Abstract
Aim:
To assess the roles of extracellular signal-regulated kinase (ERK), p38, and CD151-integrin complexes on proliferation, migration, and tube formation activities of CD151-induced human umbilical vein endothelial cells (HUVECs).
Methods:
CD151, anti-CD151 and CD151-AAA mutant were inserted into recombinant adeno-associated virus (rAAV) vectors and used to transfect HUVECs. After transfection, the expression of CD151 was measured. Proliferation was assessed using the 3-[4,5-dimethylthiazol- 2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay. Cell migration was evaluated in Boyden transwell chambers using FBS as the chemotactic stimulus. The tube formation assay was performed on matrigel. The potential involvement of various signaling pathways was explored using selective inhibitors.
Results:
CD151 gene delivery increased the expression of CD151 at both the mRNA and protein levels. Overexpression of CD151 promoted cell proliferation, migration and tube formation in vitro, and phosphorylation of ERK was also increased. Further, CD151-induced cell proliferation, migration, and tube formation were attenuated by the ERK inhibitor PD98059 (20 μmol/L) but not by a p38 inhibitor (SB203580, 20 μmol/L). Moreover, there was no significant difference in CD151 protein expression between the CD151 group and the CD151-AAA group, but the CD151-AAA mutant abrogated cellular proliferation, migration, and tube formation and decreased the phosphorylation of ERK.
Conclusion:
This study suggests that activation of the ERK signaling pathway may be involved in the angiogenic effects of CD151. Activation of ERK was dependent on the formation of CD151-integrin complexes. Therefore modulation of CD151 may be as a novel therapeutic strategy for regulating angiogenesis.
Keywords: CD151, cell migration, cell proliferation, angiogenesis, ERK, p38 MAPK, human umbilical vein endothelial cells
Introduction
As a member of the transmembrane 4 superfamily (TM4SF), CD151 (also known as platelet-endothelial cell tetraspan antigen 3, PETA-3) contains two extracellular loops, four hydrophobic transmembrane domains, and two short cytoplasmic tails1, 2. CD151 is broadly expressed in various cell types, but in endothelial cells it is characteristically localized to cell–cell junctions and endosomes3, 4. Previous studies have shown that CD151 is involved in regulating cell motility, adhesion, spreading, and morphogenesis1, 2, 3, 4, 5, 6. Over the past decade, accumulating evidence has suggested that CD151 plays a crucial role in angiogenesis7, 8, 9, 10. CD151 knock-out cells display markedly reduced endothelial events related to angiogenesis (migration, spreading, invasion, matrigel contraction, tube formation and spheroid sprouting)7. Our research group previously demonstrated that transfection with CD151 cDNA enhanced endothelial cell proliferation, migration and tube formation and significantly up-regulated endothelial nitric-oxide synthase (eNOS) expression via the PI3K/Akt signaling pathway8. Furthermore, we found that delivery of the CD151 gene increased the number of microvessels in a pig myocardial ischemia model and a rat ischemic hindlimb model, suggesting that CD151 is a potential target for therapeutic angiogenesis9, 10. However, the molecular mechanisms that govern the effects of CD151 in angiogenesis have not been well elucidated.
Several studies have demonstrated that CD151 forms complexes with the integrins α3β1, α6β4 and α6β1 and that the CD151-α3β1 integrin complex has unusually high stoichiometry, proximity, and stability3, 5, 11. Notably, the specific QRD194–196 motif of CD151 is necessary for α3β1 interaction. This motif is located in CD151's large extracellular loop12. When the key motif was mutated, CD151's ability to complex with integrin α3 was impaired12, 13. CD151 may also use the same site to form complexes with α6β1, α6β4, and other integrins5, 12, 13. In parallel, the intracellular domain of CD151 may determine the molecule's association with signaling molecules such as PtdIns 4-K and PKC11, 14. Hence, a “transmembrane linker” model for CD151 has been proposed, and CD151 signaling is thought to be mediated by CD151-integrin complexes6, 14, 15.
Mitogen-activated protein (MAP) kinases play important roles in various cellular responses16, 17, 18. In this study, using a gene transfer technique with a recombinant adeno-associated virus (rAAV) vector, we examined the roles of MAP kinases, particularly extracellular signal-regulated kinase (ERK) and p38 MAP kinase (p38), in CD151–induced endothelial cell proliferation, migration and tube formation. In addition, we mutated the QRD194–196 motif and observed the effects of this CD151 mutant. The purpose of the present study was to investigate the mechanism(s) by which CD151 induces angiogenesis.
Materials and methods
Materials
All cell culture reagents were obtained from Invitrogen (Carlsbad, CA), including Dulbecco's modified Eagle's medium (DMEM), trypsin and fetal bovine serum (FBS). The restriction enzymes were purchased from TaKaRa (Dalian, China). Antibodies against ERK1/2 (also called p42/44 MAPK), phospho-ERK1/2, p38, phospho-p38, CD151, and β-actin were purchased from Santa Cruz Biotechnology, Inc (California, USA). PD98059 and SB203580 were supplied by Calbiochem Novabiochem (Darmstadt, Germany). The enhanced chemiluminescent (ECL) substrate was obtained from Pierce (Rockford, Illinois, USA). Hybrisol solution was purchased from Intergen (Purchase, NY). Tween-20, PMSF, aprotinin, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich Chemical Co (St Louis, MO). Matrigel was purchased from B&D Biosciences (Heidelberg, Germany). [α-32P]-dCTP (3000 Ci/mmol) was obtained from Rui Fu Biological Engineering Company Nucleic Acid Laboratory (Beijing, China). All other chemicals and reagents were purchased from Sigma-Aldrich unless otherwise specified.
