Abstract
Two novel benzofuran derivatives coupled with 99mTc complexes were tested as probes for imaging cerebral β-amyloid plaques using single photon emission tomography. Although both derivatives bound to Aβ(1−42) aggregates, 99mTc-BAT-BF showed higher affinity than 99mTc-MAMA-BF. In sections of brain tissue from an animal model of AD, 99mTc-BAT-BF clearly labeled β-amyloid plaques. In biodistribution experiments using normal mice, 99mTc-BAT-BF displayed high uptake soon after its injection and washed out from the brain rapidly, a highly desirable feature for an imaging agent. 99mTc-BAT-BF may be a potential probe for imaging β-amyloid plaques in Alzheimer's brains.
Keywords: Alzheimer's disease, β-amyloid plaque, Tc-99m, single photon emission computed tomography (SPECT), imaging
Alzheimer's disease (AD) is a neurodegenerative disease of the brain associated with irreversible cognitive decline, memory impairment, and behavioral changes. The presence of β-amyloid (Aβ) aggregates in the brain is generally accepted as a hallmark of AD.1,2 Currently, the only definitive diagnosis of AD is by pathological examination of the postmortem staining of affected brain tissue, and the early appraisal of clinical symptoms for the diagnosis of AD is often difficult and unreliable. Thus, the detection of individual plaques in vivo by single photon emission tomography (SPECT) or positron emission tomography (PET) has been strongly desired to improve diagnosis and also accelerate the discovery of effective therapeutic agents for AD.3−6 Many radiolabeled probes for imaging β-amyloid based on Congo Red, thioflavin T, and DDNP have been reported. Among them, [11C]PIB,7,8 [11C]SB-13,9,10 [18F]BAY94-9172,11,12 [11C]BF-227,13 [18F]FDDNP,14−16 [123I]IMPY,17−19 and [18F]AV-4520,21 have been tested clinically and demonstrated potential utility.
We have recently reported that 125I-, 11C-, and 18F-labeled benzofuran derivatives showed high affinity for Aβ aggregates and good uptake into and rapid clearance from the brain, indicating that benzofuran can function as a promising scaffold for the development of β-amyloid imaging probes.22,23 In this study, we planned the development of novel benzofuran derivatives labeled with technetium-99 m (99mTc). 99mTc (T1/2 = 6.01 h, 141 keV) has become the most commonly used radionuclide in diagnostic nuclear medicine, because it is readily produced by an 99Mo/99mTc generator; the medium γ-ray energy that it emits is suitable for detection, and its physical half-life is compatible with the biological localization and residence time required for imaging. Its ready availability, essentially 24 h a day, and easiness of use make it the radionuclide of choice. Several 99mTc-labeled imaging probes have been developed (Figure 1),24−28 but no clinical study of them has been reported. New 99mTc-labeled imaging agents will provide simple, convenient, and widespread SPECT-based imaging methods for detecting and eventually quantifying β-amyloid plaques in living brain tissue.
Figure 1.
Chemical structure of 99mTc-labeled Aβ imaging probes reported previously.
In the present study, we synthesized two benzofuran derivatives with monoamine-monoamide dithiol (MAMA) and bis-amino-bis-thiol (BAT). MAMA and BAT were selected as a chelation ligand taking into consideration the permeability of the blood−brain barrier, because they form an electrically neutral complex with 99mTc.29 We then evaluated their biological potential as probes by testing their affinity for Aβ aggregates and β-amyloid plaques in sections of brain tissue from Tg2576 mice and their uptake by and clearance from the brain in biodistribution experiments using normal mice. To our knowledge, this is the first time that benzofurans coupled with 99mTc complexes have been proposed as probes for the detection of β-amyloid plaques in the brain.
