Abstract
Aim:
To investigate the potential antidepressant and anxiolytic effects of Neu-P11, a novel melatonin agonist, in two models of depression in rats and a model of anxiety in mice.
Methods:
In the learned helplessness test (LH), Neu-P11 or melatonin (25–100 mg/kg, ip) was administered to rats 2 h before the beginning of the dark phase once a day for 5 days and the number of escape failures and intertrial crossings during the test phase were recorded. In the forced swimming test (FST), rats received a single or repeated administration of Neu-P11 (25–100 mg/kg, ip). The total period of immobility during the test phase was assessed. In the elevated plus-maze test (EPM), mice were treated with Neu-P11 (25–100 mg/kg, ip) or melatonin in the morning or in the evening and tested 2 h later. The percentage of time spent in the open arms and the open arms entries were assessed.
Results:
In the LH test, Neu-P11 but not melatonin significantly decreased the escape deficit and had no effect on the intertrial crossings. In the FST, a single or repeated administration of Neu-P11, either in the morning or in the evening, significantly decreased the duration of immobility. In the EPM test, Neu-P11 significantly increased the percentage of time spent in the open arms and the open arms entries irrespective to the time of administration. Melatonin was effective only when administered in the afternoon.
Conclusion:
The results demonstrate that Neu-P11 exerts antidepressant and anxiolytic activities in rodent models.
Keywords: melatonin, Neu-P11, antidepressant, anxiolytic, sleep disorders, stress-related illnesses, learned helplessness, forced swimming test, elevated plus-maze
Introduction
Depression is a serious mental disorder with a high prevalence worldwide. In the United States, depression affects about 17%–21% of the population1 and costs over 83 billion dollars per year2. Depressed patients have an increased risk of suicide3, premature death4 and other morbidity5. In recent years, there has been a dramatic increase in prescriptions of antidepressants that act by an interaction with serotonin and/or norepinepherine systems6. Antidepressants currently available produce tolerability and other safety related problems7, thus there is an unmet need for antidepressants with better efficacy and reduced side effects. Clinical and epidemiological studies suggest a strong association between sleep disorders and stress-related illnesses such as depression and anxiety8, 9, 10. After an early study found that depressed patients suffer from sleep disturbances (including a reduction in slow wave sleep, shortening of rapid eye movement sleep latency and increased rapid eye movement sleep)11, numerous subsequent studies confirmed that sleep disorders are commonly present together with depression12, 13, 14, 15. Sleep disturbances appear to be a major risk factor for depression14 but the issue of whether sleep disorders have a causal role in depression remains to be resolved.
Melatonin is synthesized and secreted at night mainly by the pineal gland in vertebrates. Melatonin exerts its action by binding to the specific G protein-coupled receptors MT1 and MT2 which are widely distributed in the central nervous system16. There is increasing evidence that the timing of melatonin secretion is closely associated with the timing of sleep propensity17. Melatonin administration shifts circadian rhythms in both rodents18 and humans19 and improves sleep by shortening sleep latency and lengthening sleep duration19. Melatonin has been used successfully in treating insomnia20 and various circadian rhythm disorders such as delayed sleep phase syndrome21 and shift-work sleep disorder22. Melatonin and its agonists exert antidepressant- and/or anxiolytic effects in animal models23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34. Clinical administration of melatonin or melatonin agonists has been effective in improving depressive symptoms35, 36.
Several melatonin agonists have been developed with modified properties in comparison to melatonin. At present only two of the numerous synthesized ligands of melatonin receptors are of therapeutic importance: agomelatine (Valdoxan®) – for the treatment of depression and ramelteon (Rozerem®) – for the treatment of primary insomnia characterized by difficulty with sleep onset. Recent phase II and phase III studies have demonstrated that tasimelteon (VEC-162; a high affinity agonist of human MT1 and MT2 receptors) may have therapeutic potential for transient insomnia in circadian rhythm sleep disorders37.
Neu-P1138, a novel melatonin agonist, is currently in development for the treatment of insomnia. Neu-P11 binds with high affinity to melatonin receptors and exerts GABA enhancing properties39. Recent studies have shown that Neu-P11 promoted sleep39, inhibited weight gain and improved insulin sensitivity in high-fat/high-sucrose-fed rats38. In the present study we investigated the potential effects of Neu-P11 in two models of depression in rats and one model of anxiety in mice.
Materials and methods
Animals
Adult male Sprague Dawley rats (200–270 g) and 2–3 month old male Kunming mice were obtained from the Laboratory Animal Center of University of South China, Hengyang, Hunan, China. The rats were housed individually and the mice were housed in groups of 6 animals per cage in a temperature and humidity controlled environment with free access to food and water. The animals were maintained on a 12 h light/dark schedule, with lights on at 7 AM. After being housed, the animals were handled (5–6 min per animal per day) for 1 week to habituate them to the experimenter. Experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and experimental protocols were approved by the University of South China Animal Care and Use Committee.
Drugs
Neu-P11 and melatonin were provided by Neurim Pharmaceuticals Ltd (Israel). Imipramine and diazepam were purchased from Sigma Co (St Louis, USA). Imipramine and diazepam were used as reference drug (positive control) for antidepressant and anxiolytic effects respectively. Neu-P11 and melatonin were homogenized in one drop of Tween-80 and diluted in sterile saline containing 1% dimethyl sulfoxide (vehicle) to the final volume immediately before administration. Imipramine and diazepam were dissolved in sterile saline immediately before administration.
Learned helplessness (LH) test in rats
Apparatus and Procedure
The LH procedure involves two phases24: shock pretraining and avoidance-escape training. During the shock pretraining phase, the rats (12 per treatment group) in the helpless group were placed into fear conditioning chambers (Shanghai Jiliang Software Technology Co Ltd, Shanghai, China) as previously described40, 41 and submitted to unsignalled inescapable foot shocks (0.8 mA, 15 s duration) given according to a variable time schedule with a mean interval of 60 s (ranging from 30–90 s) for 60 min. Thirty seconds after the final shock the rats were returned to their home cages. The rats in the non-helpless control (NHC) group were placed into the same chamber for 60 min but no shock was delivered during this time. During the avoidance-escape training phase (forty-eight hours after pretraining), all the rats were exposed to an avoidance-escape task in the automated two-way shuttle-boxes (Model, DCS-2; Institute of Materia Medica, Chinese Academy of Medical Sciences). Each box (64 cm×34 cm×24 cm) was divided into two compartments of equal size by a black Plexiglas partition containing an opening of 7×7 cm equipped with an electrified grid floor made of stainless steel rods. The rats were placed individually in the shuttle-box submitted to a test session. A 5 min environmental adaptation period was set before the beginning of 30 trials. A tone signal was presented for the first 5 s of each trial during which time the rats were allowed to avoid the shock (avoidance response). If no crossing occurred within this period, a footshock (8-second duration, 0.8 mA) was delivered. A single crossing from the electrified compartment to the other compartment made within this latter period was called an escape response. If no escape response occurred, tone and shock were turned off, and this was recorded as an escape failure (learned helplessness behavior). The shuttle-box test was repeated on day 4 and day 5, with no period of adaptation. During the test session, the number of escape failures and the number of intertrial crossings were recorded.