Construction of pAAV-CD151, pAAV-anti-CD151, pAAV-CD151-AAA, and pAAV-GFP
The PzeoSV-CD151 plasmid was kindly provided by Dr Xin A ZHANG, Department of Molecular Science, University of Tennessee Health Science Center (Memphis, Tennessee, USA). The construction of pAAV-CD151, pAAV-anti-CD151, and pAAV-Green fluorescent protein (GFP) has been described previously8, 9, 10, 19. The CD151 mutant was generated by recombinant PCR. The pAAV-CD151 vector contained the full-length wild-type human CD151, and cDNA was used as the template. For the CD151-AAA mutant, the following primers were used: 5′-TGTGGAATTAATTTCCATGCCTCCAACATC-3′ (internal sense primer to amplify the 3′-region) and 5′-GGCATGGAAATTAATTCCACAAAGAGCCAC-3′ (internal antisense primer to amplify the 5′-region) of the CD151 template. Then we used either external sense (5′-GCTTAGATCTGCCACCATGGGTGAGTTCAACGAG-3′) or external antisense (5′-GACGCGGCCGCTCAGGCGTAGTCGGG-3′) primers. The final recombinant PCR was performed using purified PCR products and external sense and external antisense primers. The final PCR products were incised by the Bgl II and Not restriction enzymes, and then the incised products were purified and ligated into the AAV vector at the Bam H I and Not I restriction sites. Proper ligation was confirmed by sequencing.
Construction and preparation of recombinant adeno-associated virus
The rAAV vector pXXUF1, packaging plasmid pXX2, adenovirus helper plasmid pHelper, and a rAAV plasmid containing GFP cDNA were obtained from Dr Xiao XIAO (University of Pittsburgh, Pittsburgh, PA).
The packing and production of rAAV-GFP, rAAV-CD151, rAAV-antiCD151, and rAAV-CD151-AAA were carried out using a triple-plasmid cotransfection method in human embryonic kidney cells (293 cells)10, 20. The cells can be transfected at 70% to 80% confluence. A total of 85 mg of plasmid DNA (the molar ratios of dsAAV-GFP or dsAAV-CD151 or dsAAV-antiCD151 or rAAV-CD151-AAA to pXX2 and pHelper were 1:1:1) was dissolved in 0.25 mol/L CaCl2, and the solution was quickly mixed with 2×BES [N,N-Bis(2-hydroxyethyl) taurine]-buffered saline and added to the cells in a 15-cm plate. The cells were harvested 48 to 72 h after transfection. After three cycles of freeze-thaw and centrifugation, the rAAVs remained in the supernatant, and single-step gravity-flow column purification was applied21. The eluted rAAVs were aliquoted and stored at -80 °C. The titers of vector particles were determined by quantitative DNA dot-blot hybridization using [α-32P]- dCTP20, 21.
Isolation and culture of endothelial cells
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical veins by collagenase treatment. The cells were grown in DMEM supplemented with 20% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 U/mL heparin at 37 °C under 5% CO2 and 95% air. Only cells passaged less than five times were used for experiments.
Transfection of endothelial cells with rAAVs
Cells were grown to 60% confluence, the medium was removed, and the cells were washed three times with PBS and incubated with DMEM containing 0.5% FBS at 37 °C for 12 h. We directly added corresponding volumes of rAAV-GFP, rAAV-CD151, rAAV-antiCD151 or rAAV-CD151-AAA to the medium (about 50 virions/cell) in 6-well plates, according to the titers of vector particles. For the control group, PBS was added to the medium. Then the cells were grown under 5% CO2 and 95% air overnight. The next day, 1 mL DMEM supplemented with 20% fetal bovine serum was added to every group. Cells were incubated in the conditions above for 5 days and then subjected to the following assays.
Proliferation assay
HUVECs transfected with rAAV-CD151, rAAV-antiCD151, rAAV-CD151-AAA, or rAAV-GFP were trypsinized and seeded in 96-well plates (1×104 cells/well) . After attachment, the cells were exposed to DMEM with 0.5% FBS for 48 h and then the effects of CD151 on HUVEC proliferation were evaluated using the MTT colorimetric assay. Briefly, the medium was removed and replaced with medium containing 5 mg/mL MTT, and the cells were incubated for 4 h. The medium was then aspirated, and the product was solubilized with dimethyl sulfoxide (DMSO). Absorbance for each well was measured at 570 nm using a microplate reader (Bio-Tek Instrument, USA).
Migration assay
HUVECs transfected with rAAV-CD151, rAAV-antiCD151, rAAV-CD151-AAA, or rAAV-GFP were exposed to DMEM with 0.5% FBS for 48 h and then cell migration was assayed using a modified Boyden chamber technique. Briefly, 200 μL DMEM containing 10% FBS was added to the bottom well. Cells were resuspended in the appropriate buffer at a concentration of 106 cells/mL, and 800 μL of the cell suspension was added to the top well of the transwell chambers. A filter with 8 μm pore size separated the bottom and top wells. After incubation for 24 h at 37 °C in a 5% CO2 atmosphere, the cells that had not migrated were removed from the upper surface, and those that migrated to the lower surface of the filters were fixed in methanol and stained with hematoxylin. Migrating cells adherent to the underside of the filter were enumerated using an ocular micrometer and by counting 10 high-powered fields (HPFs, ×200). Data are presented as relative migration (number of cells/HPF).
Tube formation
HUVECs transfected with different rAAV viruses were exposed to DMEM with 0.5% FBS for 48 h, and then the cells were plated in 24-well plates with Matrigel. Matrigel (0.5 mL) was polymerized on 24-well plates, and 5×104 cells were then plated in full-growth medium for 1 h. Once the cells were seeded, the medium was replaced with medium containing 0.5% serum. Tube formation was visualized using an inverted microscope (Nikon TE 2000) equipped with digital imaging. For each treatment, 10 field images were captured, and the area containing endothelial tubes and networks that had formed was quantified using the Scion Image Analysis System (windows version of scion image, NIH) with background subtraction.
RT-PCR analysis
HUVEC RNA was extracted using Trizol (Gibco) as described previously5. RT-PCR analysis specific for human CD151 (Genbank sequence NM 004357) with an HA tag (forward primer, 5′-ATGGGTGAGTTCAACGAG-3′ reverse primer, 5′-GCCGCTCAGGCGTAGTC -3′) and β-actin (forward primer, 5′-GGAGAAGGACCCAGATC-3′ reverse primer, 5′-GATCTTCATGAGGTAGTCAG-3′) was then performed using an RT-PCR kit (Takara Biotechnology) according to the manufacturer's instructions. The conditions for PCR were one cycle of denaturation at 94 °C for 5 min followed by 35 cycles of 94 °C for 45 s, 60 °C for 40 s, and 72 °C for 1 min, with a final extension at 72 °C for 7 min. PCR products were resolved in 1.5% agarose gels and stained with ethidium bromide.
