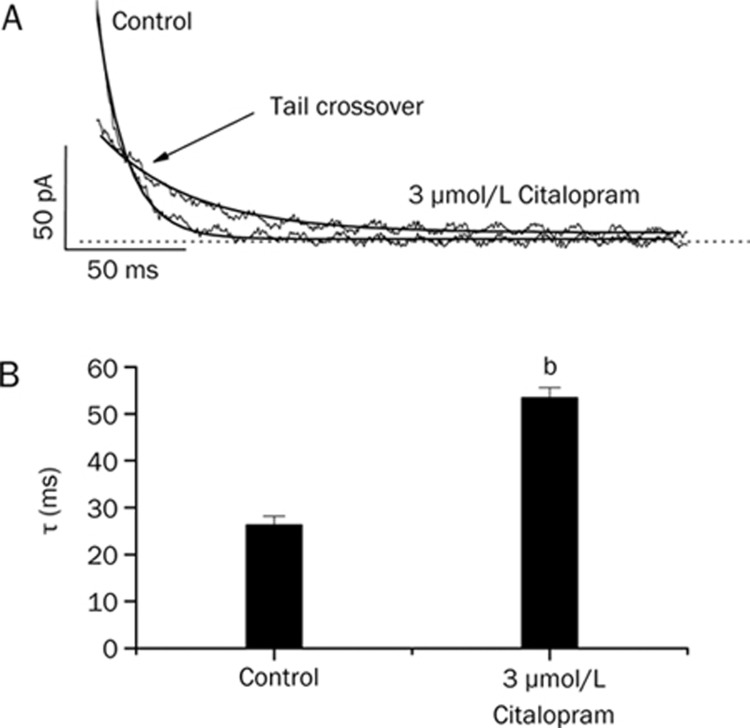
To further investigate the time-dependent inhibition of Kv1.5 by citalopram, the effect of citalopram on the Kv1.5 current deactivation kinetics was studied. Figures 4A shows the representative superimposed tail currents recorded with a 250-ms repolarizing pulse at −40 mV after a 250-ms depolarizing pulse of +50 mV from a holding potential of −80 mV, under control conditions and in the presence of 3 μmol/L citalopram. These currents were well fitted to a single exponential function. Under control conditions, the tail current declined quickly with a time constant of 26.3±1.9 ms (n=4) and was nearly completely deactivated during a 250-ms repolarizing pulse of −40 mV. In the presence of 3 μmol/L citalopram, the initial peak amplitude of the tail current was reduced and the subsequent decline of the current was slower (53.4±2.2 ms, n=4), which resulted in the tail crossover phenomenon. Figure 4B shows a summary of the time constants obtained at repolarizing pulses of −40 mV. Citalopram significantly increased the deactivation time constants of Kv1.5 at repolarizing pulses of −40 mV (n= 4, P<0.05).
Figure 4.
Effects of citalopram on deactivation kinetics of Kv1.5. (A) Tail currents were induced at the repolarizing pulses of −40 mV after a 250-ms depolarizing pulse of +50 mV from a holding potential of −80 mV in the absence and presence of 3 μmol/L citalopram. The dotted lines represent a zero current. Tail crossover phenomenon (indicated by the arrow) observed by superimposing the two tail currents. The solid lines over the current traces represent the single exponential fitting to the data. (B) Deactivation time constants (τ) obtained from (A). n=4, bP<0.05 vs control data. Data are expressed as mean±SEM.
