Abstract
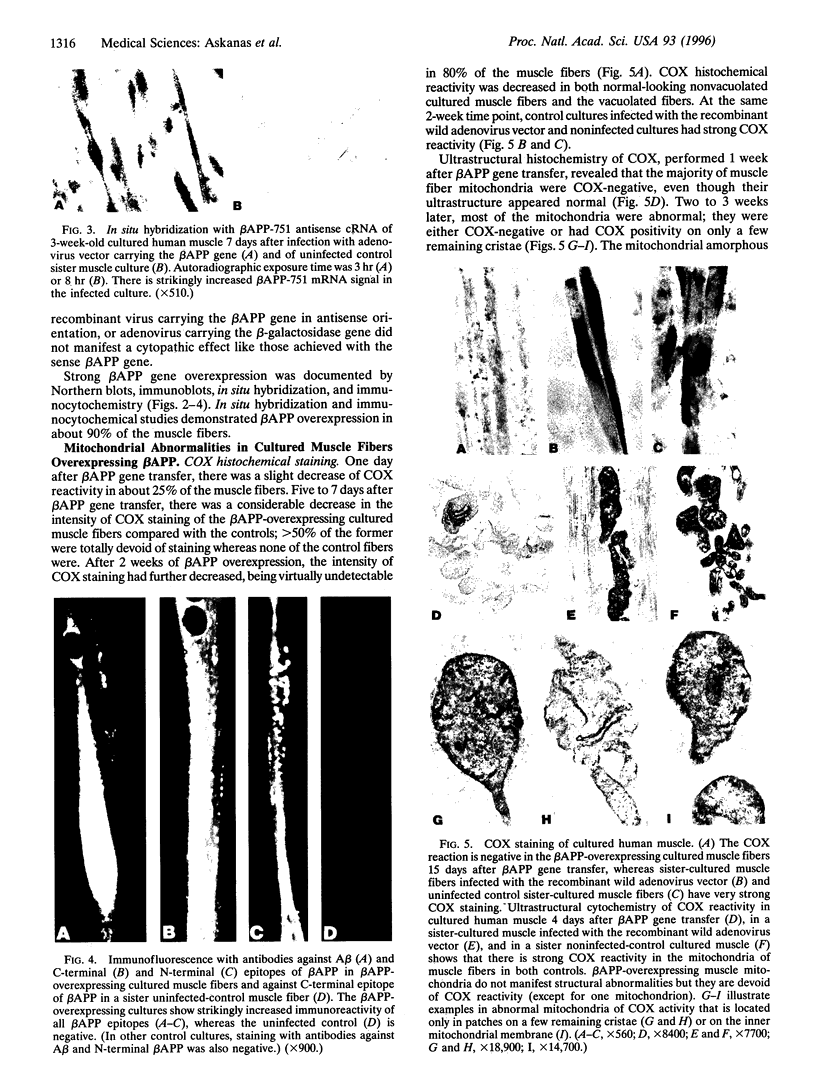
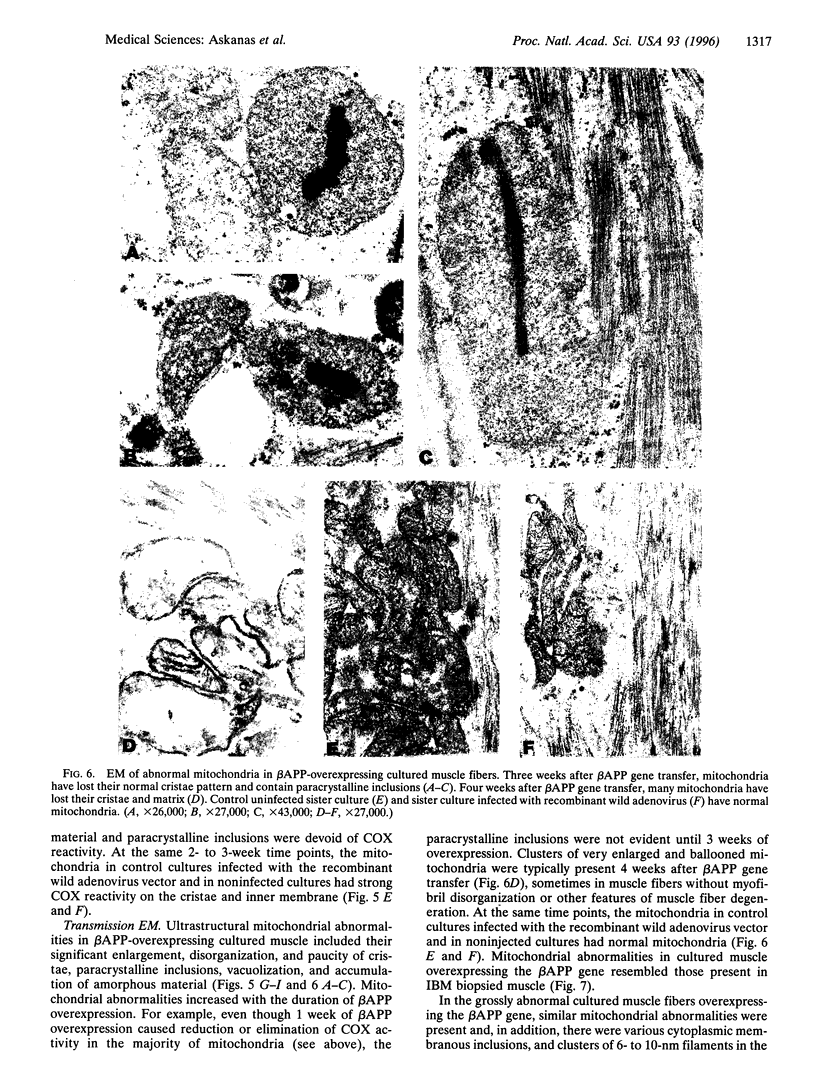
As in Alzheimer-disease (AD) brain, vacuolated muscle fibers of inclusion-body myositis (IBM) contain abnormally accumulated beta-amyloid precursor protein (beta APP), including its beta-amyloid protein epitope, and increased beta APP-751 mRNA. Other similarities between IBM muscle and AD brain phenotypes include paired helical filaments, hyperphosphorylated tau protein, apolipoprotein E, and mitochondrial abnormalities, including decreased cytochrome-c oxidase (COX) activity. The pathogenesis of these abnormalities in IBM muscle and AD brain is not known. We now report that direct transfer of the beta APP gene, using adenovirus vector, into cultured normal human muscle fibers causes structural abnormalities of mitochondria and decreased COX activity. In this adenovirus-mediated beta APP gene transfer, we demonstrated that beta APP overproduction can induce mitochondrial abnormalities. The data suggest that excessive beta APP may be responsible for mitochondrial and COX abnormalities in IBM muscle and perhaps AD brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askanas V., Alvarez R. B., Engel W. K. beta-Amyloid precursor epitopes in muscle fibers of inclusion body myositis. Ann Neurol. 1993 Oct;34(4):551–560. doi: 10.1002/ana.410340408. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K. A new program for investigating adult human skeletal muscle grown aneurally in tissue culture. Neurology. 1975 Jan;25(1):58–67. doi: 10.1212/wnl.25.1.58. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Alvarez R. B. Light and electron microscopic localization of beta-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol. 1992 Jul;141(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Bilak M., Alvarez R. B., Selkoe D. J. Twisted tubulofilaments of inclusion body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am J Pathol. 1994 Jan;144(1):177–187. [PMC free article] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Kwan H. H., Lawrence J. V. Abnormality of cultured muscle and Schwann's cells in familial lipid neuromyopathy. Muscle corrected by neural influence. Arch Neurol. 1984 Sep;41(9):932–934. doi: 10.1001/archneur.1984.04050200038015. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K. New advances in the understanding of sporadic inclusion-body myositis and hereditary inclusion-body myopathies. Curr Opin Rheumatol. 1995 Nov;7(6):486–496. doi: 10.1097/00002281-199511000-00005. [DOI] [PubMed] [Google Scholar]
- Askanas V., Gallez-Hawkins G. Synergistic influence of polypeptide growth factors on cultured human muscle. Arch Neurol. 1985 Aug;42(8):749–752. doi: 10.1001/archneur.1985.04210090013004. [DOI] [PubMed] [Google Scholar]