Western blot analysis
HUVEC protein was extracted as follows. Briefly, the medium in six-well plates was discarded, and cells were gently washed three times with cooled PBS. Lysis buffer (500 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.02% sodium azide, 0.1% SDS, 100 μg/mL phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, 1% Nonidet P-40, and 0.5% sodium deoxycholate) was added to the cells (0.25 mL/well). After incubation on ice for 30 min, the lysate was centrifuged at 12 000×g at 4 °C for 10 min. The protein concentration of the supernatant was determined using the Bradford method. Lysates (25 μg protein/ lane) were resolved by SDS-PAGE gel, transferred to nitrocellulose membranes, and blocked with 5% nonfat dry milk in 10 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl, and 0.1% Tween 20. The membranes were then incubated with primary antibodies against CD151, ERK1/2, phospho-ERK1/2, p38, and phospho-p38 overnight at 4 °C. Peroxidase-conjugated secondary antibodies were applied for 2 to 3 h. The ECL system was used to visualize the separated proteins. Blots were stripped and reprobed with β-actin as a loading control. The intensities of the various protein bands were quantified by densitometry (GeneTools analysis software).
Evaluation of signaling pathways
To examine the signaling mechanisms through which CD151 enhances endothelial cell proliferation, migration, and tube formation, inhibitors of ERK (PD98059, 20 μmol/L) and p38 (SB203580, 20 μmol/L) were added to cultured HUVECs for 8 h. The effects of these inhibitors on endothelial cell proliferation, migration, and tube formation were observed.
Statistical analysis
Data were analyzed using SPSS 13.0 statistical software (SPSS Inc, USA). Each variable was examined to determine whether it was normally distributed using the Shapiro-Wilk test. Data were expressed as means±SEM. Comparisons between two groups were performed using Student's t-tests. Three or more groups were compared by analysis of variance followed by the Newman-Keuls test. P values less than 0.05 were considered significant.
Results
Expression of CD151 in different groups
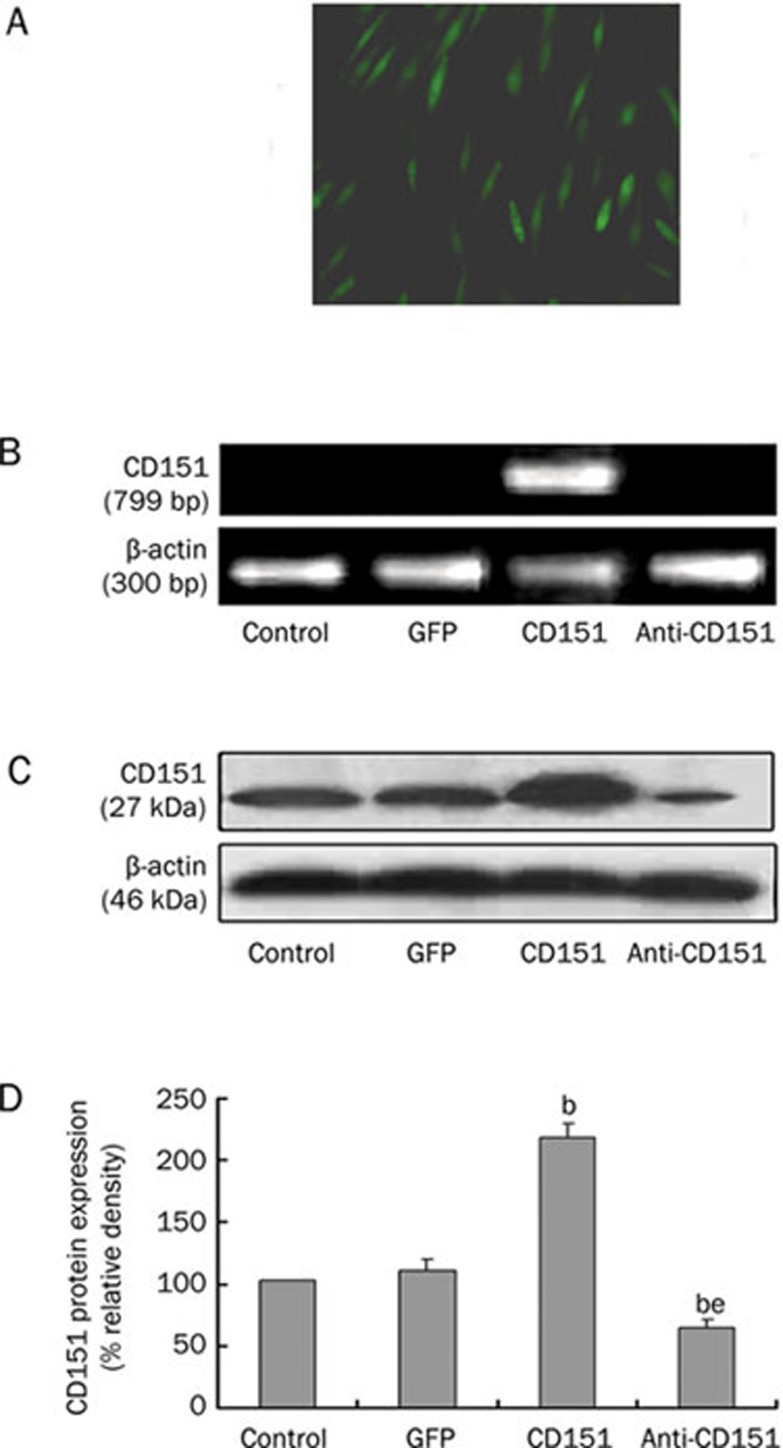
HUVECs transfected with rAAV-GFP were observed with an inverted fluorescence microscope (Figure 1A). In our experiment, CD151 was linked to an HA-tag, and RT-PCR analysis was specific for CD151 with this tag. Therefore, CD151 mRNA was detected in the CD151 group (positive), but not in the control, GFP and anti-CD151 groups (Figure 1B). Similar levels of β-actin mRNA were detected in all groups, confirming the integrity of prepared RNA.
Figure 1.
