Abstract
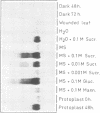
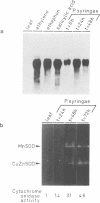
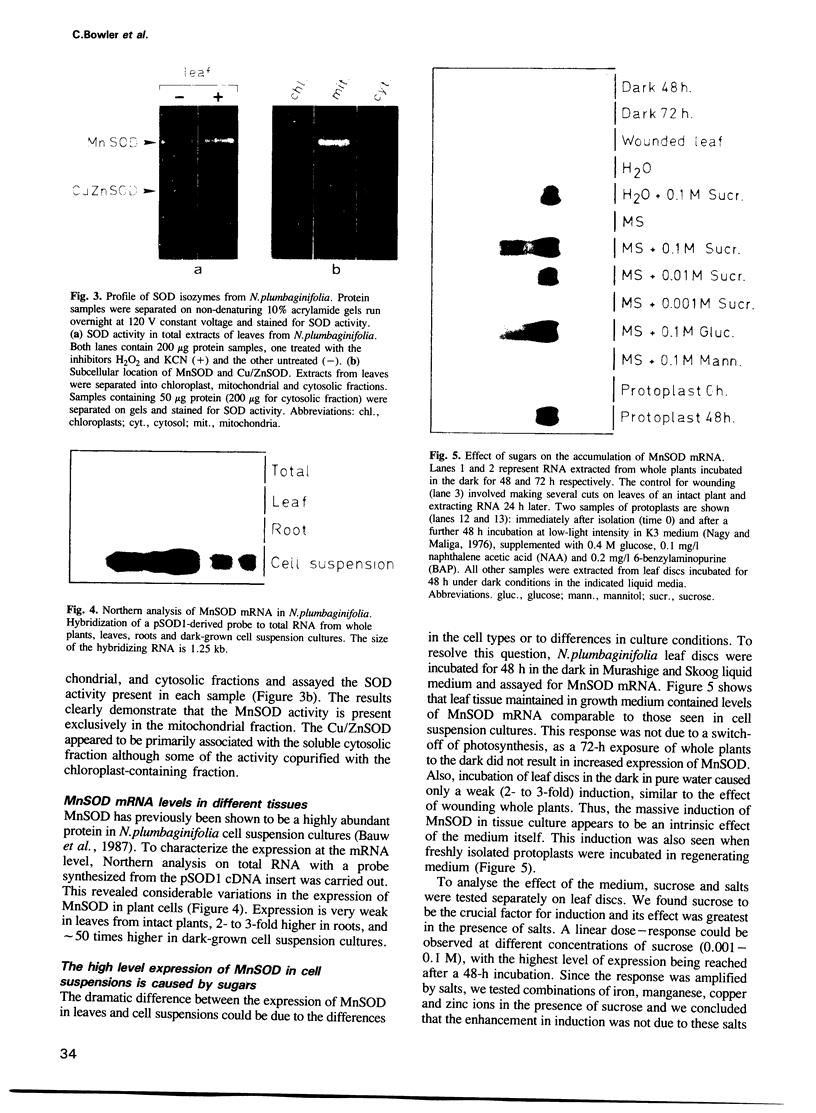
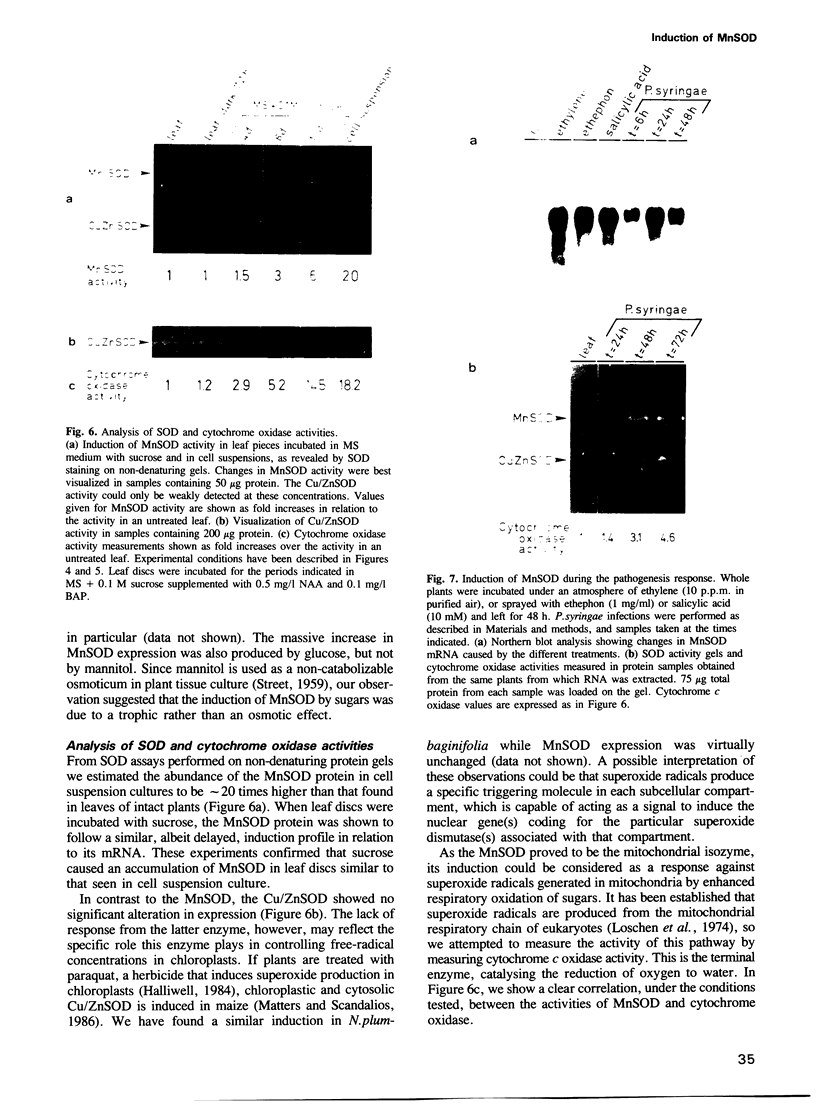
Superoxide dismutases (SODs) are metalloproteins that catalyse the dismutation of superoxide radicals to oxygen and hydrogen peroxide. The enzyme has been found in all aerobic organisms examined, where it plays a major role in the defence against toxic reduced oxygen species which are generated in many biological oxidations. Here we report the complete primary structure of a plant manganese superoxide dismutase (MnSOD), deduced from a cDNA clone of Nicotiana plumbaginifolia. The plant protein is highly homologous to MnSODs from other organisms and also contains an N-terminal leader sequence resembling a transit peptide for mitochondrial targeting. The location of the mature protein within the mitochondria has been demonstrated by subcellular fractionation experiments. We have analysed the expression profile of this MnSOD and found that it is dramatically induced during stress conditions, most notably in tissue culture as a result of sugar metabolism and also as part of the pathogenesis response of the plant, being induced by ethylene, salicylic acid, and Pseudomonas syringae infection. This induction is always accompanied by an increase in cytochrome oxidase activity, which suggests a specific protective role for MnSOD during conditions of increased mitochondrial respiration.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Barra D., Schinina M. E., Simmaco M., Bannister J. V., Bannister W. H., Rotilio G., Bossa F. The primary structure of human liver manganese superoxide dismutase. J Biol Chem. 1984 Oct 25;259(20):12595–12601. [PubMed] [Google Scholar]
- Bauw G., De Loose M., Inzé D., Van Montagu M., Vandekerckhove J. Alterations in the phenotype of plant cells studied by NH(2)-terminal amino acid-sequence analysis of proteins electroblotted from two-dimensional gel-separated total extracts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4806–4810. doi: 10.1073/pnas.84.14.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beck Y., Oren R., Amit B., Levanon A., Gorecki M., Hartman J. R. Human Mn superoxide dismutase cDNA sequence. Nucleic Acids Res. 1987 Nov 11;15(21):9076–9076. doi: 10.1093/nar/15.21.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biliński T., Krawiec Z., Liczmański A., Litwińska J. Is hydroxyl radical generated by the Fenton reaction in vivo? Biochem Biophys Res Commun. 1985 Jul 31;130(2):533–539. doi: 10.1016/0006-291x(85)90449-8. [DOI] [PubMed] [Google Scholar]
