Abstract
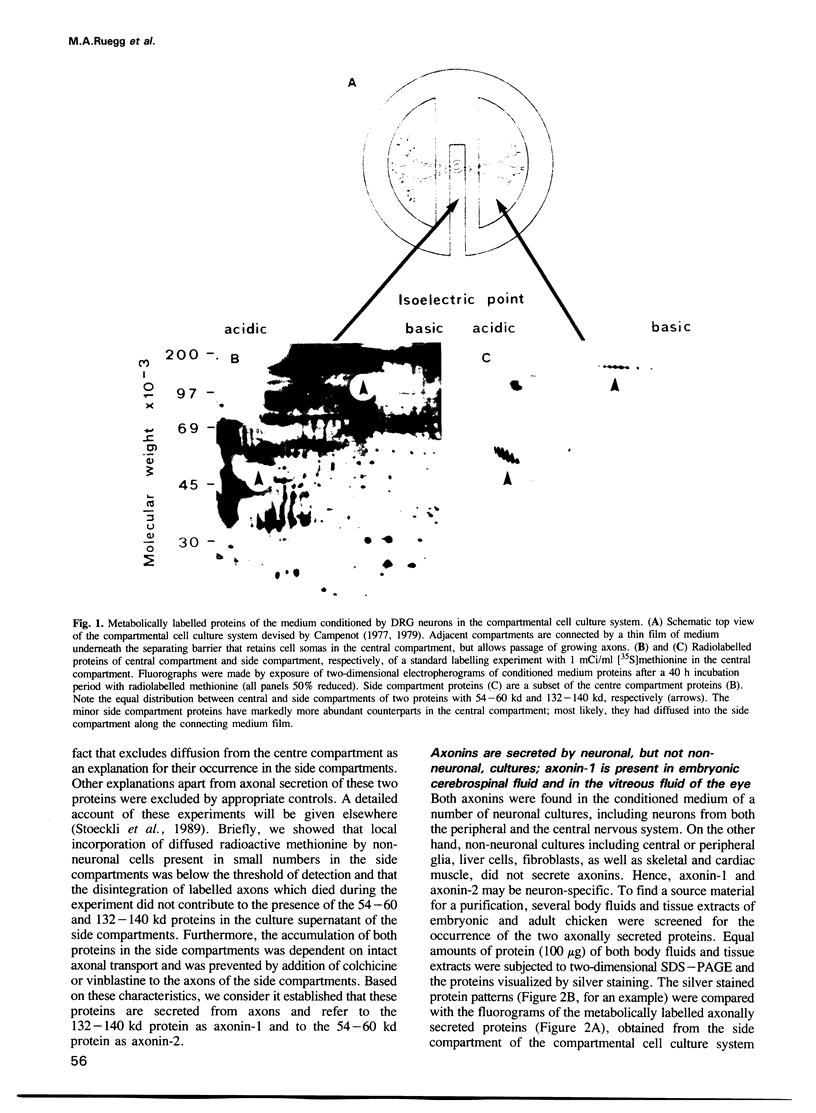
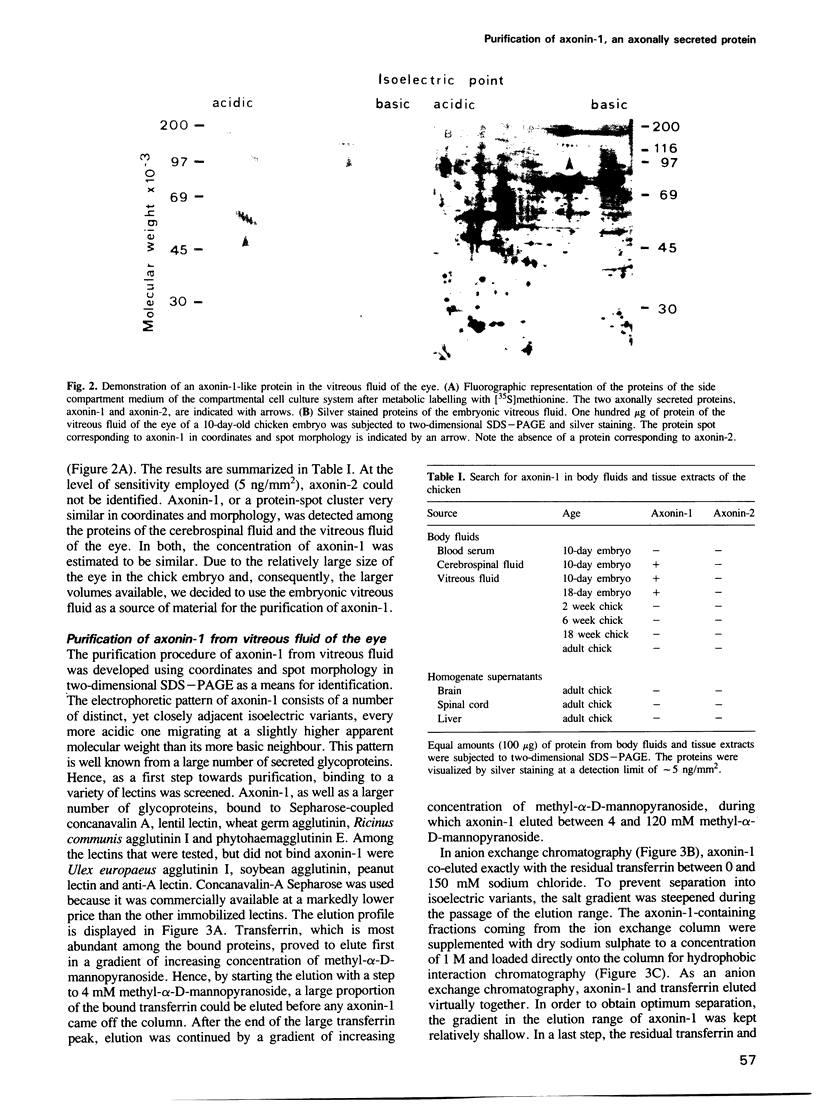
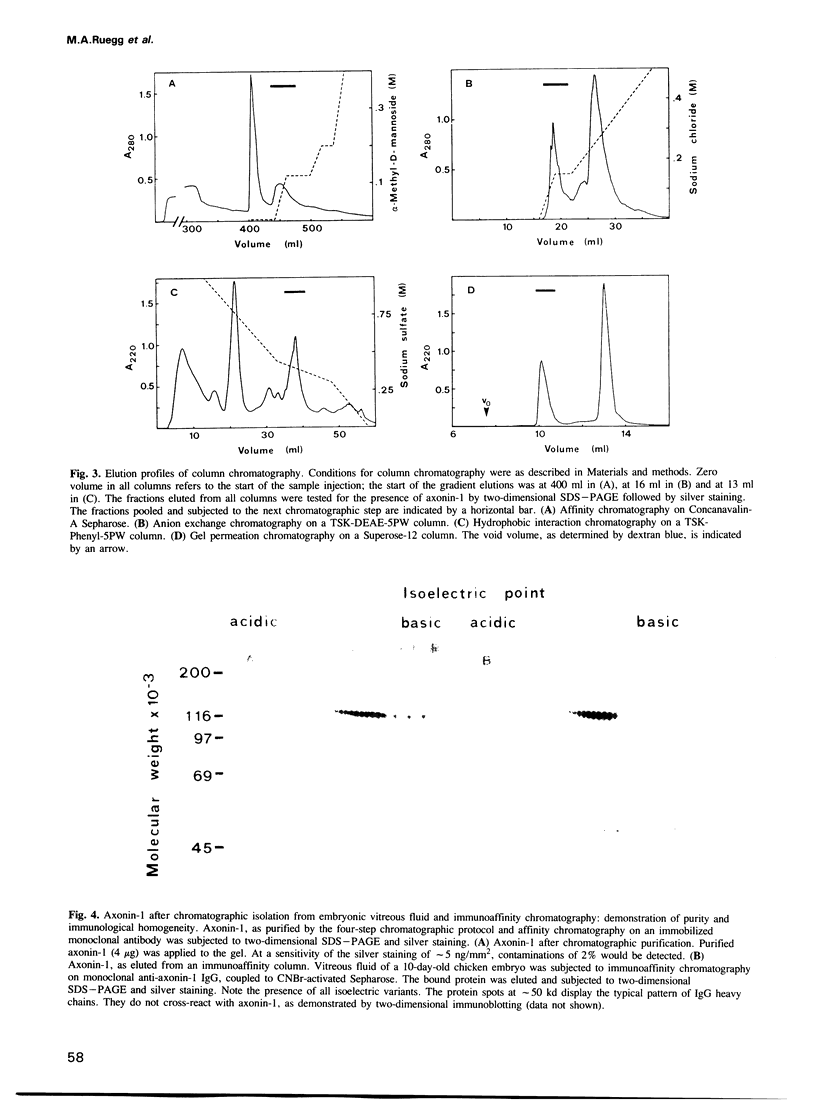
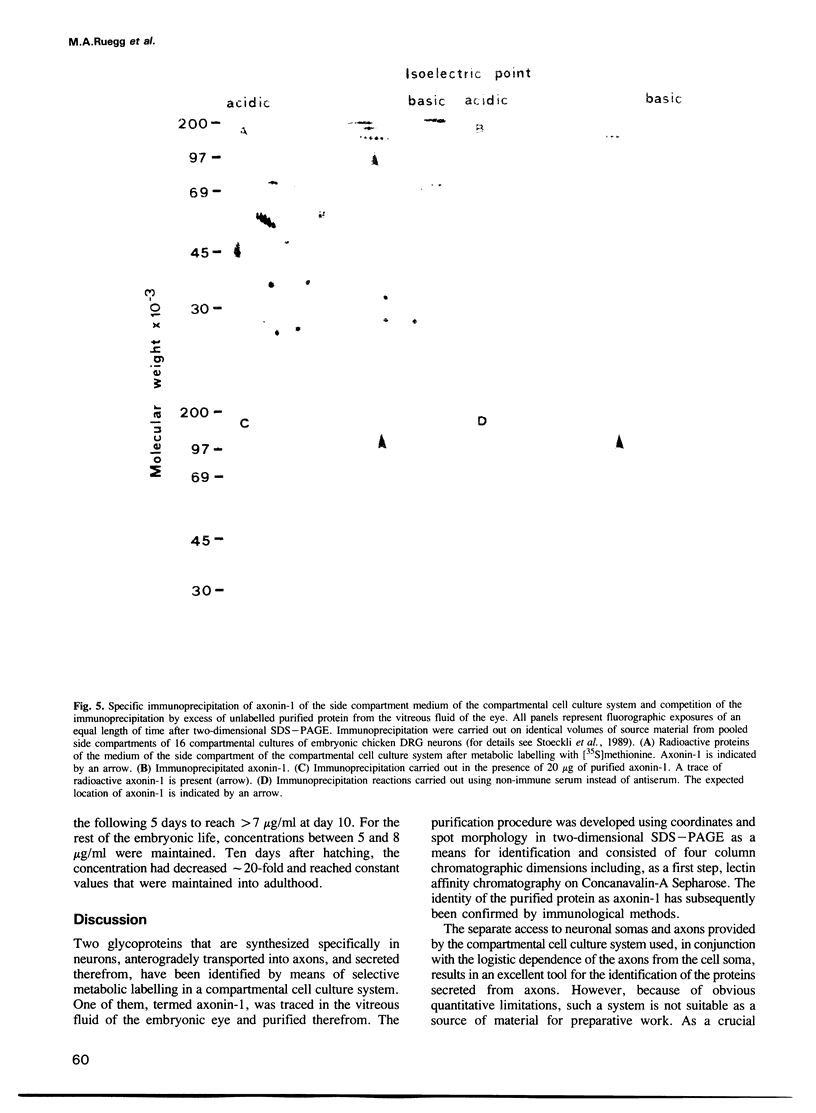
Using selective metabolic labelling in a compartmental cell culture system two proteins, denoted axonin-1 and axonin-2, were found to be secreted by axons of dorsal root ganglia neurons from chicken embryos. Based on its characteristic coordinates and spot morphology in two-dimensional gel electrophoresis, axonin-1 was detected in the cerebrospinal fluid and the vitreous fluid, axonin-1 was purified 476-fold to homogeneity by a four-step chromatographic procedure. The identity of the purified protein as axonin-1 was confirmed by immunological methods. Axonin-1 is a glycoprotein that subdivides into at least 16 immunologically similar isoelectric variants; their molecular weight range extends from 132 to 140 kd and their pI range from 5.3 to 