CD151 transfection increases the expression of human CD151 mRNA and protein. (A) HUVECs transfected with rAAV-GFP observed with inverted fluorescence microscope 7 days after transfection. (B) Detection of human CD151 gene expression by RT-PCR. The anticipated sizes of PCR products for CD151 and β-actin were 799 and 300 bp, respectively. The human CD151 mRNA was detected in CD151 group, but not in the control, GFP and anti-CD151 groups. (C) and (D) Western blot analysis for CD151 in HUVECs transfected with rAAV-GFP, rAAV-CD151 or rAAV-anti-CD151. β-actin was used as an internal loading control. The mean density of CD151 in control group was defined as 100%. bP<0.05 vs the control group and GFP group. eP<0.05 vs the CD151 group. n=3. Data are mean±SEM.
Western blot analysis showed that expression of CD151 protein increased significantly in the CD151 group compared with the control group and the GFP group, but it decreased dramatically in the anti-CD151 group (Figures 1C and 1D). There were no significant differences in the expression of CD151 between the control group and the GFP group. These results indicate that rAAV-CD151 transfection increases CD151 expression.
Effects of CD151 on the proliferation, migration and tube formation of HUVECs
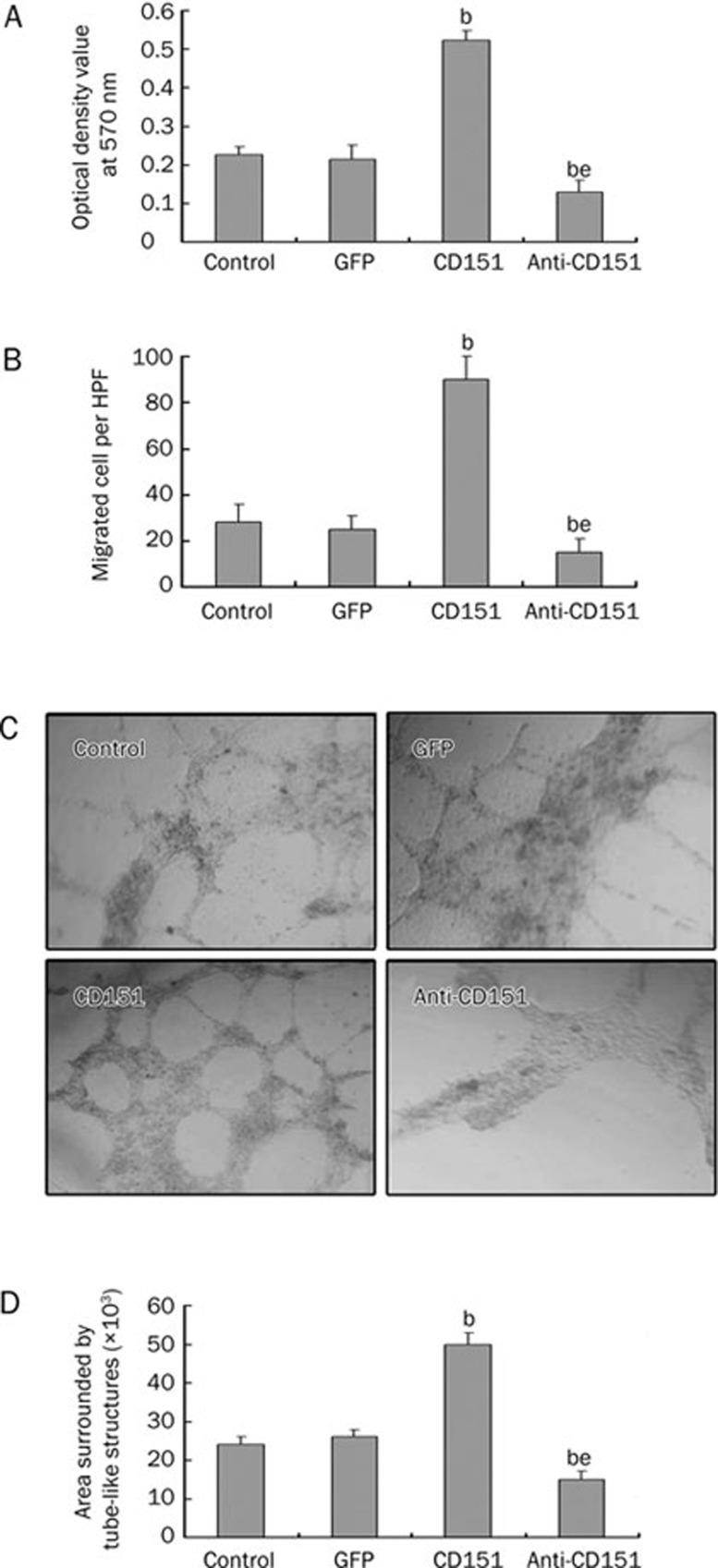
Endothelial cell proliferation, migration and tube formation are important processes in angiogenesis and vessel sprouting22. To determine the effects of rAAV-mediated CD151 gene transfection on HUVEC proliferation, migration and tube formation, we conducted experiments using the MTT assay, transwell Boyden chambers, and matrigel, respectively8.
First, the MTT assays showed that rAAV-CD151 transfection significantly enhanced the proliferation of HUVECs, whereas proliferation was significantly inhibited in the anti-CD151 group (Figure 2A). Next, in Boyden chamber assays, we observed that the number of migrated cells on the underside of the filter in the CD151 group was much greater than that in the control group and the GFP group (Figures 2B). In contrast, the anti-CD151 group showed less migration. Finally, we examined the effect of CD151 on tube formation. Matrigel tests demonstrated that rAAV-mediated CD151 gene transfection significantly increased tube formation compared with the control group and the GFP group. In contrast, the anti-CD151 group exhibited decreased tube formation (Figure 2C and 2D).
Figure 2.
CD151 gene delivery promotes the proliferation, migration, tube formation of HUVECs. (A) MTT assay results after HUVECs transfected with rAAV-CD151, rAAV-anti-CD151 and rAAV-GFP. rAAV-CD151 transfection significantly enhanced HUVECs proliferation as compared with the control and GFP groups. (B) Transwell boyden chamber assays showed rAAV-CD151 transfection promoted the migration of HUVECs. (C) and (D) Matrigel test demonstrated that rAAV-CD151 transfection dramatically increased the tube formation of HUVECs. (C) Representative photomicrographs showing effect of CD151 on tube formation (×100). (D) Quantitative analysis of the effect of CD151 on tube formation. bP<0.05 vs the control group and GFP group. eP<0.05 vs the CD151 group. n=3. Mean±SEM.