Experiment 1A
Experiment 1A was designed to assess the antidepressant effects of Neu-P11 in the LH model. Neu-P11 (25, 50, 100 mg/kg) and the reference compound imipramine (32 mg/kg) were administered ip repeatedly on 5 consecutive days (once per day). On day 1, Neu-P11 and imipramine were administered 6 h after the shock pretraining. On day 2–5, Neu-P11 was administered 2 h before the beginning of the dark phase, and imipramine was administered 60 min before the escape test. The same administration protocol was used for the administration of vehicle in the helpless control (HC) and NHC animals. Escape tests were performed in the morning on days 3–5.
Experiment 1B
Experiment 1B was designed to assess the antidepressant-like effects of melatonin in the LH model. In this experiment, training and drug administration schedule were as described in experiment 1A except that rats received melatonin (25, 50, 100 mg/kg) instead of Neu-P11.
Forced swimming test (FST) in rats
Apparatus and procedure
Four black Plexiglas cylinders were used for the FST. Each cylinder (height 40 cm, diameter 25 cm) was situated in a sound-attenuating cabinet which was located in an isolated room. Illumination was provided by a 15 W white house light mounted on the ceiling of cabinet, and a 65 dB background noise was supplied by a ventilation fan in the cabinet. The FST employed was similar to that described elsewhere25. Rats (12 per treatment group) were dropped individually into cylinders containing 20 cm of water maintained at about 25 °C and left for 15 min (pretest phase) or 5 min (test phase). The total period of immobility of each rat during the test phase was detected by a motion detector and analyzed by software provided by Shanghai Jiliang Software Technology Co Ltd, Shanghai, China.
Experiment 2A
Experiment 2A was designed to assess the antidepressant-like effects of single administration of Neu-P11 in the FST model. Vehicle, Neu-P11 (25, 50, 100 mg/kg) and imipramine (32 mg/kg) were administered ip at about 8:00 AM or 22:00 PM, Each group was divided into two subgroups for the FST, (n=12 per subgroup). Twenty-four hours after an initial 15 min pretest, the subgroups were submitted to a 5 min test 2 and 12 hours after administration respectively. The FST was performed in the morning.
Experiment 2B
Experiment 2B was designed to assess the antidepressant effects of repeated administration of Neu-P11 in the FST model. The subgroups who had received drug injection 12 h before the test phase were used for testing the effects of repeated Neu-P11 administration. Vehicle, Neu-P11 (25, 50, 100 mg/kg) and imipramine (32 mg/kg) were injected repeatedly on 6 consecutive days (once per day) 2 h before the beginning of the dark phase. The FST was performed in the morning of day 7.
Elevated plus-maze (EPM) test in mice
Apparatus and procedure
The EPM consisted of two open arms (30 cm×5 cm) and two closed arms (30 cm×5 cm×25 cm) joined to a common central platform (5 cm×5 cm) thus forming a cross. The entire apparatus was elevated 45 cm and a light was placed above the maze. The mice (12 per group) were taken from their home cages and transported to the apparatus for 5 min. Testing commenced with placing a mouse on the central platform facing an open arm. A mouse was considered to have entered an arm when all four legs were in the arm. The parameters observed were number of entries in the open and closed arms and time spent in the open arms. The number of entries in the open arms and time spent in the open arms were expressed as a percentage. The number of entries into the closed arms was considered as the locomotor activity index and the percentage of the time spent in the open arms and the percentage of the open arms entries as the anxiety index42, 43, 44.
Experiment 3A
Experiment 3A was designed to assess the dose-dependent anxiolytic effects of Neu-P11 in the EPM model. Vehicle, Neu-P11 (25, 50, 100 mg/kg) and the reference compound diazepam (2 mg/kg) were administered ip 2 h before the EPM test. The EPM test was performed in the morning.
Experiment 3B
Experiment 3B was designed to assess the time-dependent anxiolytic effects of Neu-P11 in the EPM model. Vehicle and Neu-P11 (50 mg/kg) were administered ip 30 min, 1 h, 2 h, or 3 h before the EMP test. The EPM test was performed in the morning.
Experiment 3C
Experiment 3C was designed to compare the effects of Neu-P11 to that of melatonin in the EPM model. Vehicle, Neu-P11 (50 mg/kg) and melatonin (50 mg/kg) were administered ip 2 h before the EMP test in the morning and in the afternoon respectively.
Statistical analyses
Statistical analyses were performed using one-way ANOVA or two-way ANOVA with or without repeated-measures (RM) (Sigma Stat 3.2). When a significant difference was found by two-way ANOVA a one-way ANOVA was performed. Post-hoc comparisons were performed with the Tukey HSD method. All data were represented as mean±SEM. Significant level was set at P<0.05.
Results
Learned helplessness test in rats
Experiment 1A
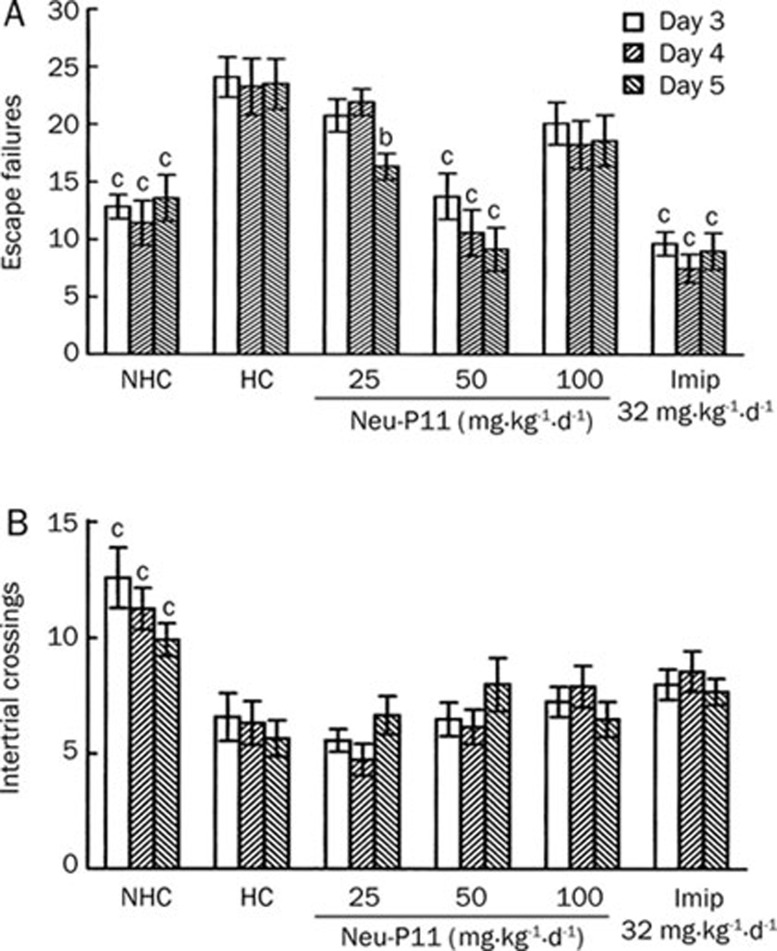
As shown in Figure 1A the HC rats presented an escape deficit on all 3 days of testing compared with the NHC rats. Imipramine treatment reversed the escape deficit observed in the HC rats over the 3 days of testing. The rats treated with Neu-P11 at 50 mg/kg showed a significant decrease of escape failures compared with HC animals over the 3 days of testing. We also found that compared with the HC rats, the rats treated with Neu-P11 at 25 mg/kg showed a significant decrease of escape failures on day 5. There was no significant difference in the level of escape failures between the rats treated with Neu-P11 at 100 mg/kg and the HC animals over all 3 days of testing. Only the NHC rats showed a significant increase in intertrial crossings over the 3 days of testing compared with HC animals (Figure 1B).