The synthesis of the 99mTc/Re benzofuran derivatives is outlined in Scheme 1. The key step in the formation of the benzofuran backbone was readily achieved by reacting 2-hydroxy-5-methoxybenzaldehyde with 4-nitrobenzylbromide to produce compound 1 in a yield of 75%. The amino derivative 2 was prepared from 1 by reduction with SnCl2 in a yield of 95%. Conversion of 2 to the dimethylamino derivative 3 was achieved by an efficient method with paraformaldehyde, sodium cyanoborohydride, and acetic acid (78% yield). The O-methyl group of 3 was removed by reacting with BBr3 to give 4 in a yield of 63%. After a trimethylene group was introduced into 4 as a linker by reacting with 1,3-dibromopropane, the chelation ligands were conjugated with 5. The thiol-protected chelation ligands (PMB-BAT and TRT-MAMA) were synthesized according to methods reported previously with some slight modifications. Then, 5 was joined to PMB-BAT or TRT-MAMA to generate the compounds 6 (PMB-BAT-BF) and 9 (TRT-MAMA-BF), respectively. After deprotection of the thiol group in 6 and 9, the Re complexes (7 and 10) were directly prepared by a reaction with (PPh3)2ReOCl3. The corresponding 99mTc complex, 8 (99mTc-BAT-BF) or 11 (99mTc-MAMA-BF), was prepared by a ligand exchange reaction employing the precursor 99mTc-glucoheptonate (GH). The resulting mixture was analyzed by reversed-phase high-performance liquid chromatography (HPLC), showing that a single radioactive complex formed with radiochemical purity higher than 95% after purification by HPLC. The identity of the complex was established by comparative HPLC using the corresponding Re complexes as a reference. The retention times for 99mTc-BAT-BF and 99mTc-MAMA-BF on HPLC (radioactivity) were 13.2 and 10.3 min, respectively. The retention times of the corresponding Re complexes (7 and 10) on HPLC (UV detection) were 11.4 and 9.4 min, respectively.
Scheme 1. Synthesis of Re- and 99mTc-benzofuran Derivatives.
Reagents: (a) DMF, K2CO3, 4-nitrobenzylbromide. (b) EtOH, SnCl2. (c) CH3COOH, (CH2O)n, NaBH3CN. (d) CH2Cl2, BBr3. (e) CH3CN, K2CO3, 1,3-dibromopropane. (f) CH3CN, DIPEA, PMB-BAT. (g) TFA, MeSO3H, anisole. (h) CH2Cl2/MeOH, (PPh3)2ReOCl3, AcONa. (i) CH3CN, 0.1 N HCl, 99mTc-GH. (j) CH3CN, TRT-MAMA, DIPEA. (k) TFA, Et3SiH.
To evaluate the binding affinity of Re-BAT-BF (7) and Re-MAMA-BF (10), inhibition assays with [125I]IMPY and Aβ(1−42) aggregates were performed (Figure 2).18 Both ligands inhibited the binding of [125I]IMPY to Aβ(1−42) aggregates in a dose-dependent manner, indicating an affinity for Aβ aggregates. Their Ki values were 11.5 and 24.4 nM, respectively, suggesting that Re-BAT-BF displayed higher affinity than Re-MAMA-BF (Table 1). The results also indicated that 99mTc-BAT-BF and 99mTc-MAMA-BF would bind Aβ aggregates. Indeed, in subsequent assays, 99mTc-BAT-BF and 99mTc-MAMA-BF showed higher affinity than 99mTc-BAT and 99mTc-MAMA (Figure S1 in the Supporting Information). These results strongly support our previous report that benzofuran derivatives have considerable tolerance for structural modifications.22,23
Figure 2.

Inhibition curves of Re-BAT-BF (7) (pink circle) and Re-MAMA-BF (10) (green square) for the binding of [125I]IMPY to Aβ(1−42) aggregates.
Table 1. Inhibition Constants for the Binding of [125I]IMPY to Aβ(1−42) Aggregates.
| compound | Ki (nM)a |
|---|---|
| Re-BAT-BF (7) | 11.5 ± 0.56 |
| Re-MAMA-BF (10) | 24.4 ± 0.77 |
Values are the means ± standard errors of the mean of three independent determinations.