These results suggest that CD151 transfection promotes endothelial cell proliferation, migration and tube formation, but anti-CD151 transfection inhibits those effects.
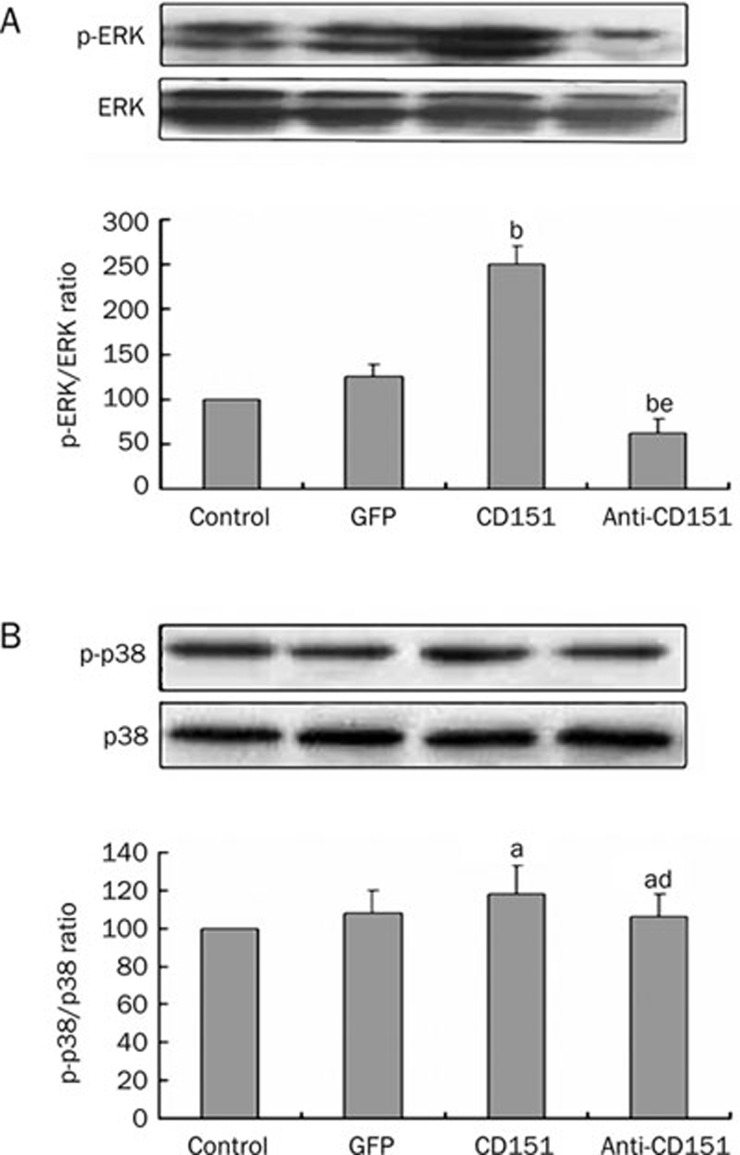
Effects of CD151 on the phosphorylation of ERK and p38
Western blot analysis revealed that transfection with rAAV-CD151 increased expression of phosphorylated ERK compared with the control group and the GFP group, whereas transfection with rAAV-anti-CD151 decreased expression of phosphorylated ERK (Figure 3A). However, there were no significant differences in the expression of phosphorylated p38 among the four groups (Figure 3B), suggesting that overexpression of CD151 may not influence the phosphorylation of p38. These data suggest that CD151 could activate the ERK signaling pathway, but it likely does not involve the p38 pathway.
Figure 3.
Effects of rAAV-CD151 transfection on the phosphorylation of ERK and p38. (A) Western blot analysis for ERK, phosphorylated ERK in control group and the rAAV-GFP, rAAV-CD151 and rAAV-anti-CD151 groups. rAAV-CD151 transfection increased the phosphorylation of ERK. (B) Western blot analysis showed rAAV-CD151 transfection did not influence the phosphorylation of p38. aP>0.05, bP<0.05 vs the control group and GFP group; dP>0.05, eP<0.05 vs the CD151 group. n=3. Mean±SEM.
Roles of ERK and p38 signaling pathways in CD151-induced cell proliferation, migration and tube formation
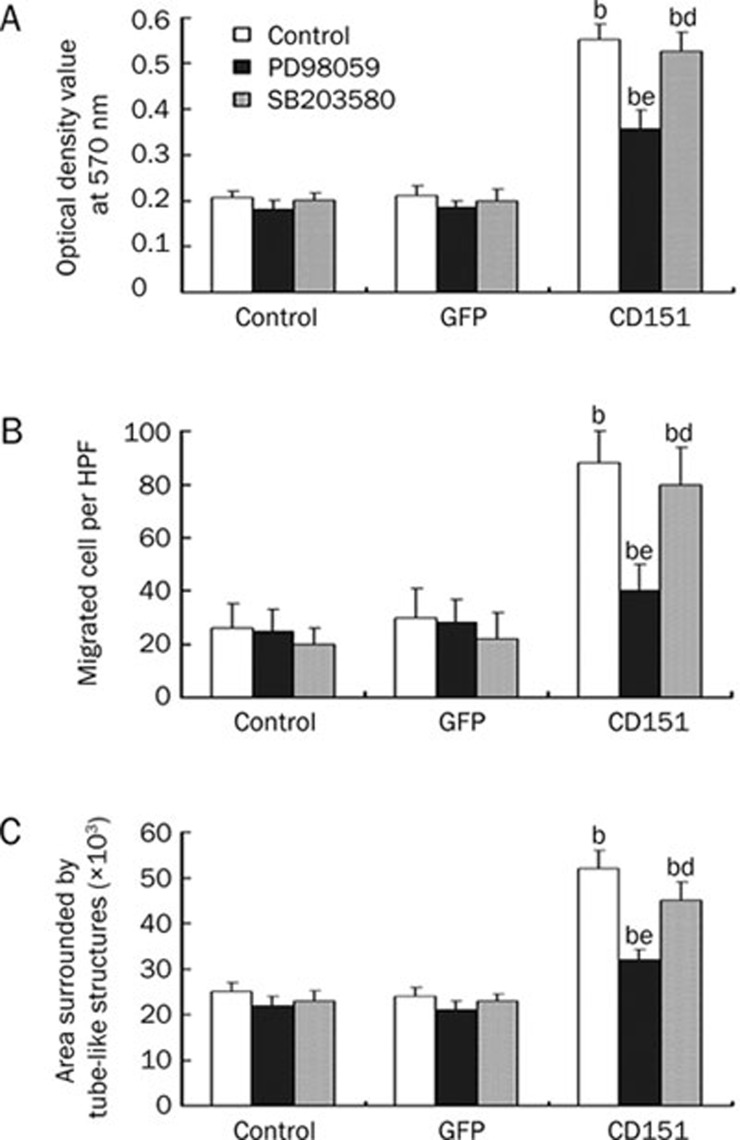
To investigate the roles of ERK and p38 in CD151-induced proliferation, migration, and tube formation of endothelial cells, we applied inhibitors of ERK (PD98059) and p38 (SB203580) to HUVEC cultures following transfection with rAAV- CD151.