Use-dependent inhibition of Kv1.5 by citalopram
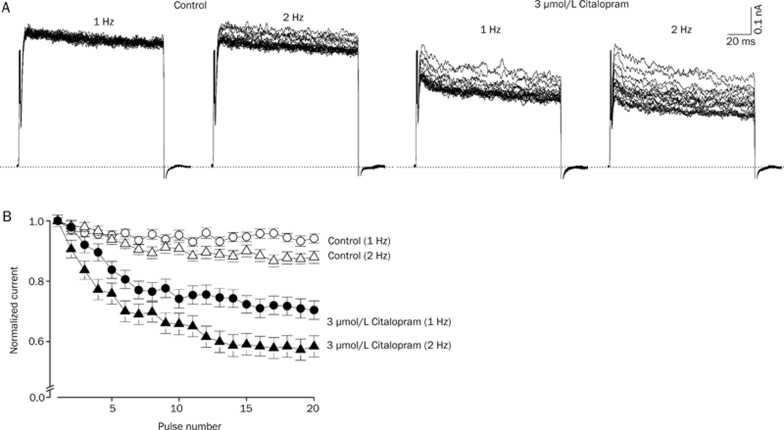
Figure 5A shows the original current traces in the absence and presence of 3 μmol/L citalopram obtained from applying 20 repetitive 125-ms depolarizing pulses of +50 mV from a holding potential of −80 mV at 1 and 2 Hz. After the 2-min exposure to 3 μmol/L citalopram with a holding potential of −80 mV, this repetitive pulse protocol was applied. Figure 5B shows the normalized current amplitudes at two different frequencies, 1 and 2 Hz in the absence and presence of 3 μmol/L citalopram during application of the pulse trains. Under control conditions, the peak amplitude of the Kv1.5 current slightly decreased by 6.8%±1.5% (n=4) at 1 Hz and by 12.1±2.1% (n=4) at 2 Hz in a weak frequency-dependent manner. In the presence of 3 μmol/L citalopram, the peak amplitude of Kv1.5 progressively decreased by 30.6%±3.1% (n=4) and 42.6%±3.5% (n=4) at 1 Hz and 2 Hz, respectively in a frequency- and use-dependent manner. These results suggest that citalopram-induced inhibition of Kv1.5 current is use-dependent.
Figure 5.
Effects of repetitive depolarization on citalopram-induced inhibition of Kv1.5 currents. (A) Original current traces under control conditions and in the presence of 3 μmol/L citalopram obtained from applying 20 repetitive 125-ms depolarizing pulses of +50 mV from a holding potential of −80 mV at 1 or 2 Hz. The dotted lines represent a zero current. (B) Plot of normalized current at two different frequencies, 1 and 2 Hz under control conditions (open circle and open triangle, n=4) and in the presence of 3 μmol/L citalopram (closed circle and closed triangle, n=4) as a function of the number of pulses. The peak amplitudes of the current at every pulse were normalized to the peak amplitudes of current obtained at the first pulse. Data are expressed as mean±SEM.
Discussion
The present study shows the effects of citalopram on the Kv1.5 expressed in CHO cells, using the patch-clamp technique.
The characteristics of the citalopram-induced inhibition of Kv1.5 were as follows. 1) The inhibition of Kv1.5 by citalopram is associated with time-dependent development of block with no effect on the activation kinetics of Kv1.5, suggesting that citalopram does not bind to the closed or resting state of Kv1.5 and the drug preferentially interacts with the open state of Kv1.5. 2) The inhibition of Kv1.5 induced by citalopram was highly voltage-dependent and increased steeply in the voltage range of channel activation. The voltage-dependent inhibition of Kv1.5 by citalopram implies that the inhibition of Kv1.5 by citalopram occurs preferentially after the channels are open21, 25, 26. 3) Citalopram slowed the deactivation time course, resulting in a tail crossover phenomenon. This can be explained if the dissociation rate of citalopram is lower than the transition rate between the open and the closed (or resting) state under control conditions. This tail crossover phenomenon suggests an interaction between citalopram and the open state of Kv1.5 as previously reported21, 26, 27. 4) The actions of citalopram in inhibiting Kv1.5 were use-dependent, with effects enhanced at higher rates of channel activation. One of the features of open channel blockers is a use-dependent inhibition because the blockers would have a higher chance to bind to channel pores as the channels open more frequently21, 25, 26, 28. This is consistent with the actions of citalopram on the open state of Kv1.5. The above summarized results suggest that citalopram acts on the Kv1.5 as an open channel blocker.