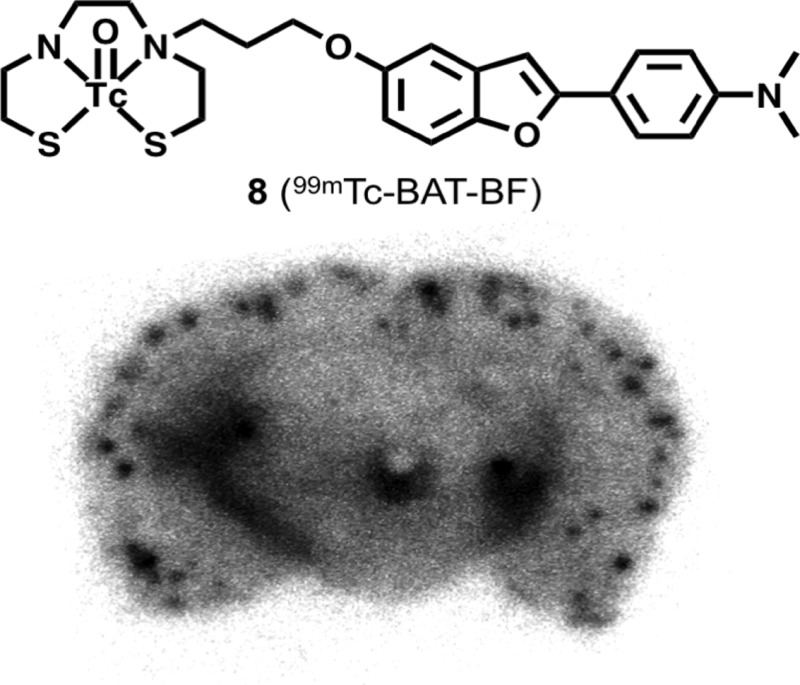
Next, the affinity of 99mTc-BAT-BF for β-amyloid plaques was investigated in vitro using sections of Tg2576 mouse brain (Figure 3). Autoradiographic images showed many radioactive spots in the brain tissue. Furthermore, the radioactivity of 99mTc-BAT-BF corresponded with the areas of staining with thioflavin-S, a pathological dye commonly used for β-amyloid plaques. In contrast, normal mouse brain displayed no remarkable accumulation of 99mTc-BAT-BF (data not shown). The results suggest that 99mTc-BAT-BF binds affinity for β-amyloid plaques in the mouse brain in addition to binding synthetic Aβ aggregates.
Figure 3.

Autoradiography of 99mTc-BAT-BF in sections from Tg2576 mouse brain (A). Labeled plaques were confirmed by the staining of the adjacent sections with thioflavin-S (B).
The biodistribution of 99mTc-BAT-BF and 99mTc-MAMA-BF was examined in normal mice (Table 2). A biodistribution experiment provides important information on uptake in the brain. The ideal imaging probe should penetrate the blood−brain barrier to deliver a sufficient dose into the brain but be rapidly cleared from normal regions so as to achieve in a high signal-to-noise ratio. 99mTc-BAT-BF showed greater uptake (1.34%ID/g) than 99mTc-MAMA-BF (0.74% ID/g) at 2 min postinjection. The uptake of 99mTc-BAT-BF peaked at 10 min postinjection, reaching 1.37% ID/g, and about 60% of radioactivity accumulated at 2 min postinjection had been washed out from the brain by 60 min. The uptake of 99mTc-MAMA-BF peaked 30 min after the injection at 1.23% ID/g, and the washout from the brain was slower than that of 99mTc-BAT-BF throughout the time course, which is unsuitable for imaging in vivo. The log P values of 99mTc-BAT-BF and 99mTc-MAMA-BF were 3.33 and 3.01, respectively. Although lipophilicity is just one of the factors affecting the uptake of a compound into the brain,4 it may explain the good uptake of 99mTc-BAT-BF.
Table 2. Biodistribution of Radioactivity after Injection of 99mTc-Labeled Benzofuran Derivatives in Normal Micea.