We have shown that CD151 can promote endothelial cell proliferation, migration and tube formation. Treatment with the ERK inhibitor PD98059 resulted in attenuated effects of CD151 on proliferation, migration and tube formation (Figure 4A–4C). This suggests that ERK is involved in CD151-mediated cell responses. In contrast, treatment with the p38 inhibitor SB203580 did not significantly inhibit CD151-induced cell proliferation, migration and tube formation (Figure 4A–4C). Combined, these data suggest that CD151 enhances endothelial cell proliferation, migration and tube formation via the ERK signaling pathway, but p38 is likely not involved.
Figure 4.
rAAV-CD151 transfection promotes the proliferation, migration, tube formation of HUVECs via ERK signaling pathway, but not via p38. (A) MTT assays showing effects of inhibitors of ERK (PD98059, 20 μmol/L), and p38 inhibitor (SB203580, 20 μmol/L) on CD151-induced HUVECs proliferation. (B) Transwell Boyden chamber assays showing effects of inhibitors of ERK (PD98059, 20 μmol/L) and p38 inhibitor (SB203580, 20 μmol/L) on CD151 enhancement of HUVECs migration. (C) The effects of inhibitors of ERK (PD98059, 20 μmol/L), and p38 inhibitor (SB203580, 20 μmol/L) on CD151-induced HUVEC tube formation on matrigel. bP<0.05 vs the control and GFP groups. dP>0.05, eP<0.05 vs the CD151 group without inhibitors. n=3. Mean±SEM.
Effects of CD151-AAA mutant on CD151-induced cell proliferation, migration and tube formation
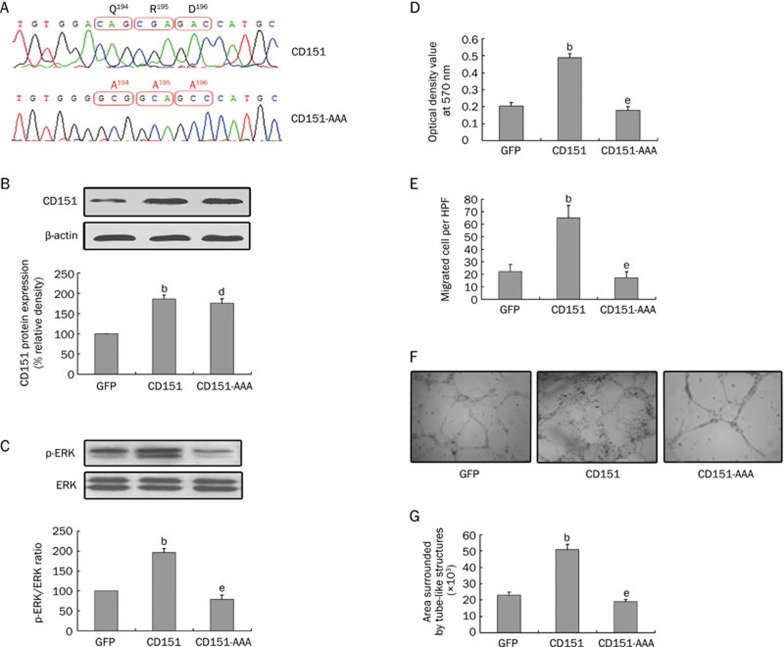
To investigate the function of CD151-integrin complexes, we first mutated the QRD194–196 motif of CD151 (glutamine, arginine, aspartic acid) to AAA194–196(three consecutive alanines), resulting in a mutant to which integrins cannot bind (Figure 5A). Western blot analysis showed that there was no significant difference in the expression of CD151 between the CD151 group and the CD151-AAA group, suggesting that the CD151-AAA mutant does not influence the expression of CD151 protein (Figure 5B). However, the level of phosphorylated ERK was significantly decreased in the CD151-AAA group compared with the CD151 group (Figure 5C). These data suggest that the CD151-AAA mutant results in reduced activation of ERK via phosphorylation.
Figure 5.
The effects of CD151-AAA on the proliferation, migration and tube formation of HUVECs. (A) The gene sequences of CD151 and CD151-AAA. The CD151 mutation (CD151-AAA) was undergone as follows: change the motif of QRD194–196 to AAA194–196. (B) Western blot analysis demonstrated there was no significant difference of CD151 protein expression between the CD151 group and the CD151-AAA group. (C) The effect of CD151-AAA on the phosphorylation of ERK. CD151 promoted the phosphorylation of ERK, but the CD151-AAA mutant abrogated this effect. (D) MTT assay showed that the CD151-induced proliferation of HUVECs was impaired in the CD151-AAA group. (E) Transwell Boyden chamber assays demonstrated the promoted migration of HUVECs was impaired in the CD151-AAA group. (F) and (G) The rAAV-CD151-AAA transfection abrogated the tube formation on matrigel. (F) Representative photomicrographs of tube formation. (G) Quantitative analysis of HUVECs tube formation. bP<0.05 vs the GFP group. dP>0.05, eP<0.05 vs the CD151 group. n=3. Mean±SEM.
Next, we further studied the proliferation, migration and tube formation of HUVECs transfected with rAAV-CD151-AAA. CD151-induced proliferation of HUVECs was impaired in the CD151-AAA group (Figure 5D). Similarly, transwell Boyden chamber assays demonstrated that the increase in migration observed in HUVECs treated with CD151 was also impaired in the CD151-AAA group (Figure 5E). CD151-AAA transfection decreased tube formation on matrigel compared with the CD151 group (Figure 5F and 5G). Together, these data suggest that delivery of the CD151-AAA mutant abrogates endothelial cell proliferation, migration and tube formation, which are promoted by CD151 gene delivery.