- Boutry M., Nagy F., Poulsen C., Aoyagi K., Chua N. H. Targeting of bacterial chloramphenicol acetyltransferase to mitochondria in transgenic plants. Nature. 1987 Jul 23;328(6128):340–342. doi: 10.1038/328340a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bridges S. M., Salin M. L. Distribution of iron-containing superoxide dismutase in vascular plants. Plant Physiol. 1981 Aug;68(2):275–278. doi: 10.1104/pp.68.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C. J., Walker J. E. Superoxide dismutase from Bacillus stearothermophilus. Complete amino acid sequence of a manganese enzyme. Biochemistry. 1980 Jun 24;19(13):2873–2882. doi: 10.1021/bi00554a009. [DOI] [PubMed] [Google Scholar]
- Cannon R. E., White J. A., Scandalios J. G. Cloning of cDNA for maize superoxide dismutase 2 (SOD2). Proc Natl Acad Sci U S A. 1987 Jan;84(1):179–183. doi: 10.1073/pnas.84.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Loose M., Alliotte T., Gheysen G., Genetello C., Gielen J., Soetaert P., Van Montagu M., Inzé D. Primary structure of a hormonally regulated beta-glucanase of Nicotiana plumbaginifolia. Gene. 1988 Oct 15;70(1):13–23. doi: 10.1016/0378-1119(88)90100-x. [DOI] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Plant defense genes are regulated by ethylene. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5202–5206. doi: 10.1073/pnas.84.15.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Geller B. L., Winge D. R. Subcellular distribution of superoxide dismutases in rat liver. Methods Enzymol. 1984;105:105–114. doi: 10.1016/s0076-6879(84)05014-x. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksenzenko M., Konstantinov A. A., Khomutov G. B., Tikhonov A. N., Ruuge E. K. Effect of electron transfer inhibitors on superoxide generation in the cytochrome bc1 site of the mitochondrial respiratory chain. FEBS Lett. 1983 May 2;155(1):19–24. doi: 10.1016/0014-5793(83)80200-2. [DOI] [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Lowry C. V., Zitomer R. S. Oxygen regulation of anaerobic and aerobic genes mediated by a common factor in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6129–6133. doi: 10.1073/pnas.81.19.