Figure 1.
Effects of Neu-P11 on the number of escape failures (A) and intertrial crossings (B) over the 3 days of testing in the learned helplessness model in rats. Neu-P11 (25, 50, or 100 mg/kg) and imipramine (32 mg/kg) were injected repeatedly on 5 consecutive days as described in the methods section. Escape tests were performed on day 3−5. bP<0.05, cP<0.01 vs the helpless control rats. NHC, non-helpless control; HC, helpless control; Imip, imipramine.
Experiment 1B
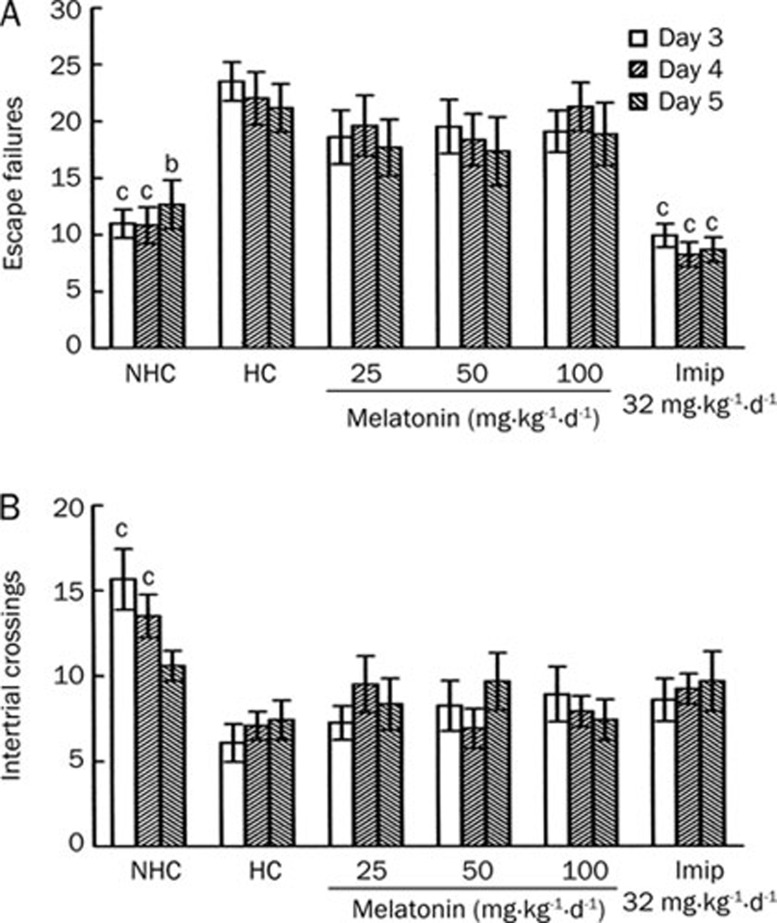
As observed in experiment 1A, compared with the NHC rats, the HC rats presented an escape deficit over all 3 days of testing, and imipramine treatment reversed the escape deficit observed in the HC rats over the 3 days of testing (Figure 2A). There was no significant difference in the level of escape failures between the rats treated with melatonin at any dose and the HC rats over all 3 days of testing. Only the NHC rats showed a significant increase in intertrial crossings compared with the HC rats on day 1 and 2 although this was not seen on day 3 (Figure 2B).
Figure 2.
Effects of melatonin on the number of escape failures (A) and intertrial crossings (B) over the 3 days of testing in the learned helplessness model in rats. Melatonin (25, 50, and 100 mg/kg) and imipramine (32 mg/kg) were injected repeatedly on 5 consecutive days as described in the methods section. Escape tests were performed on day 3–5. bP<0.05, cP<0.01 vs the helpless control rats. NHC, non-helpless control; HC, helpless control; Imip, imipramine.
Forced swimming test (FST) in rats
Experiment 2A
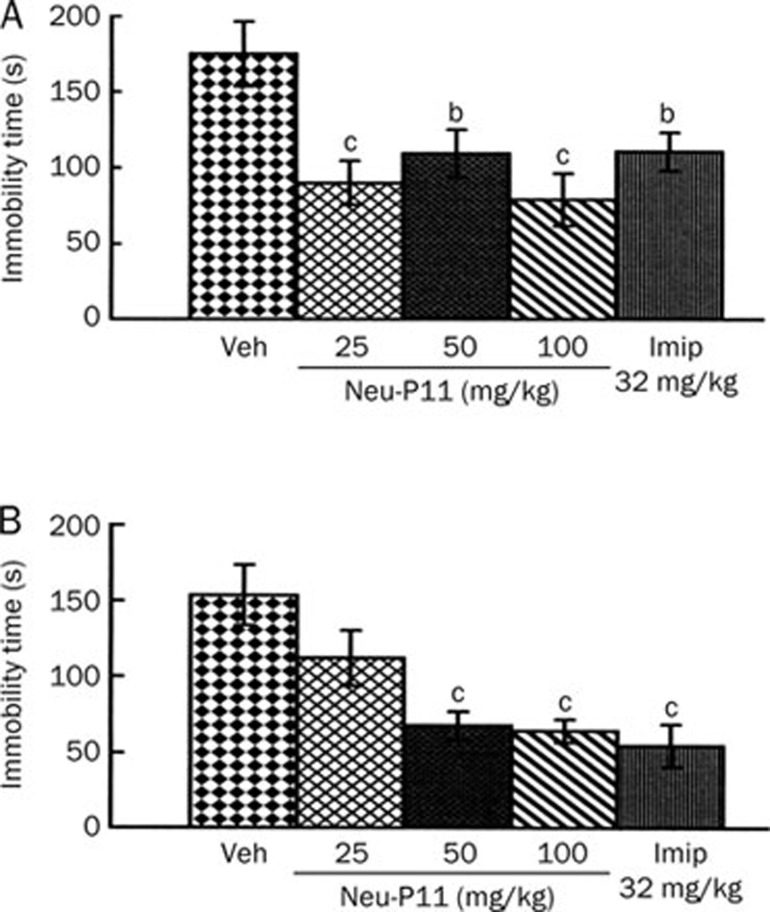
The effects of a single administration of Neu-P11 and imipramine are shown in Figure 3. When drugs were administered 2 h before test phase (Figure 3A), the rats treated with Neu-P11 at 25, 50, and 100 mg/kg showed less levels of immobility time compared with the vehicle control rats. The positive control rats treated with imipramine also presented a similar decrease of the immobility time. When drugs were injected 12 hours before test phase (Figure 3B), the rats treated with Neu-P11 at 50 and 100 mg/kg and imipramine showed lower levels of immobility time compared with the vehicle control rats.
Figure 3.