Discussion
Angiogenesis is the formation of new blood vessels from preexisting vessels. It is a physiological or pathological neovascularization process in response to tissue ischemia and/or tumor growth22. Angiogenesis is a complex process involving extracellular matrix degradation, endothelial cell proliferation and migration, formation of tube structures and morphological differentiation22, 23. Recently, mounting evidence supports the hypothesis that CD151 plays an important role in angiogenesis, in addition to its recognized ability to regulate the motility and adhesion of cells7, 8, 9, 10.
CD151 is broadly expressed in various cell types3, 4. Using an in vitro study, our group demonstrated that rAAV-mediated CD151 gene transfer increased the expression of CD151. To differentiate between endogenous and exogenous CD151, we constructed an HA-tag linked to the transfected CD151, and the reverse primer for RT-PCR was designed specifically for the HA-tag. Therefore, the CD151 mRNA was only detected in the CD151 group, but not in other groups (Figure 1B). This interesting technique could be used to differentiate between the endogenous gene and the transfected gene, which is also expressed in basal conditions. In the present study, we also observed that CD151 gene delivery promoted endothelial cell proliferation, migration and tube formation, which is consistent with our previous data8, 24. However, the molecular mechanisms underlying these effects are not known.
Previous studies have shown that activation of ERK is generally associated with cell proliferation, angiogenesis and tumor metastasis17, 25. p38 is usually activated by inflammatory cytokines and leads to cell apoptosis16, 18. Interestingly, several recent papers revealed different data about the activation of ERK or p38 in the CD151 signaling pathway6, 7, 26. In MelJuSo melanoma cells, inhibitors and small interfering RNAs targeted to p38 were shown to abrogate CD151-enhanced cell migration and adhesion6. However, another study found that absence of CD151 did not affect phosphorylation of components of the MAPK pathway, such as p38 and ERK7. It seems likely that ERK and p38 would show different responses in the CD151-related signaling pathway, depending on the cellular context. The present work demonstrated that overexpression of CD151 increased the expression of phospho-ERK, indicating that CD151 gene transfection could activate the ERK pathway.
To further determine the role of ERK in CD151-mediated biological processes, we examined the influence of the ERK inhibitor PD98059. The addition of PD98059 to cultures of HUVECs attenuated the CD151-induced proliferation, migration and tube formation of these cells. Therefore, ERK up-regulation may mediate, at least in part, the angiogenic effects of CD151. In contrast, we found that CD151 gene transfection did not influence the activation of p38, and an inhibitor of p38 (SB203580) did not significantly attenuate the CD151-mediated angiogenic effects.
The mechanisms by which CD151 could activate ERK are not currently known. Earlier studies have shown that CD151 forms multimolecular complexes with many other transmembrane proteins11, 14, 27. In particular, CD151 forms strong complexes with some integrins via the QRD motif194–196 12, 13. The formation of CD151-integrin complexes is reported to function as a “signal bridge” linking the “outside-in” signal transduction6, 28, 29, 30. Based on these data, we hypothesized that CD151 may also exert angiogenic effects through the formation of CD151-integrin complexes. Therefore, we mutated the QRD194–196 motif of CD151 to AAA194–196 in the present study, interfering with the formation of complexes between CD151 and integrins12, 13. Surprisingly, we found that the level of phospho-ERK in the CD151 mutant group was significantly decreased, but the level of CD151 protein was not significantly different in the CD151 and CD151 mutant (CD151-AAA) groups. These results provide perhaps the clearest evidence that impaired CD151-integrin complexes could significantly attenuate the phosphorylation of ERK, indicating that the formation of CD151-integrin complexes may be necessary for the activation of ERK.
Furthermore, the present study also revealed that cell proliferation, migration, and tube formation in the CD151 mutant group (CD151-AAA) were all decreased compared with the CD151 group. When CD151 could not complex with integrins, the effects of CD151 were all diminished. It seems that CD151-mediated promotion of angiogenesis depends on the formation of CD151-integrin complexes. Taken together, these data suggest that CD151 may activate the ERK signaling pathway and promote angiogenesis by forming complexes with integrins.
In summary, our data demonstrate that CD151 overexpression promotes endothelial cell proliferation, migration and tube formation via activation of the ERK signaling pathway. Furthermore, these effects are likely dependent on the formation of CD151-integrin complexes. To our knowledge, this is the most recent evidence showing ERK activation and the effects of CD151-integrin complexes in CD151-induced angiogenesis. This study provides clues to better understand the function and molecular mechanisms of CD151. Our observations may also lend insight that could assist in the development of CD151 as a novel therapeutic strategy for regulating angiogenesis.
Author contribution
Zheng-xiang LIU designed the research and handled funding; Hou-juan ZUO, Jing-yang LIN, Zhao-yu LIU performed the research, analyzed data and drafted the manuscript; Wei-feng LIU and Tao LIU performed the research; Jun YANG and Yu LIU analyzed data and drafted part of the manuscript; Dao-wen WANG contributed new reagents and analytical tools and revised the manuscript.
Acknowledgments
The project was supported by a grant from the National Natural Science Foundation of China (No 30670856).
We are grateful to Dr Xin ZHANG for providing the PzeoSV-CD151 plasmid (Department of Molecular Science, University of Tennessee Health Science Center).