| time after injection (min) |
||||
|---|---|---|---|---|
| organ | 2 | 10 | 30 | 60 |
| 99mTc-BAT-BF (8) | ||||
| blood | 4.40 (0.27) | 1.96 (0.06) | 1.93 (0.26) | 2.15 (0.91) |
| liver | 21.94 (5.94) | 20.87 (1.28) | 19.65 (1.31) | 15.09 (3.83) |
| kidney | 10.28 (1.76) | 7.90 (0.40) | 4.27 (0.18) | 2.70 (0.57) |
| intestineb | 1.45 (0.18) | 3.68 (0.52) | 7.42 (1.62) | 9.02 (1.93) |
| spleen | 5.20 (1.01) | 3.09 (0.23) | 1.69 (0.21) | 1.16 (0.14) |
| lung | 26.70 (2.27) | 6.48 (1.33) | 3.51 (0.64) | 2.36 (0.48) |
| stomachb | 1.33 (0.57) | 1.90 (0.43) | 4.09 (1.37) | 4.17 (1.92) |
| pancreas | 4.14 (0.77) | 4.57 (0.24) | 2.98 (0.38) | 1.42 (0.15) |
| heart | 17.60 (2.60) | 8.29 (0.97) | 3.28 (1.35) | 1.51 (0.25) |
| brain | 1.34 (0.12) | 1.37 (0.18) | 0.94 (0.20) | 0.56 (0.07) |
| 99mTc-MAMA-BF (11) | ||||
| blood | 4.13 (0.42) | 1.78 (0.25) | 2.15 (0.12) | 2.24 (0.24) |
| liver | 20.17 (3.81) | 21.62 (2.62) | 23.32 (1.59) | 20.16 (2.13) |
| kidney | 7.37 (1.06) | 8.09 (1.16) | 5.11 (0.29) | 3.28 (0.45) |
| intestineb | 0.95 (0.22) | 2.13 (0.19) | 4.75 (0.93) | 5.73 (0.66) |
| spleen | 4.48 (0.56) | 3.69 (0.34) | 3.49 (0.61) | 2.59 (0.65) |
| lung | 24.04 (5.17) | 7.59 (2.13) | 4.24 (0.35) | 3.54 (1.26) |
| stomachb | 0.73 (0.21) | 2.35 (0.58) | 4.94 (0.57) | 2.81 (0.51) |
| pancreas | 2.70 (0.47) | 4.00 (1.28) | 5.48 (0.61) | 3.76 (0.36) |
| heart | 12.28 (2.20) | 10.48 (1.79) | 5.05 (0.90) | 2.16 (0.34) |
| brain | 0.74 (0.15) | 0.99 (0.22) | 1.23 (0.09) | 0.89 (0.08) |
Each value represents the mean (SD) for five mice. Expressed as % injected dose per gram.
Expressed as % injected dose per organ.
In conclusion, we successfully designed and synthesized novel benzofuran derivatives conjugated with 99mTc or Re complexes for the detection of β-amyloid plaques in the brain. In experiments in vitro, Re-BAT-BF bound to Aβ aggregates with greater affinity than did Re-MAMA-BF, and 99mTc-BAT-BF clearly labeled β-amyloid plaques in sections of brain tissue from Tg2576 mice. In addition, 99mTc-BAT-BF displayed good uptake into and a rapid washout from the brain after its injection in normal mice. The combination of good affinity for β-amyloid plaques, uptake, and clearance makes 99mTc-BAT-BF a promising probe for the detection of β-amyloid plaques in the brain. The results of the present study should provide useful information for the development of 99mTc-labeled probes for the imaging of β-amyloid plaques in the brain.