Effects of a single drug administration on the immobility time of rats in the forced swimming test. Neu-P11 (25, 50, and 100 mg/kg) and imipramine (32 mg/kg) were injected 2 h (A) or 12 h (B) before test phase, respectively. bP<0.05, cP<0.01 vs vehicle-treated rats. Veh, vehicle; Imip, imipramine.
Experiment 2B
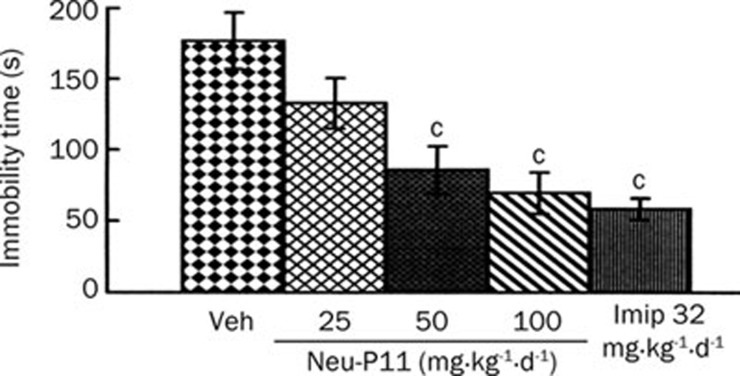
Repeated administration of Neu-P11 at 50 and 100 mg/kg significantly reduced the immobility time of rats compared with vehicle control (Figure 4). The repeated administration of imipramine produced a similar effect (Figure 4).
Figure 4.
Effects of a repeated drug administration on the immobility time of rats in the forced swimming test. Neu-P11 (25, 50, and 100 mg/kg) and imipramine (32 mg/kg) were injected repeatedly on 6 consecutive days (once per day), and the test was performed on day 7. cP<0.01 vs vehicle-treated rats. Veh, vehicle; Imip, imipramine.
Elevated plus-maze (EPM) test in mice
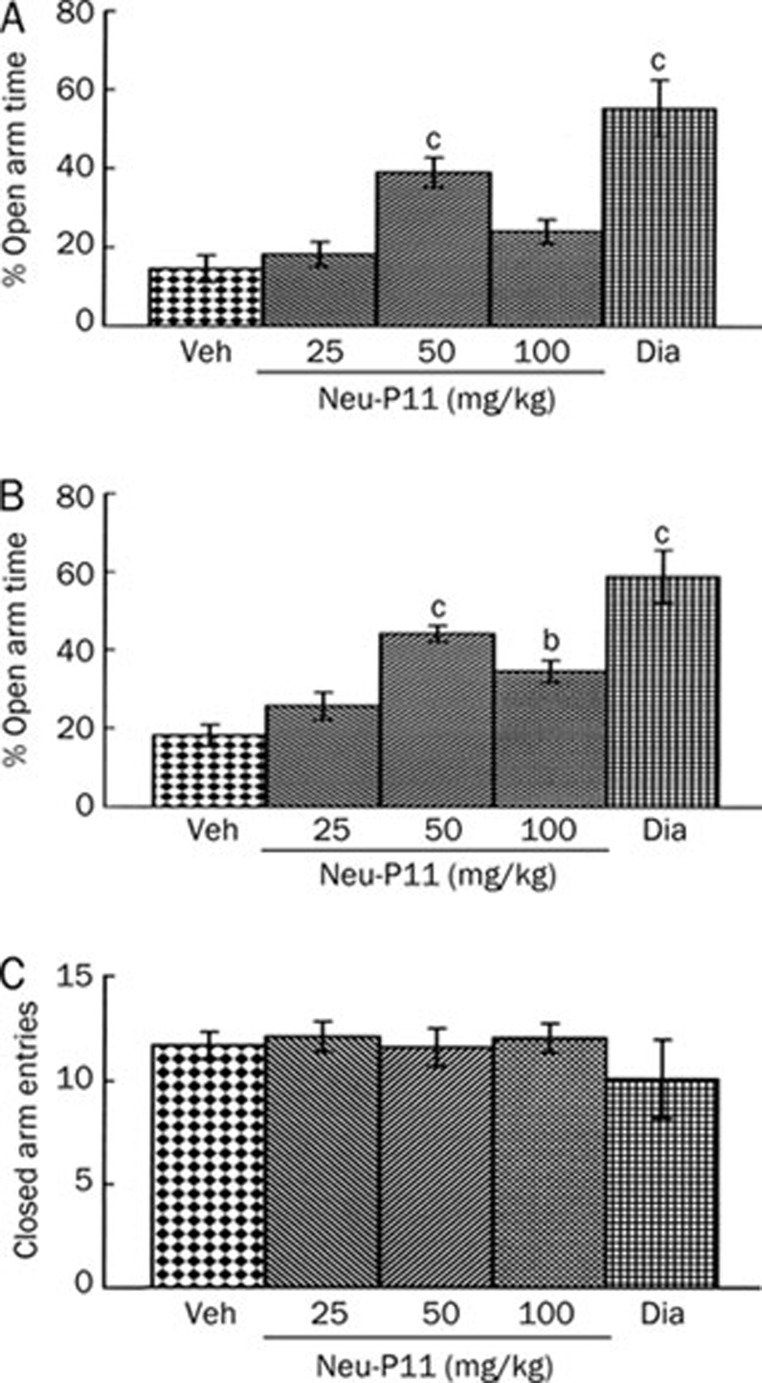
Experiment 3A
The dose-effects of Neu-P11 at 25, 50, and 100 mg/kg in the EPM test are shown in Figure 5. Compared with the vehicle control the mice treated with Neu-P11 at 50 mg/kg and diazepam showed higher levels of the percentage of time spent in the open arms (Figure 5A). The mice treated with Neu-P11 at 50 and 100 mg/kg and diazepam showed higher levels of the percentage of the open arms entries compared with the vehicle control (Figure 5B). There was no significant difference among the five groups for the number of the closed arms entries (Figure 5C).
Figure 5.
Dose-effects of a single drug administration on the percentage of time spent in the open arms (A), the percentage of the open arms entries (B) and the number of the closed arms entries (C) in the elevated plus-maze model in mice. Neu-P11 (25, 50, and 100 mg/kg) and diazepam (2 mg/kg) were injected 2 h prior to a 5 min test. bP<0.05, cP<0.01 vs vehicle-treated mice. Veh, vehicle; Dia, diazepam.