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marres C. A., Van Loon A. P., Oudshoorn P., Van Steeg H., Grivell L. A., Slater E. C. Nucleotide sequence analysis of the nuclear gene coding for manganese superoxide dismutase of yeast mitochondria, a gene previously assumed to code for the Rieske iron-sulphur protein. Eur J Biochem. 1985 Feb 15;147(1):153–161. doi: 10.1111/j.1432-1033.1985.tb08731.x. [DOI] [PubMed] [Google Scholar]
- Matters G. L., Scandalios J. G. Effect of the free radical-generating herbicide paraquat on the expression of the superoxide dismutase (Sod) genes in maize. Biochim Biophys Acta. 1986 Jun 3;882(1):29–38. doi: 10.1016/0304-4165(86)90051-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Parker M. W., Blake C. C. Iron- and manganese-containing superoxide dismutases can be distinguished by analysis of their primary structures. FEBS Lett. 1988 Mar 14;229(2):377–382. doi: 10.1016/0014-5793(88)81160-8. [DOI] [PubMed] [Google Scholar]
- Raskin I., Ehmann A., Melander W. R., Meeuse B. J. Salicylic Acid: a natural inducer of heat production in arum lilies. Science. 1987 Sep 25;237(4822):1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- Schatz G. 17th Sir Hans Krebs lecture. Signals guiding proteins to their correct locations in mitochondria. Eur J Biochem. 1987 May 15;165(1):1–6. doi: 10.1111/j.1432-1033.1987.tb11186.x. [DOI] [PubMed] [Google Scholar]
- Sobel E., Martinez H. M. A multiple sequence alignment program. Nucleic Acids Res. 1986 Jan 10;14(1):363–374. doi: 10.1093/nar/14.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings W. C., Pattridge K. A., Strong R. K., Ludwig M. L. The structure of manganese superoxide dismutase from Thermus thermophilus HB8 at 2.4-A resolution. J Biol Chem. 1985 Dec 25;260(30):16424–16432. [PubMed] [Google Scholar]
- Steinman H. M. The amino acid sequence of mangano superoxide dismutase from Escherichia coli B. J Biol Chem. 1978 Dec 25;253(24):8708–8720. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Loon A. P., Pesold-Hurt B., Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]