6.2. In the vitreous fluid of the embryo, axonin-1 could first be detected on the embryonic day 5 and highest concentrations were measured during the second half of embryonic life; in the vitreous fluid of the adult chicken, concentrations were approximately 20 times lower. The early onset of secretion and the time course of expression suggest a role for axonin-1 in the development of the nervous system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L., Anderson N. G. High resolution two-dimensional electrophoresis of human plasma proteins. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5421–5425. doi: 10.1073/pnas.74.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Campenot R. B. Independent control of the local environment of somas and neurites. Methods Enzymol. 1979;58:302–307. doi: 10.1016/s0076-6879(79)58146-4. [DOI] [PubMed] [Google Scholar]
- Campenot R. B. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lemkin P. F., Lipkin L. E. GELLAB: A computer system for 2D gel electrophoresis analysis. II. Pairing spots. Comput Biomed Res. 1981 Aug;14(4):355–380. doi: 10.1016/0010-4809(81)90006-9. [DOI] [PubMed] [Google Scholar]
- Marshall T., Williams K. M. Artifacts associated with 2-mercaptoethanol upon high resolution two-dimensional electrophoresis. Anal Biochem. 1984 Jun;139(2):502–505. doi: 10.1016/0003-2697(84)90041-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Ochs D. Protein contaminants of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1983 Dec;135(2):470–474. doi: 10.1016/0003-2697(83)90714-5. [DOI] [PubMed] [Google Scholar]
- Rosa P., Hille A., Lee R. W., Zanini A., De Camilli P., Huttner W. B. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985 Nov;101(5 Pt 1):1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settleman J., Fonseca R., Nolan J., Angeletti R. H. Relationship of multiple forms of chromogranin. J Biol Chem. 1985 Feb 10;260(3):1645–1651. [PubMed] [Google Scholar]
- Sonderegger P., Fishman M. C., Bokoum M., Bauer H. C., Neale E. A., Nelson P. G. A few axonal proteins distinguish ventral spinal cord neurons from dorsal root ganglion neurons. J Cell Biol. 1984 Jan;98(1):364–368. doi: 10.1083/jcb.98.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger P., Fishman M. C., Bokoum M., Bauer H. C., Nelson P. G. Axonal proteins of presynaptic neurons during synaptogenesis. Science. 1983 Sep 23;221(4617):1294–1297. doi: 10.1126/science.6612344. [DOI] [PubMed] [Google Scholar]
- Sonderegger P., Lemkin P. F., Lipkin L. E., Nelson P. G. Differential modulation of the expression of axonal proteins by non-neuronal cells of the peripheral and central nervous system. EMBO J. 1985 Jun;4(6):1395–1401. doi: 10.1002/j.1460-2075.1985.tb03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]