- Askanas V., Mirabella M., Engel W. K., Alvarez R. B., Weisgraber K. H. Apolipoprotein E immunoreactive deposits in inclusion-body muscle diseases. Lancet. 1994 Feb 5;343(8893):364–365. doi: 10.1016/s0140-6736(94)91208-4. [DOI] [PubMed] [Google Scholar]
- Askanas V., Serdaroglu P., Engel W. K., Alvarez R. B. Immunolocalization of ubiquitin in muscle biopsies of patients with inclusion body myositis and oculopharyngeal muscular dystrophy. Neurosci Lett. 1991 Sep 2;130(1):73–76. doi: 10.1016/0304-3940(91)90230-q. [DOI] [PubMed] [Google Scholar]
- Becker T. C., Noel R. J., Coats W. S., Gómez-Foix A. M., Alam T., Gerard R. D., Newgard C. B. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43(Pt A):161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- Bilak M., Askanas V., Engel W. K. Strong immunoreactivity of alpha 1-antichymotrypsin co-localizes with beta-amyloid protein and ubiquitin in vacuolated muscle fibers of inclusion-body myositis. Acta Neuropathol. 1993;85(4):378–382. doi: 10.1007/BF00334447. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A. Structure and assembly of cytochrome c oxidase. Arch Biochem Biophys. 1990 Aug 1;280(2):252–262. doi: 10.1016/0003-9861(90)90327-u. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K., Giordano T., Brady D. R., Stoll J., Martin L. J., Rapoport S. I. Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Brain Res Mol Brain Res. 1994 Jul;24(1-4):336–340. doi: 10.1016/0169-328x(94)90147-3. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Moraes C. T. Mitochondrial encephalomyopathies. Arch Neurol. 1993 Nov;50(11):1197–1208. doi: 10.1001/archneur.1993.00540110075008. [DOI] [PubMed] [Google Scholar]
- ENGEL W. K., CUNNINGHAM G. G. RAPID EXAMINATION OF MUSCLE TISSUE. AN IMPROVED TRICHROME METHOD FOR FRESH-FROZEN BIOPSY SECTIONS. Neurology. 1963 Nov;13:919–923. doi: 10.1212/wnl.13.11.919. [DOI] [PubMed] [Google Scholar]
- Gorycki M. A., Askanas V. Improved technique for electron microscopy of cultured cells. Stain Technol. 1977 Sep;52(5):249–254. doi: 10.3109/10520297709116788. [DOI] [PubMed] [Google Scholar]
- Ishii T., Kametani F., Haga S., Sato M. The immunohistochemical demonstration of subsequences of the precursor of the amyloid A4 protein in senile plaques in Alzheimer's disease. Neuropathol Appl Neurobiol. 1989 Mar-Apr;15(2):135–147. doi: 10.1111/j.1365-2990.1989.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Mann D. M., Odaka A., Suzuki N., Ihara Y. Amyloid beta protein (A beta) deposition: A beta 42(43) precedes A beta 40 in Down syndrome. Ann Neurol. 1995 Mar;37(3):294–299. doi: 10.1002/ana.410370305. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Mori H., Selkoe D. J. Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature. 1989 Sep 21;341(6239):226–230. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- Mendell J. R., Sahenk Z., Gales T., Paul L. Amyloid filaments in inclusion body myositis. Novel findings provide insight into nature of filaments. Arch Neurol. 1991 Dec;48(12):1229–1234. doi: 10.1001/archneur.1991.00530240033013. [DOI] [PubMed] [Google Scholar]
- Mutisya E. M., Bowling A. C., Beal M. F. Cortical cytochrome oxidase activity is reduced in Alzheimer's disease. J Neurochem. 1994 Dec;63(6):2179–2184. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- Oldfors A., Moslemi A. R., Fyhr I. M., Holme E., Larsson N. G., Lindberg C. Mitochondrial DNA deletions in muscle fibers in inclusion body myositis. J Neuropathol Exp Neurol. 1995 Jul;54(4):581–587. doi: 10.1097/00005072-199507000-00012. [DOI] [PubMed] [Google Scholar]
- Parker W. D., Jr, Mahr N. J., Filley C. M., Parks J. K., Hughes D., Young D. A., Cullum C. M. Reduced platelet cytochrome c oxidase activity in Alzheimer's disease. Neurology. 1994 Jun;44(6):1086–1090. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- Parker W. D., Jr, Parks J., Filley C. M., Kleinschmidt-DeMasters B. K. Electron transport chain defects in Alzheimer's disease brain. Neurology. 1994 Jun;44(6):1090–1096. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Rifai Z., Welle S., Kamp C., Thornton C. A. Ragged red fibers in normal aging and inflammatory myopathy. Ann Neurol. 1995 Jan;37(1):24–29. doi: 10.1002/ana.410370107. [DOI] [PubMed] [Google Scholar]
- Sarkozi E., Askanas V., Johnson S. A., Engel W. K., Alvarez R. B. beta-Amyloid precursor protein mRNA is increased in inclusion-body myositis muscle. Neuroreport. 1993 Jun;4(6):815–818. doi: 10.1097/00001756-199306000-00055. [DOI] [PubMed] [Google Scholar]
- Sarkozi E., Askanas V., Johnson S. A., McFerrin J., Engel W. K. Expression of beta-amyloid precursor protein gene is developmentally regulated in human muscle fibers in vivo and in vitro. Exp Neurol. 1994 Jul;128(1):27–33. doi: 10.1006/exnr.1994.1109. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Amyloid beta-protein precursor: new clues to the genesis of Alzheimer's disease. Curr Opin Neurobiol. 1994 Oct;4(5):708–716. doi: 10.1016/0959-4388(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Hagen T. M., Ames B. N. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonian N. A., Hyman B. T. Functional alterations in Alzheimer's disease: selective loss of mitochondrial-encoded cytochrome oxidase mRNA in the hippocampal formation. J Neuropathol Exp Neurol. 1994 Sep;53(5):508–512. doi: 10.1097/00005072-199409000-00010. [DOI] [PubMed] [Google Scholar]