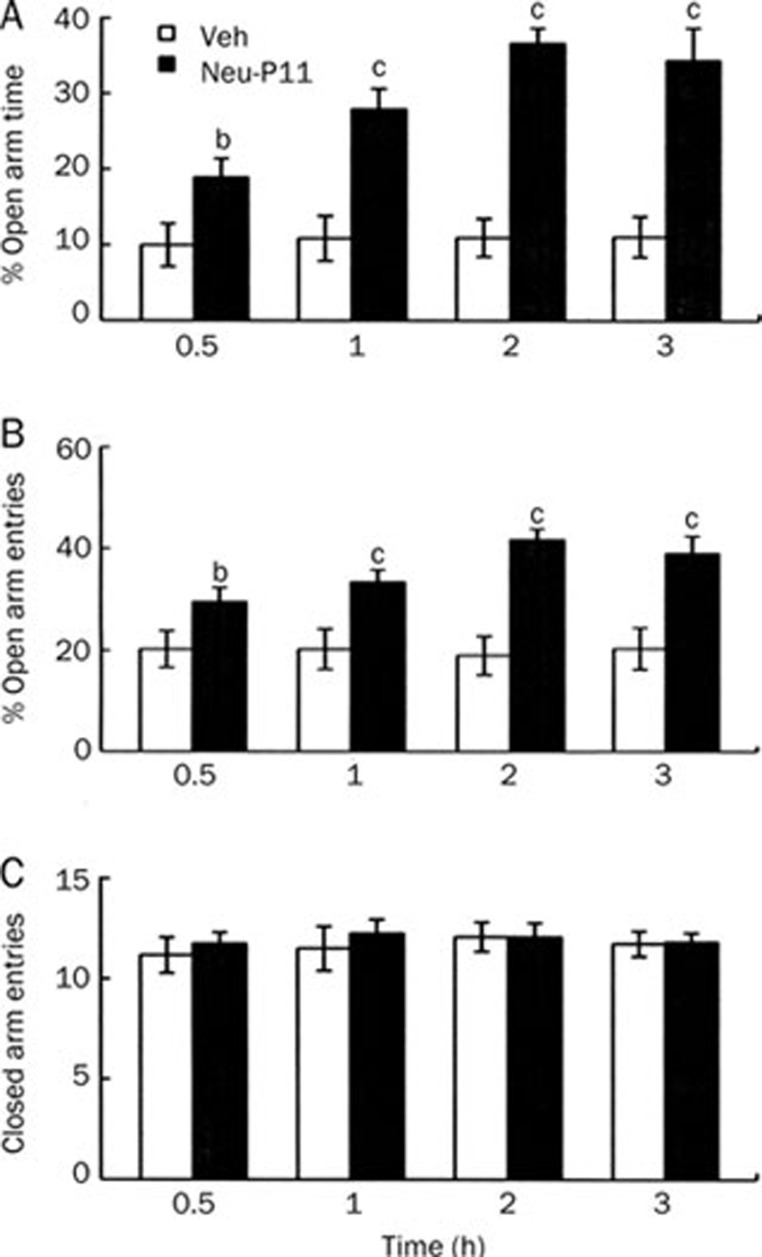
Experiment 3B
The time-effects of 50 mg/kg Neu-P11 in the EPM test are shown in Figure 6. Compared with the respective vehicle control the mice treated with Neu-P11 30 min, 1 h, 2 h or 3 h before the EPM test showed more time spent in the open arms (Figure 6A) and more open arms entries (Figure 6B). There was no significant effect of treatment for the number of the closed arms entries (Figure 6C).
Figure 6.
Time-effects of a single administration on the percentage of time spent in the open arms (A), the percentage of the open arms entries (B) and the number of the closed arms entries (C) in the elevated plus-maze model in mice. Neu-P11 (50 mg/kg) was injected 0.5 h, 1 h, 2 h, or 3 h prior to a 5-min test. bP<0.05, cP<0.01 vs vehicle-treated mice. Veh, vehicle.
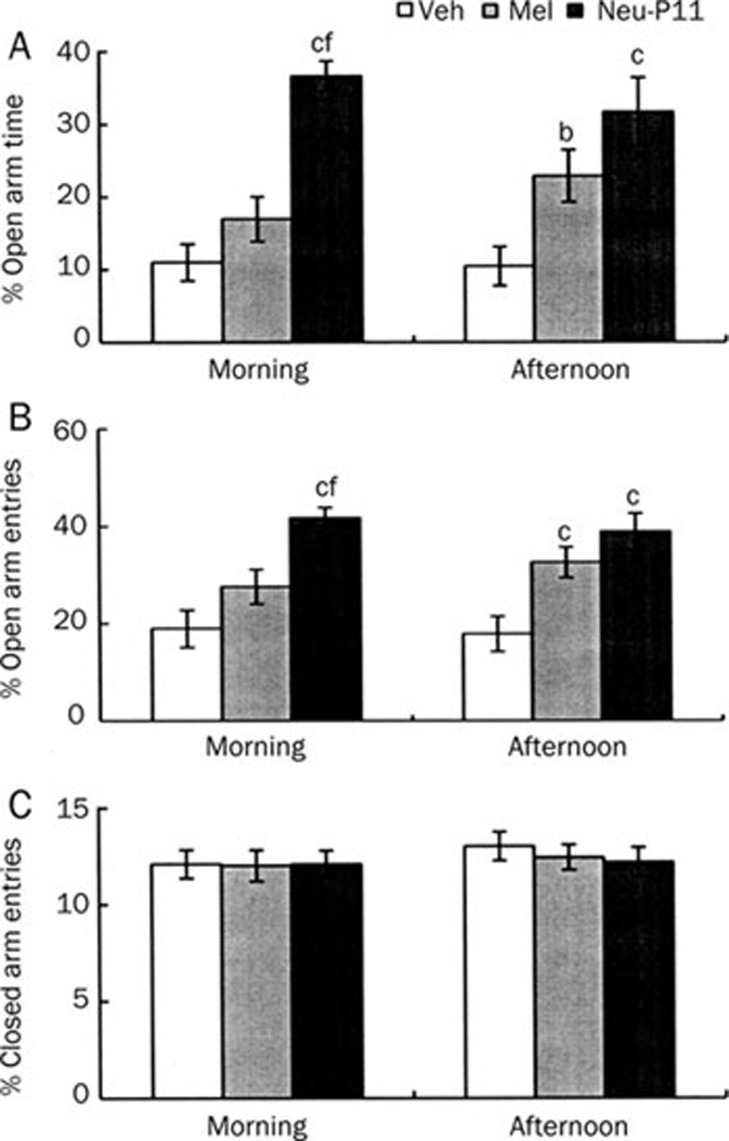
Experiment 3C
The effects of Neu-P11 and melatonin, both at 50 mg/kg in the EPM test are shown in Figure 7. Compared with the vehicle control the mice treated with Neu-P11 but not melatonin showed longer time spent in the open arms during the morning test session (Figure 7A). The mice treated with Neu-P11 spent more time in the open arms than the mice treated with melatonin. The mice treated with melatonin and the mice treated with Neu-P11 showed longer time spent in the open arms compared with the vehicle mice during the afternoon test session. The mice treated with Neu-P11 but not melatonin showed more open arms entries during the morning test compared with the vehicle control (Figure 7B). The mice treated with Neu-P11 presented higher levels of the open arms entries than mice treated with melatonin. both the mice treated with melatonin and Neu-P11 showed higher levels of the open arms entries compared with the vehicle control during the afternoon test. There was no significant effect of treatment for the number of the closed arms entries (Figure 7C).
Figure 7.
Effects of a single administration of Neu-P11 and melatonin on the percentage of time spent on the open arms (A), the percentage of the open arms entries (B) and the number of the closed arms entries (C) in the elevated plus-maze model in mice. Neu-P11 (50 mg/kg) and melatonin (50 mg/kg) was injected 2 h prior to a 5 min test in the morning or in the afternoon, respectively. bP<0.05, cP<0.01 vs vehicle-treated mice at the same test session. fP<0.01 vs melatonin-treated mice at the same test session. Veh, vehicle; Mel, melatonin.