An additional shallow degree of inhibition was detected in the voltage range positive to 0 mV despite Kv1.5 being fully activated at this voltage range. This weak but significant voltage-dependent inhibition was considered the consequence of the effects of the transmembrane electrical field on the interaction between citalopram and Kv1.5. If a positively charged drug moves into the transmembrane electric field from the inside, then inhibition should increase upon depolarization due to electrostatic repulsion between a positively charged citalopram and membrane depolarizing potential. This will occur in the voltage range where channels are in the opening state and should also occur over the voltage range where channels are fully activated. At an intracellular pH of 7.3, citalopram is mainly positively charged because the drug is a weak base with a pKa=9.5929. Given the assumption that citalopram interacts intracellularly with Kv1.5, the positively charged drug appears to move into the transmembrane electric field from the inside. Inhibition should then increase at more depolarizing potentials because of electrostatic repulsion between the charge and the potentials, over the voltage range where the channels are fully activated. The δ value of 0.19 for the shallow voltage dependence indicates that the positively charged citalopram senses 19% of the applied transmembrane electrical field as referenced from the intracellular side. This value is similar to the δ values of 0.16 − 0.19 obtained in previous experiments with open channel blockers of Kv1.530, 31, 32.
Although citalopram is thought to be safer than TCA in the risk of cardiovascular causes, accumulating evidence indicates that citalopram may lead to cardiovascular risk within the clinical range9, 10, 33. On the basis of pharmacokinetics of citalopram, the therapeutic plasma concentrations of citalopram is reported to be less than 1 μmol/L in human depressed patients34, 35, 36, 37. In the present study, the IC50 value (2.9 μmol/L) of citalopram for blocking Kv1.5 is higher than therapeutic plasma concentrations. However, significant inhibitions of Kv1.5 currents at concentrations of 0.3 or 1 μmol/L were detected. Furthermore, the effects of citalopram on Kv1.5 were examined in a CHO cell line in the present study. The phospholipid composition of the cell may be different from human native cardiac myocytes and the differences of membrane composition may affect citalopram-induced Kv1.5 blockade. And also, drug concentrations in tissues may be higher than in plasma due to its high lipophilicity and affinity for adipose tissues. Therefore, it is possible that in the present study, the extent of the blocking effects of citalopram on Kv1.5 under physiological conditions may be underestimated. That is, the citalopram-induced block of Kv1.5 channel could be clinically relevant in the upper range of therapeutic plasma concentrations that are observed in treatment with citalopram for depressed patients. Finally, in cardiomyocytes, it is most likely that citalopram may lead to cardiac disorder through block of Kv1.5 channel resulting in a significant prolongation of the action potential or affecting cardiac excitability. However, we cannot completely rule out the possibility that the effect of citalopram on Kv1.5 expressed in cell line may be different from human native cardiac myocytes. Thus, in the further study, it is highly required whether citalopram can induce the prolongation of the action potential or arrhythmia phenomenon in the native cardiac myocytes or animal heart.
In conclusion, the present study has described the effects of citalopram on the Kv1.5 channel expressed in Chinese hamster ovary cells. Detailed study of the interaction kinetics between citalopram and Kv1.5 suggests that citalopram is an open-channel blocker for Kv1.5 in a concentration-, voltage-, time-, and use-dependent manner. Thus, much caution about arrhythmogenic risk is required when using citalopram in the treatment with depressed patients.
Author contribution
Hyang Mi LEE designed research, performed research, analyzed data and wrote the paper. Sang June HAHN analyzed data and wrote the paper. Bok Hee CHOI designed research, performed research, analyzed data and wrote the paper.
Acknowledgments
We thank Dr Leonard KACZMAREK (Yale University School of Medicine, USA) for the Kv1.5 cDNA.