Supporting Information Available
Procedures for the preparation of new ligands, analysis of data, experiments in vitro, and biodistribution experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
This study was supported by Grants-in-Aid for Scientific Research (B), Young Scientists (A), and Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Supplementary Material
References
- Hardy J. A.; Higgins G. A. Alzheimer's disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Alzheimer's disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Imaging Alzheimer's amyloid. Nat. Biotechnol. 2000, 18, 823–824. [DOI] [PubMed] [Google Scholar]
- Mathis C. A.; Wang Y.; Klunk W. E. Imaging β-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr. Pharm. Des. 2004, 10, 1469–1492. [DOI] [PubMed] [Google Scholar]
- Nordberg A. PET imaging of amyloid in Alzheimer's disease. Lancet Neurol. 2004, 3, 519–527. [DOI] [PubMed] [Google Scholar]
- Kung H. F.; Choi S. R.; Qu W.; Zhang W.; Skovronsky D. 18F Stilbenes and Styrylpyridines for PET Imaging of Aβ Plaques in Alzheimer's Disease: A Miniperspective. J. Med. Chem. 2010, 53, 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis C. A.; Wang Y.; Holt D. P.; Huang G. F.; Debnath M. L.; Klunk W. E. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J. Med. Chem. 2003, 46, 2740–2754. [DOI] [PubMed] [Google Scholar]
- Klunk W. E.; Engler H.; Nordberg A.; Wang Y.; Blomqvist G.; Holt D. P.; Bergstrom M.; Savitcheva I.; Huang G. F.; Estrada S.; Ausen B.; Debnath M. L.; Barletta J.; Price J. C.; Sandell J.; Lopresti B. J.; Wall A.; Koivisto P.; Antoni G.; Mathis C. A.; Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [DOI] [PubMed] [Google Scholar]
- Ono M.; Wilson A.; Nobrega J.; Westaway D.; Verhoeff P.; Zhuang Z. P.; Kung M. P.; Kung H. F. 11C-labeled stilbene derivatives as Aβ-aggregate-specific PET imaging agents for Alzheimer's disease. Nucl. Med. Biol. 2003, 30, 565–571. [DOI] [PubMed] [Google Scholar]
- Verhoeff N. P.; Wilson A. A.; Takeshita S.; Trop L.; Hussey D.; Singh K.; Kung H. F.; Kung M. P.; Houle S. In-vivo imaging of Alzheimer disease β-amyloid with [11C]SB-13 PET. Am. J. Geriatr. Psychiatry 2004, 12, 584–595. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Oya S.; Kung M. P.; Hou C.; Maier D. L.; Kung H. F. F-18 polyethyleneglycol stilbenes as PET imaging agents targeting Aβ aggregates in the brain. Nucl. Med. Biol. 2005, 32, 799–809. [DOI] [PubMed] [Google Scholar]
- Rowe C. C.; Ackerman U.; Browne W.; Mulligan R.; Pike K. L.; O'Keefe G.; Tochon-Danguy H.; Chan G.; Berlangieri S. U.; Jones G.; Dickinson-Rowe K. L.; Kung H. P.; Zhang W.; Kung M. P.; Skovronsky D.; Dyrks T.; Holl G.; Krause S.; Friebe M.; Lehman L.; Lindemann S.; Dinkelborg L. M.; Masters C. L.; Villemagne V. L. Imaging of amyloid β in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: Proof of mechanism. Lancet Neurol. 2008, 7, 129–135. [DOI] [PubMed] [Google Scholar]
- Kudo Y.; Okamura N.; Furumoto S.; Tashiro M.; Furukawa K.; Maruyama M.; Itoh M.; Iwata R.; Yanai K.; Arai H. 2-(2-[2-Dimethylaminothiazol-5-yl]ethenyl)-6-(2-[fluoro]ethoxy)benzoxazole: A novel PET agent for in vivo detection of dense amyloid plaques in Alzheimerʼs disease patients. J. Nucl. Med. 2007, 48, 553–561. [DOI] [PubMed] [Google Scholar]
- Agdeppa E. D.; Kepe V.; Liu J.; Flores-Torres S.; Satyamurthy N.; Petric A.; Cole G. M.; Small G. W.; Huang S. C.; Barrio J. R. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for β-amyloid plaques in Alzheimer's disease. J. Neurosci. 2001, 21, RC189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoghi-Jadid K.; Small G. W.; Agdeppa E. D.; Kepe V.; Ercoli L. M.; Siddarth P.; Read S.; Satyamurthy N.; Petric A.; Huang S. C.; Barrio J. R. Localization of neurofibrillary tangles and β-amyloid plaques in the brains of living patients with Alzheimer disease. Am. J. Geriatr. Psychiatry 2002, 10, 24–35. [PubMed] [Google Scholar]