Discussion
In the present study, we investigated the effects of Neu-P11 in two models of depression (LH and FST) in rats and one model of anxiety (EPM) in mice. Neu-P11 significantly decreased the level of escape failures in the LH model and reduced the duration of immobility in the FST model. In the EMP model, Neu-P11 significantly increased the percentage of the entries to and time spent in the open arms. The results suggest that Neu-P11 exerts antidepressant and anxiolytic activities in rodent models. Imipramine and diazepam were chosen as two positive reference compounds.
Neu-P11 has been reported previously to decrease body weight gain and improve insulin sensitivity and metabolic profiles in obese rats at 10 mg/kg in 8-weeks study38. In this study, the observed decrease in body weight with Neu-P11 was ∼5% in the first treatment week, similar to the effect of melatonin. It could be argued that Neu-P11-induced changes in body weight may produce a change in locomotor activity. However, our results did not support this argument. Melatonin appears to affect body weight of obese rats but did not affect body weight in normal rats45. In the present study we tested normal rats or mice exposed to Neu-p11 and melatonin for a relatively short time (0–6 days). Our drug treatment procedure did not affect locomotor activity indexes, number of intertrial crossings and number of closed arm entries in the LH model and EPM model, respectively. We therefore surmise that the antidepressant and anxiolytic effects of Neu-P11 are unlikely to be attributed to a change in locomotor activity that followed Neu-P11 injection.
The dose response of Neu-P11 in the LH and EPM models appears to be bell-shaped. This explains why the 50 mg/kg dose appears to be superior to the 100 mg/kg. This phenomenon remains poorly understood although many of the most important biological events show receptor desensitization. The bell-shaped curve of Neu-P11 dose-response could be attributed either to down regulation of melatonin receptors by the high dose or cross talk of neu-P11 in high dose with other unidentified systems that might affect the behavior of the rats.
Previous studies have shown that the antidepressant activity of melatonin depend on the type of the animal model and experimental procedure adopted. Melatonin has induced antidepressant-like effects in the tail suspension28 and chronic mild stress31 models but not in the learned helplessness model24. In the forced swimming model, repeated29, 33 but not single25 administration of melatonin exerted antidepressant-like activities. A similar dependence was found for the anxiolytic-like activity of melatonin, for example melatonin was effective in the animal model for a 'trait' anxiety but not for a 'state' anxiety32. Melatonin also appeared only to be active in depression and anxiety models when administered in the afternoon or dark phase of the light/dark cycle31, 32.
The present study demonstrated that the novel melatonin agonist Neu-P11 has antidepressant and anxiolytic properties similar to those of melatonin. Neu-P11 presented some activities distinguishable from those of melatonin. Neu-P11 was active persistently both in the LH and FST models. Neu-P11 activity was independent of the procedures of drug administration (single or repeated) in the FST test. Neu-P11 persistently displayed antidepressant properties (in the FST model) and anxiolytic properties in the EPM model whether administered during the morning or afternoon. The differential effects of Neu-P11 and melatonin in the antidepressant and anxiolytic activities can be explained by the following points: Neu-P11 may interact with serotoninergic receptors with better affinities as compared to melatonin. Neu-P11 metabolism may result in active metabolites that exert antidepressant and anxiolytic activities. Melatonin and Neu-P11 may have different pharmacokinetic values, that is, higher metabolic stability of Neu-P11 may result in longer or stronger effects compared to melatonin. Further studies are needed to examine these possibilities.
The exact mechanisms underlying the antidepressant and anxiolytic activities of melatonin agonists are not well established. In previous studies, γ-aminobutiric acid (GABA), serotonin (5-HT), glutamate and nitric oxide (NO) pathways have been indicated in brain melatonin effects46, 47, 48, 49. There is increasing evidence that melatonin is involved in the regulation of GABA-benzodiazepine receptor complex. Melatonin administration has been shown to modify GABA-benzodiazepine binding to brain membranes50, 51 and increase of GABA turnover rate and GABA-induced chloride influx52, 53. In line with the GABAergic hypothesis of mental disorders, several reports have indicated that GABA-mimetic drugs have antidepressant and anxiolytic properties26, 33, 54 and administration of a benzodiazepine receptor antagonist blunted melatonin activity26, 33. Recent evidence also shows that the antidepressant effect of melatonin could be mediated through an interaction with N-methyl-D-aspartate (NMDA) receptors and the L-arginine/NO pathway55.
It is well documented that 5-HT neurotransmission is involved in anxiety and depression. Preclinical and clinical studies have shown that 5-HT1A receptor agonists have antidepressant activity56, 57. Intracerebroventricular injection of mRNA antisense to 5-HT2A receptors or systemic injection of a selective 5-HT2A receptor antagonist produces antidepressant effects in rodent models58, 59. Agomelatine, a potent melatonin receptor agonist, also acts as an antagonist at 5-HT2C receptors and exerts antidepressant activities in several animal models23, 24, 25, 31. Several antidepressants such as tricyclics, mianserin, mirtazapine and trazodone have been known to display moderate to high affinity for 5-HT2C receptors60, 61. Melatonin has been shown to act as a 5-HT2A antagonist62 and regulates both spontaneous efflux and evoked release of 5-HT in the hippocampus63. These studies suggest that an interaction with the central serotonergic system is involved in the antidepressant and anxiolytic activities of melatonin and its analogues.
In summary we have demonstrated that Neu-P11, a novel melatonin agonist, exerts antidepressant and anxiolytic activities with persistent and time-independent characters in several rodent models. We suggest that these central nervous system effects of Neu-P11 are due to an interaction with central neurotransmitters such as GABA and 5-HT. Although the mechanisms by which Neu-P11 exerts its effects are under investigation, the current data suggest that Neu-P11 may serve as a novel agent for the treatment of affective disorders.
Author contribution
Shao-wen TIAN and Moshe LAUDON designed the study, performed the research and wrote the paper. Li HAN, Jun GAO, Fu-lian HUANG, Yu-feng YANG, and Hai-feng DENG performed some of the research.
Acknowledgments
This study was supported by A Project 30770689 Supported by National Natural Science Foundation of China and Neurim Pharmaceuticals Research Grant to S Tian. The authors wish to thank Dr Matthew LEWIS for his editorial help.
References
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–51. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000. J Clin Psychiatry. 2003;64:1465–75. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Murphy SL. Deaths: final data for 1998. Natl Vital Stat Rep. 2000;48:1–106. [PubMed] [Google Scholar]
- Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Shapiro PA. Depression and the course of coronary artery disease. Am J Psychiatry. 1998;155:4–11. doi: 10.1176/ajp.155.1.4. [DOI] [PubMed] [Google Scholar]
- Lenox RH, Frazer A.Mechanism of action of antidepressants and mood stabilizersIn: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: the fifth generation of progress. Philadelphia: Williams & Wilkins; 2002. p 1139–63.