References
- Henry JA, Alexander CA, Sener EK. Relative mortality from overdose of antidepressants. BMJ. 1995;310:221–4. doi: 10.1136/bmj.310.6974.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JA. Epidemiology and relative toxicity of antidepressant drugs in overdose. Drug Saf. 1997;16:374–90. doi: 10.2165/00002018-199716060-00004. [DOI] [PubMed] [Google Scholar]
- Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048–52. doi: 10.1016/s0140-6736(00)02035-3. [DOI] [PubMed] [Google Scholar]
- Scherer D, von Lowenstern K, Zitron E, Scholz EP, Bloehs R, Kathofer S, et al. Inhibition of cardiac hERG potassium channels by tetracyclic antidepressant mianserin. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:73–83. doi: 10.1007/s00210-008-0289-4. [DOI] [PubMed] [Google Scholar]
- Roose SP, Spatz E. Treatment of depression in patients with heart disease. J Clin Psychiatry. 1999;60:34–7. [PubMed] [Google Scholar]
- Keller MB. Citalopram therapy for depression: a review of 10 years of European experience and data from US clinical trials. J Clin Psychiatry. 2000;61:896–908. [PubMed] [Google Scholar]
- Parker NG, Brown CS. Citalopram in the treatment of depression. Ann Pharmacother. 2000;34:761–71. doi: 10.1345/aph.19137. [DOI] [PubMed] [Google Scholar]
- Spadone C. Efficacy of escitalopram and severity of depression: New data. Encephale. 2009;35:577–85. doi: 10.1016/j.encep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Grundemar L, Wohlfart B, Lagerstedt C, Bengtsson F, Eklundh G. Symptoms and signs of severe citalopram overdose. Lancet. 1997;349:1602. doi: 10.1016/S0140-6736(05)61630-3. [DOI] [PubMed] [Google Scholar]
- Personne M, Persson H, Shoberg E. Citalopram toxicity. Lancet. 1997;350:518–9. doi: 10.1016/s0140-6736(05)63109-1. [DOI] [PubMed] [Google Scholar]
- Cascade E, Kalali AH, Kennedy SH. Real-World data on SSRI antidepressant side effects. Psychiatry (Edgmont) 2009;6:16–8. [PMC free article] [PubMed] [Google Scholar]
- Khawaja IS, Feinstein RE. Cardiovascular effects of selective serotonin reuptake inhibitors and other novel antidepressants. Heart Dis. 2003;5:153–60. doi: 10.1097/01.hdx.0000061695.97215.64. [DOI] [PubMed] [Google Scholar]
- Pacher P, Kecskemeti V. Cardiovascular effects of selective serotonin reuptake inhibitor antidepressants. Orv Hetil. 2004;145:425–31. [PubMed] [Google Scholar]
- Rodriguez de la Torre B, Dreher J, Malevany I, Bagli M, Kolbinger M, Omran H, et al. Serum levels and cardiovascular effects of tricyclic antidepressants and selective serotonin reuptake inhibitors in depressed patients. Ther Drug Monit. 2001;23:435–40. doi: 10.1097/00007691-200108000-00019. [DOI] [PubMed] [Google Scholar]
- Witchel HJ, Pabbathi VK, Hofmann G, Paul AA, Hancox JC. Inhibitory actions of the selective serotonin re-uptake inhibitor citalopram on HERG and ventricular L-type calcium currents. FEBS Lett. 2002;512:59–66. doi: 10.1016/s0014-5793(01)03320-8. [DOI] [PubMed] [Google Scholar]
- Zahradnik I, Minarovic I, Zahradnikova A. Inhibition of the cardiac L-type calcium channel current by antidepressant drugs. J Pharmacol Exp Ther. 2008;324:977–84. doi: 10.1124/jpet.107.132456. [DOI] [PubMed] [Google Scholar]
- Colatsky TJ, Follmer CH, Starmer CF. Channel specificity in antiarrhythmic drug action. Mechanism of potassium channel block and its role in suppressing and aggravating cardiac arrhythmias. Circulation. 1990;82:2235–42. doi: 10.1161/01.cir.82.6.2235. [DOI] [PubMed] [Google Scholar]