References
- Fitter S, Tetaz TJ, Berndt MC, Ashman LK. Molecular cloning of cDNA encoding a novel platelet-endothelial cell tetra-span antigen, PETA-3. Blood. 1995;86:1348–55. [PubMed] [Google Scholar]
- Hasegawa H, Utsunomiya Y, Kishimoto K, Yanagisawa K, Fujita S. SFA-1, a novel cellular gene induced by human T-cell leukemia virus type 1, is a member of the transmembrane 4 superfamily. J Virol. 1996;70:3258–63. doi: 10.1128/jvi.70.5.3258-3263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincock PM, Mayrhofer G, Ashman LK. Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9, CD63, and alpha5beta1 integrin. J Histochem Cytochem. 1997;45:515–25. doi: 10.1177/002215549704500404. [DOI] [PubMed] [Google Scholar]
- Sincock PM, Fitter S, Parton RG, Berndt MC, Gamble JR, Ashman LK. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J Cell Sci. 1999;112:833–44. doi: 10.1242/jcs.112.6.833. [DOI] [PubMed] [Google Scholar]
- Zhang XA, Kazarov AR, Yang X, Bontrager AL, Stipp CS, Hemler ME. Function of the tetraspanin CD151-alpha6beta1 integrin complex during cellular morphogenesis. Mol Biol Cell. 2002;13:1–11. doi: 10.1091/mbc.01-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong IK, Jin YJ, Byun HJ, Jeoung DI, Kim YM, Lee H. Homophilic interactions of tetraspanin CD151 up-regulate motility and matrix metalloproteinase-9 expression of human melanoma cells through adhesion-dependent c-Jun activation signaling pathways. J Biol Chem. 2006;281:24279–92. doi: 10.1074/jbc.M601209200. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Kazarov AR, Butterfield CE, Hopkins BD, Benjamin LE, Kaipainen A, et al. Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood. 2007;109:1524–32. doi: 10.1182/blood-2006-08-041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZZ, Liu ZX. CD151 gene delivery increases eNOS activity and induces ECV304 migration, proliferation and tube formation. Acta Pharmacol Sin. 2007;28:66–72. doi: 10.1111/j.1745-7254.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- Zuo HJ, Liu ZX, Liu XC, Yang J, Liu T, Wen S, et al. Assessment of myocardial blood perfusion improved by CD151 in a pig myocardial infarction model. Acta Pharmacol Sin. 2009;30:70–7. doi: 10.1038/aps.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan RF, Liu ZX, Liu XC, Song YE, Wang DW. CD151 promotes neovascularization and improves blood perfusion in a rat hind-limb ischemia model. J Endovasc Ther. 2005;12:469–78. doi: 10.1583/04-1478R.1. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Berditchevski F, Harler MB, Reichner J, Hemler ME. Highly stoichiometric, stable, and specific association of integrin alpha3beta1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell. 1998;9:2751–65. doi: 10.1091/mbc.9.10.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Kazarov AR, Desai B, Lee RT, Hemler ME. Direct extracellular contact between integrin α3β1 and TM4SF protein CD151. J Biol Chem. 2000;275:9230–8. doi: 10.1074/jbc.275.13.9230. [DOI] [PubMed] [Google Scholar]
- Kazarov AR, Yang X, Stipp CS, Sehgal B, Hemler ME. An extracellular site on tetraspanin CD151 determines alpha 3 and alpha 6 integrin- dependent cellular morphology. J Cell Biol. 2002;158:1299–309. doi: 10.1083/jcb.200204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XA, Bontrager AL, Hemler ME. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J Biol Chem. 2001;276:25005–13. doi: 10.1074/jbc.M102156200. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Integrin-associated proteins. Curr Opin Cell Biol. 1998;10:578–85. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao T, Miyazaki H, et al. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler Thromb Vasc Biol. 2003;23:795–801. doi: 10.1161/01.ATV.0000066132.32063.F2. [DOI] [PubMed] [Google Scholar]
- Zhou QM, Wang S, Zhang H, Lu YY, Wang XF, Motoo Y, et al. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol Sin. 2009;30:1648–58. doi: 10.1038/aps.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R, Liu Z, Song Y, Zhang X. Effects of rAAV-CD151 and rAAV- antiCD151 on the migration of human tongue squamous carcinoma cell line Tca8113. J Huazhong Univ Sci Tech Med Sci. 2004;24:556–9. doi: 10.1007/BF02911353. [DOI] [PubMed] [Google Scholar]
- Wang T, Li H, Zhao C, Chen C, Li J, Chao J, et al. Recombinant adeno-associated virus-mediated kallikrein gene therapy reduces hypertension and attenuates its cardiovascular injuries. Gene Ther. 2004;11:1342–50. doi: 10.1038/sj.gt.3302294. [DOI] [PubMed] [Google Scholar]
- Wang H, Lin L, Jiang J, Wang Y, Lu ZY, Bradbury JA, et al. Up-regulation of endothelial nitric-oxide synthase by endothelium-derived hyperpolarizing factor involves mitogen-activated protein kinase and protein kinase C signaling pathways. J Pharmacol Exp Ther. 2003;307:753–64. doi: 10.1124/jpet.103.052787. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Buschmann I, Schaper W. Arteriogenesis versus angiogenesis: Two mechanisms of vessel growth. News Physiol Sci. 1999;14:121–25. doi: 10.1152/physiologyonline.1999.14.3.121. [DOI] [PubMed] [Google Scholar]
- Zheng ZZ, Liu ZX. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates CD151-induced endothelial cell proliferation and cell migration. Int J Biochem Cell Biol. 2007;39:340–8. doi: 10.1016/j.biocel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, et al. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther. 2005;314:522–32. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- Sawada S, Yoshimoto M, Odintsova E, Hotchin NA, Berditchevski F. The tetraspanin CD151 functions as a negative regulator in the adhesion-dependent activation of Ras. J Biol Chem. 2003;278:26323–6. doi: 10.1074/jbc.C300210200. [DOI] [PubMed] [Google Scholar]
- Ashman LK. Cd151. J Biol Regul Homeost Agents. 2002;16:223–6. [PubMed] [Google Scholar]
- Johnson JL, Winterwood N, DeMali KA, Stipp CS. Tetraspanin CD151 regulates RhoA activation and the dynamic stability of carcinoma cell-cell contacts. J Cell Sci. 2009;122:2263–73. doi: 10.1242/jcs.045997. [DOI] [PubMed] [Google Scholar]
- Shigeta M, Sanzen N, Ozawa M, Gu J, Hasegawa H, Sekiguchi K. CD151 regulates epithelial cell-cell adhesion through PKC- and Cdc42-dependent actin cytoskeletal reorganization. J Cell Biol. 2003;163:165–76. doi: 10.1083/jcb.200301075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo H, Liu Z, Liu X, Yang J, Liu T, Wen S, et al. CD151 gene delivery after myocardial infarction promotes functional neovascularization and activates FAK signaling. Mol Med. 2009;15:307–15. doi: 10.2119/molmed.2009.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]