- Nash J, Nutt D. Antidepressants. Psychiatry. 2007;6:289–94. [Google Scholar]
- Hudson JL, Gradisar M, Gamble A, Schniering CA, Rebelo I. The sleep patterns and problems of clinically anxious children. Behav Res Ther. 2009;47:339–44. doi: 10.1016/j.brat.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Srinivasan V, Pandi-Perumal SR, Trakht I, Spence DW, Hardeland R, Poeggeler B, et al. Pathophysiology of depression: role of sleep and the melatonergic system. Psychiat Res. 2009;165:201–14. doi: 10.1016/j.psychres.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Nakao M, Nomura K, Yano E. Association of metabolic syndrome with depression and anxiety in Japanese men. Diabetes Metab. 2009;35:32–6. doi: 10.1016/j.diabet.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Spiker DG, Coble PA, Neil JF, Ulrich R, Shaw DH. Sleep and treatment prediction in endogenous depression. Am J Psychiat. 1981;138:429–34. doi: 10.1176/ajp.138.4.429. [DOI] [PubMed] [Google Scholar]
- Armitage R, Hoffmann RF. Sleep EEG, depression and gender. Sleep Med Rev. 2001;5:237–46. doi: 10.1053/smrv.2000.0144. [DOI] [PubMed] [Google Scholar]
- Hubain P, Le Bon O, Vandenhende F, Van Wijnendaele R, Linkowski P. Major depression in males: effects of age, severity and adaptation on sleep variables. Psychiat Res. 2006;145:169–77. doi: 10.1016/j.psychres.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Lustberg L, Reynolds CF. Depression and insomnia: questions of cause and effect. Sleep Med Rev. 2000;4:253–62. doi: 10.1053/smrv.1999.0075. [DOI] [PubMed] [Google Scholar]
- Riemann D, Berger M, Voderholzer U. Sleep and depression-results from psychobiological studies: an overview. Biol Psychol. 2001;57:67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–10. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- Redman J, Armstrong S, Ng KT. Free-running activity rhythms in the rat: entrainment by melatonin. Science. 1983;219:1089–91. doi: 10.1126/science.6823571. [DOI] [PubMed] [Google Scholar]
- Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9:25–39. doi: 10.1016/j.smrv.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Nir T, Laudon M, Zisapel N. Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J Sleep Res. 2007;16:372–80. doi: 10.1111/j.1365-2869.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- Dahlitz M, Alvarez B, Vignau J, English J, Arendt J, Parkes JD. Delayed sleep phase syndrome response to melatonin. Lancet. 1991;337:1121–4. doi: 10.1016/0140-6736(91)92787-3. [DOI] [PubMed] [Google Scholar]
- Srinivasan V, Smits G, Kayumov L, Pandi-Perumal SR, Cardinali DP, Thorpy MJ.Melatonin in circadian rhythm sleep disordersIn: Cardinali DP, Pandi-Perumal SR, editors. Neuroendocrine correlates of sleep/wakefulness. New York: Springer; 2006. p 269–94.
- Barden N, Shink E, Labbé M, Vacher R, Rochford J, Mocaёr E. Antidepressant action of agomelatine (S 20098) in a transgenic mouse model. Prog Neuro-Psychoph Biol Psychiat. 2005;29:908–16. doi: 10.1016/j.pnpbp.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Bertaina-Anglade V, Rochelle CD, Boyer PA, Mocaёr E. Antidepressant-like effects of agomelatine (S 20098) in the learned helplessness model. Behav Pharmacol. 2006;17:703–13. doi: 10.1097/FBP.0b013e3280116e5c. [DOI] [PubMed] [Google Scholar]
- Bourin M, Mocaer E, Porsolt R. Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors. J Psychiatry Neurosci. 2004;29:126–33. [PMC free article] [PubMed] [Google Scholar]
- Golombek DA, Martini M, Cardinali DP. Melatonin as an anxiolytic in rats: time dependence and interaction with the central GABAergic system. Eur J Pharmacol. 1993;237:231–6. doi: 10.1016/0014-2999(93)90273-k. [DOI] [PubMed] [Google Scholar]
- Kopp C, Vogel E, Rettori MC, Delagrange P, Misslin R. Anxiolytic-like properties of melatonin receptor agonists in mice: involvement of mt1 and/or MT2 receptors in the regulation of emotional responsiveness. Neuropharmacology. 2000;39:1865–71. doi: 10.1016/s0028-3908(99)00263-4. [DOI] [PubMed] [Google Scholar]
- Mantovani M, Michel Bonetti K, Calixto JB, Santos AR, Rodrigues ALS. Antidepressant-like effect of melatonin in the tail suspension test in mice: evidence for involvement of N-methyl-D-aspartate receptors and the L-arginine-nitric oxide pathway. Neurosci Lett. 2003;343:1–4. doi: 10.1016/s0304-3940(03)00306-9. [DOI] [PubMed] [Google Scholar]
- Micale V, Arezzi A, Rampello L, Drago F. Melatonin affects the immobility time of rats in the forced swim test: The role of serotonin neurotransmission. Eur Neuropsychopharm. 2006;16:538–45. doi: 10.1016/j.euroneuro.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Pucilowski O, Retton MC, Delagrande P, Guardiola-Lemaitre B. Effects of melatonin receptor ligands on swim test immobility. Neuroreport. 1998;9:249–53. doi: 10.1097/00001756-199801260-00014. [DOI] [PubMed] [Google Scholar]
- Papp M, Gruca P, Boyer PA, Mocaёr E. Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacol. 2003;28:694–703. doi: 10.1038/sj.npp.1300091. [DOI] [PubMed] [Google Scholar]
- Papp M, Litwa E, Gruca P, Mocaёr E. Anxiolytic-like activity of agomelatine and melatonin in three animal models of anxiety. Behav Pharmacol. 2006;17:9–18. doi: 10.1097/01.fbp.0000181601.72535.9d. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Kaur G, Kulkarni SK. Anti-depressant action of melatonin in chronic forced swimming-induced behavioral despair in mice, role of peripheral benzodiazepine receptor modulation. Eur Neuropsychopharm. 2000;10:473–81. doi: 10.1016/s0924-977x(00)00115-2. [DOI] [PubMed] [Google Scholar]
- Shaji AV, Kulkarni SK. Central nervous system depressant activities of melatonin in rats and mice. Ind J Exp Biol. 1998;36:257–63. [PubMed] [Google Scholar]
- Dalton EJ, Rotondi D, Levitan RD, Kennedy SH, Brown GM. Use of slow-release melatonin in treatment-resistant depression. J Psychiatry Neurosci. 2000;25:48–52. [PMC free article] [PubMed] [Google Scholar]
- Norman TR, Burrows GD. Emerging treatments for major depression. Expert Rev Neurother. 2007;7:203–13. doi: 10.1586/14737175.7.2.203. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. J Pharmacol Rep. 2009;61:383–410. doi: 10.1016/s1734-1140(09)70081-7. [DOI] [PubMed] [Google Scholar]