- Schumacher SM, McEwen DP, Zhang L, Arendt KL, Van Genderen KM, Martens JR. Antiarrhythmic drug-induced internalization of the atrial-specific K+ channel Kv1.5. Circ Res. 2009;104:1390–8. doi: 10.1161/CIRCRESAHA.108.192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–76. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res. 1996;78:689–96. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- Choi BH, Choi JS, Jeong SW, Hahn SJ, Yoon SH, Jo YH, et al. Direct block by bisindolylmaleimide of rat Kv1.5 expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 2000;293:634–40. [PubMed] [Google Scholar]
- Swanson R, Marshall J, Smith JS, Williams JB, Boyle MB, Folander K, et al. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990;4:929–39. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpon E, Valenzuela C, Gay P, Franqueza L, Snyders DJ, Tamargo J. Block of human cardiac Kv1.5 channels by loratadine: voltage-, time- and use-dependent block at concentrations above therapeutic levels. Cardiovasc Res. 1997;35:341–50. doi: 10.1016/s0008-6363(97)00121-1. [DOI] [PubMed] [Google Scholar]
- Sung MJ, Hahn SJ, Choi BH. Effect of psoralen on the cloned Kv3.1 currents. Arch Pharm Res. 2009;32:407–12. doi: 10.1007/s12272-009-1314-y. [DOI] [PubMed] [Google Scholar]
- Ahn HS, Kim SE, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, et al. Open channel block of Kv1.3 by rosiglitazone and troglitazone: Kv1.3 as the pharmacological target for rosiglitazone. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:305–9. doi: 10.1007/s00210-006-0118-6. [DOI] [PubMed] [Google Scholar]
- Wu J, Ding WG, Matsuura H, Tsuji K, Zang WJ, Horie M. Inhibitory actions of the phosphatidylinositol 3-kinase inhibitor LY294002 on the human Kv1.5 channel. Br J Pharmacol. 2009;156:377–87. doi: 10.1111/j.1476-5381.2008.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasskog T, Berger U, Samuelsen PJ, Kallenborn R, Jensen E. Selective serotonin reuptake inhibitors in sewage influents and effluents from Tromsø, Norway. J Chromatogr A. 2006;1115:187–95. doi: 10.1016/j.chroma.2006.02.091. [DOI] [PubMed] [Google Scholar]
- Snyders J, Knoth KM, Roberds SL, Tamkun MM. Time-, voltage-, and state-dependent block by quinidine of a cloned human cardiac potassium channel. Mol Pharmacol. 1992;41:322–30. [PubMed] [Google Scholar]
- Franqueza L, Valenzuela C, Delpon E, Longobardo M, Caballero R, Tamargo J. Effects of propafenone and 5-hydroxy-propafenone on hKv1.5 channels. Br J Pharmacol. 1998;125:969–78. doi: 10.1038/sj.bjp.0702129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela C, Delpon E, Franqueza L, Gay P, Perez O, Tamargo J, et al. Class III antiarrhythmic effects of zatebradine: time-, state-, use-, and voltage-dependent block of hKv1.5 Channels. Circulation. 1996;94:562–70. doi: 10.1161/01.cir.94.3.562. [DOI] [PubMed] [Google Scholar]
- Ostrom M, Eriksson A, Thorson J, Spigset O. Fatal overdose with citalopram. Lancet. 1996;348:339–40. doi: 10.1016/s0140-6736(05)64513-8. [DOI] [PubMed] [Google Scholar]
- Fredricson Overo K. Kinetics of citalopram in man; plasma levels in patients. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:311–8. doi: 10.1016/s0278-5846(82)80181-4. [DOI] [PubMed] [Google Scholar]
- Macek J, Ptácek P, Klíma J. Rapid determination of citalopram in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2001;755:279–85. doi: 10.1016/s0378-4347(01)00121-9. [DOI] [PubMed] [Google Scholar]
- Bjerkenstedt L, Flyckt L, Fredricson Overo K, Lingjarde O. Relationship between clinical effects, serum drug concentration and serotonin uptake inhibition in depressed patients treated with citalopram. Eur J Clin Pharmacol. 1985;28:553–57. doi: 10.1007/BF00544066. [DOI] [PubMed] [Google Scholar]
- Sindrup SH, Bjerre U, Dejgaard A, Brosen K, Aaes-Jorgensen T, Gram LF. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther. 1992;52:547–52. doi: 10.1038/clpt.1992.183. [DOI] [PubMed] [Google Scholar]