- Small G. W.; Kepe V.; Ercoli L. M.; Siddarth P.; Bookheimer S. Y.; Miller K. J.; Lavretsky H.; Burggren A. C.; Cole G. M.; Vinters H. V.; Thompson P. M.; Huang S. C.; Satyamurthy N.; Phelps M. E.; Barrio J. R. PET of brain amyloid and tau in mild cognitive impairment. N. Engl. J. Med. 2006, 355, 2652–2663. [DOI] [PubMed] [Google Scholar]
- Kung M. P.; Hou C.; Zhuang Z. P.; Zhang B.; Skovronsky D.; Trojanowski J. Q.; Lee V. M.; Kung H. F. IMPY: An improved thioflavin-T derivative for in vivo labeling of β-amyloid plaques. Brain Res. 2002, 956, 202–210. [DOI] [PubMed] [Google Scholar]
- Zhuang Z. P.; Kung M. P.; Wilson A.; Lee C. W.; Plossl K.; Hou C.; Holtzman D. M.; Kung H. F. Structure-activity relationship of imidazo[1,2-a]pyridines as ligands for detecting β-amyloid plaques in the brain. J. Med. Chem. 2003, 46, 237–243. [DOI] [PubMed] [Google Scholar]
- Newberg A. B.; Wintering N. A.; Clark C. M.; Plossl K.; Skovronsky D.; Seibyl J. P.; Kung M. P.; Kung H. F. Use of 123I IMPY SPECT to differentiate Alzheimer's disease from controls. J. Nucl. Med. 2006, 47, 78P. [PubMed] [Google Scholar]
- Zhang W.; Kung M. P.; Oya S.; Hou C.; Kung H. F. 18F-labeled styrylpyridines as PET agents for amyloid plaque imaging. Nucl. Med. Biol. 2007, 34, 89–97. [DOI] [PubMed] [Google Scholar]
- Choi S. R.; Golding G.; Zhuang Z.; Zhang W.; Lim N.; Hefti F.; Benedum T. E.; Kilbourn M. R.; Skovronsky D.; Kung H. F. Preclinical properties of 18F-AV-45: A PET agent for Aβ plaques in the brain. J. Nucl. Med. 2009, 50, 1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M.; Kung M. P.; Hou C.; Kung H. F. Benzofuran derivatives as Aβ-aggregate-specific imaging agents for Alzheimer's disease. Nucl. Med. Biol. 2002, 29, 633–642. [DOI] [PubMed] [Google Scholar]
- Ono M.; Kawashima H.; Nonaka A.; Kawai T.; Haratake M.; Mori H.; Kung M. P.; Kung H. F.; Saji H.; Nakayama M. Novel benzofuran derivatives for PET imaging of β-amyloid plaques in Alzheimer's disease brains. J. Med. Chem. 2006, 49, 2725–2730. [DOI] [PubMed] [Google Scholar]
- Han H.; Cho C. G.; Lansbury P. T. Jr. Technetium complexes for the quantitation of brain amyloid. J. Am. Chem. Soc. 1996, 118, 4506–4507. [Google Scholar]
- Dezutter N. A.; Dom R. J.; de Groot T. J.; Bormans G. M.; Verbruggen A. M. 99mTc-MAMA-chrysamine G, a probe for β-amyloid protein of Alzheimer's disease. Eur. J. Nucl. Med. 1999, 26, 1392–1399. [DOI] [PubMed] [Google Scholar]
- Chen X.; Yu P.; Zhang L.; Liu B. Synthesis and biological evaluation of 99mTc, Re-monoamine-monoamide conjugated to 2-(4-aminophenyl)benzothiazole as potential probes for β-amyloid plaques in the brain. Bioorg. Med. Chem. Lett. 2008, 18, 1442–1445. [DOI] [PubMed] [Google Scholar]
- Serdons K.; Verduyckt T.; Cleynhens J.; Terwinghe C.; Mortelmans L.; Bormans G.; Verbruggen A. Synthesis and evaluation of a 99mTc-BAT-phenylbenzothiazole conjugate as a potential in vivo tracer for visualization of amyloid β. Bioorg. Med. Chem. Lett. 2007, 17, 6086–6090. [DOI] [PubMed] [Google Scholar]
- Zhuang Z. P.; Kung M. P.; Hou C.; Ploessl K.; Kung H. F. Biphenyls labeled with technetium 99m for imaging β-amyloid plaques in the brain. Nucl. Med. Biol. 2005, 32, 171–184. [DOI] [PubMed] [Google Scholar]
- Oya S.; Plossl K.; Kung M. P.; Stevenson D. A.; Kung H. F. Small and neutral Tc(v)O BAT, bisaminoethanethiol (N2S2) complexes for developing new brain imaging agents. Nucl. Med. Biol. 1998, 25, 135–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