- She M, Deng X, Guo Z, Laudon M, Hu Z, Liao D, et al. Neu-P11, a novel melatonin agonist, inhibits weight gain and improves insulin sensitivity in high-fat/high-sucrose-fed rats. Pharmacol Res. 2009;59:248–53. doi: 10.1016/j.phrs.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Laudon M, Urade Y, Huang Z. Neu-P11, a novel melatonin agonist: effects on sleep and EEG power spectra in rats. Sleep. 2008;31 Suppl:A34–35. [Google Scholar]
- Fu J, Li P, Ouyang X, Gu C, Song Z, Gao J, et al. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 2007;144:1186–92. doi: 10.1016/j.neuroscience.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Gu C, Li P, Hu B, Ou-yang X, Fu J, Gao J, et al. Chronic morphine selectively impairs cued fear extinction in rats: implications for anxiety disorders associated with opiate use. Neuropsychopharmacology. 2008;33:666–73. doi: 10.1038/sj.npp.1301441. [DOI] [PubMed] [Google Scholar]
- Raupp IM, Sereniki A, Virtuoso S, Ghislandi C, Silva ELC, Trebien HA, et al. Anxiolytic-like effect of chronic treatment with Erythrina velutina extract in the elevated plus-maze test. J Ethnopharmacol. 2008;118:295–9. doi: 10.1016/j.jep.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Vargas KM, Da Cunha C, Andreatini R. Amphetamine and pentylenetetrazole given post-trial 1 enhance one-trial tolerance to the anxiolytic effect of diazepam in the elevated plus-maze in mice. Prog Neuro-Psychoph Biol Psychiat. 2006;30:1394–402. doi: 10.1016/j.pnpbp.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang H, Ye N, Zhang J, Wu Q, Sun P, et al. Synthesis of dihydrofuroaporphine derivatives: identification of a potent and selective serotonin 5-HT1a receptor agonist. J Med Chem. 2010;53:1319–28. doi: 10.1021/jm9015763. [DOI] [PubMed] [Google Scholar]
- Mustonen AM, Nieminen P, Hyvärinen H. Effects of continuous light and melatonin treatment on energy metabolism of the rat. J Endocrinol Invest. 2002;25:716–23. doi: 10.1007/BF03345106. [DOI] [PubMed] [Google Scholar]
- Gaffori O, Van Ree JM. Serotonin and antidepressant drugs antagonize melatonin-induced behavioural changes after injection into the nucleus accumbens of rats. Neuropharmacology. 1985;24:237–44. doi: 10.1016/0028-3908(85)90080-2. [DOI] [PubMed] [Google Scholar]
- Eison AS, Freeman P, Guss VB, Mullins UL, Wright RN. Melatonin agonists modulate 5-HT2a receptor-mediated neurotransmission: behavioral and biochemical studies in the rat. J Pharmacol Exp Ther. 1995;273:304–8. [PubMed] [Google Scholar]
- Raghavendra V, Kaur G, Kulkarni SK. Anti-depressant action of melatonin in chronic forced swimming-induced behavioral despair in mice, role of peripheral benzodiazepine receptor modulation. Eur Neuropsychopharmacol. 2000;10:473–81. doi: 10.1016/s0924-977x(00)00115-2. [DOI] [PubMed] [Google Scholar]
- Hill MN, Brotto LA, Lee TT, Gorzalka BB. Corticosterone attenuates the antidepressant-like effects elicited by melatonin in the forced swim test in both male and female rats. Prog Neuro-psychopharmacol Biol Psychiatry. 2003;27:905–11. doi: 10.1016/S0278-5846(03)00149-0. [DOI] [PubMed] [Google Scholar]
- AcuÑa-Castroviejo D, Rosenstein RE, Romeo HE, Cardinali DP. Changes in gamma-aminobutyric acid high affinity binding to cerebral cortex membranes after pinealectomy or melatonin administration to rats. Neuroendocrinology. 1986;43:24–31. doi: 10.1159/000124504. [DOI] [PubMed] [Google Scholar]
- Niles LP, Pickering DS, Arxiszewski MA. Effect of chronic melatonin administration on GABA and diazepam binding in rat brain. J Neural Transm. 1987;70:117–24. doi: 10.1007/BF01252513. [DOI] [PubMed] [Google Scholar]
- Rosenstein RE, Cardinali DP. Melatonin increases in vivo GABA accumulation in rat hypothalamus, cerebellum, cerebral cortex and pineal gland. Brain Res. 1986;398:403–6. doi: 10.1016/0006-8993(86)91505-2. [DOI] [PubMed] [Google Scholar]
- Rosenstein RE, Estevez AG, Cardinali DP. Time-dependent effect of melatonin on glutamic acid decarboxylase activity and 36Cl− influx in rat hypothalamus. J Neuroendocrinol. 1989;1:443–7. doi: 10.1111/j.1365-2826.1989.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Joly D, Deportere H, Zivkovic B, Lloyd KG.Behavioral profile of progabide in test for anxiolytic and anti-depressant drugsIn: Bartholini G, Lloyd KG, Morselli PL, editors. GABA and mood disorders. New York: Raven Press; 1996. p 77–84.
- Mantovani M, Michel Bonetti K, Calixto JB, Santos AR, Rodrigues ALS. Antidepressant-like effect of melatonin in the tail suspension test in mice: evidence for involvement of N-methyl-D-aspartate receptors and the L-arginine-nitric oxide pathway. Neurosci Lett. 2003;343:1–4. doi: 10.1016/s0304-3940(03)00306-9. [DOI] [PubMed] [Google Scholar]
- Deakin J. A review of clinical efficacy of 5-HT1a agonists in anxiety and depression. J Psychopharmacol. 1993;7:283–9. doi: 10.1177/026988119300700308. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Commissaris RC, De La Garza II R, File SE, Knapp DJ, Seiden LS. Involvement of 5-HT1a receptors in animal tests of anxiety and depression: evidence from genetic models. Stress. 2003;6:101–10. doi: 10.1080/1025389031000111311. [DOI] [PubMed] [Google Scholar]
- Sibille E, Sarnyai Z, Benjamin D, Gal J, Baker H, Toth M. Antisense inhibition of 5-hydroxytryptamine2a receptor induces an antidepressant-like effect in mice. Mol Pharmacol. 1997;52:1056–63. doi: 10.1124/mol.52.6.1056. [DOI] [PubMed] [Google Scholar]
- Patel JG, Bartoszyk GD, Edwards E, Ashby CR., Jr The highly selective 5-hydroxytryptamine (5-HT) 2a receptor antagonist, EMD 281014, significantly increases swimming and decreases immobility in male congenital learned helpless rats in the forced swim test. Synapse. 2004;52:73–5. doi: 10.1002/syn.10308. [DOI] [PubMed] [Google Scholar]
- Jenck F, Bos M, Wichmann J, Stadler H, Martin JR, Moreau JL. The role of 5-HT2c receptors in affective disorders. Exp Opin Invest Drugs. 1998;7:1587–99. doi: 10.1517/13543784.7.10.1587. [DOI] [PubMed] [Google Scholar]
- Pinder RM. The pharmacologic rationale for the clinical use of antidepressants. J Clin Psychiatry. 1997;58:501–8. doi: 10.4088/jcp.v58n1108. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Brotto LA, Hong JJ. Corticosterone regulation of 5-HT2a receptor-mediated behaviors: attenuation by melatonin. Physiol Behav. 1999;67:439–42. doi: 10.1016/s0031-9384(99)00096-7. [DOI] [PubMed] [Google Scholar]
- Monnet FP. Melatonin modulates [3H]serotonin release in the rat hippocampus: effects of circadian rhythm. J Neuroendocrinol. 2002;14:194–9. doi: 10.1046/j.0007-1331.2001.00761.x. [DOI] [PubMed] [Google Scholar]