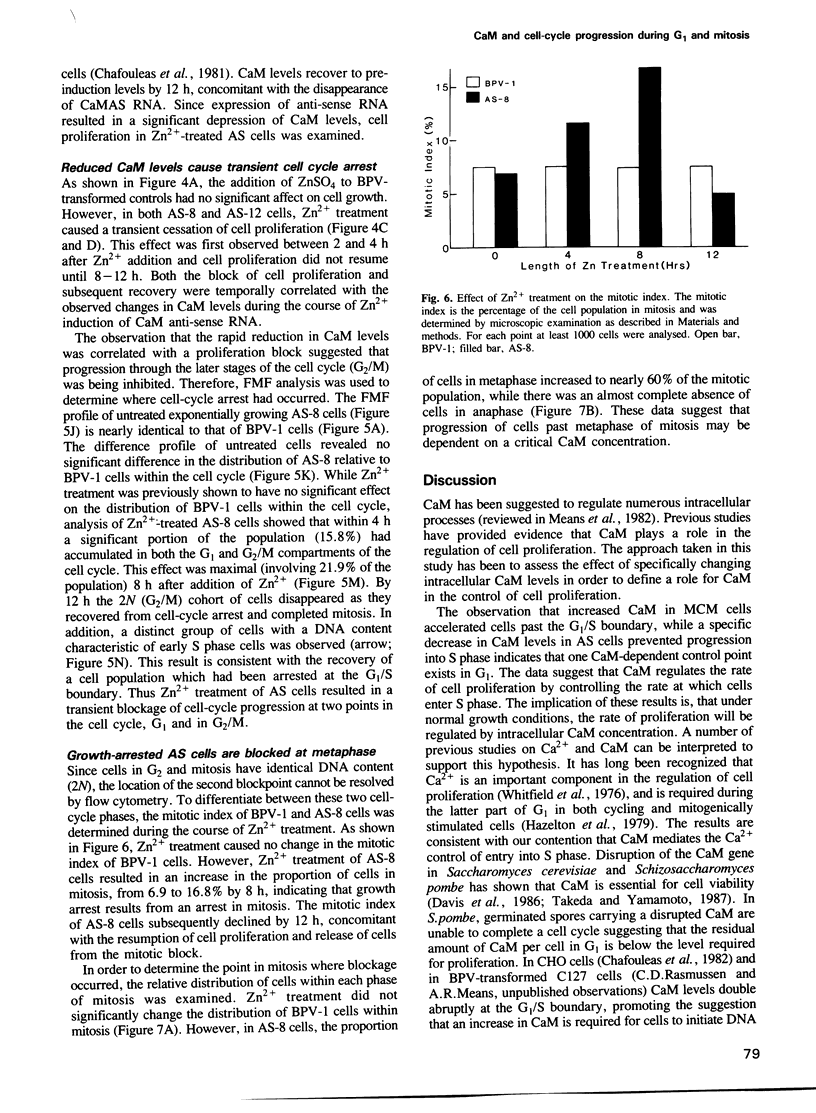
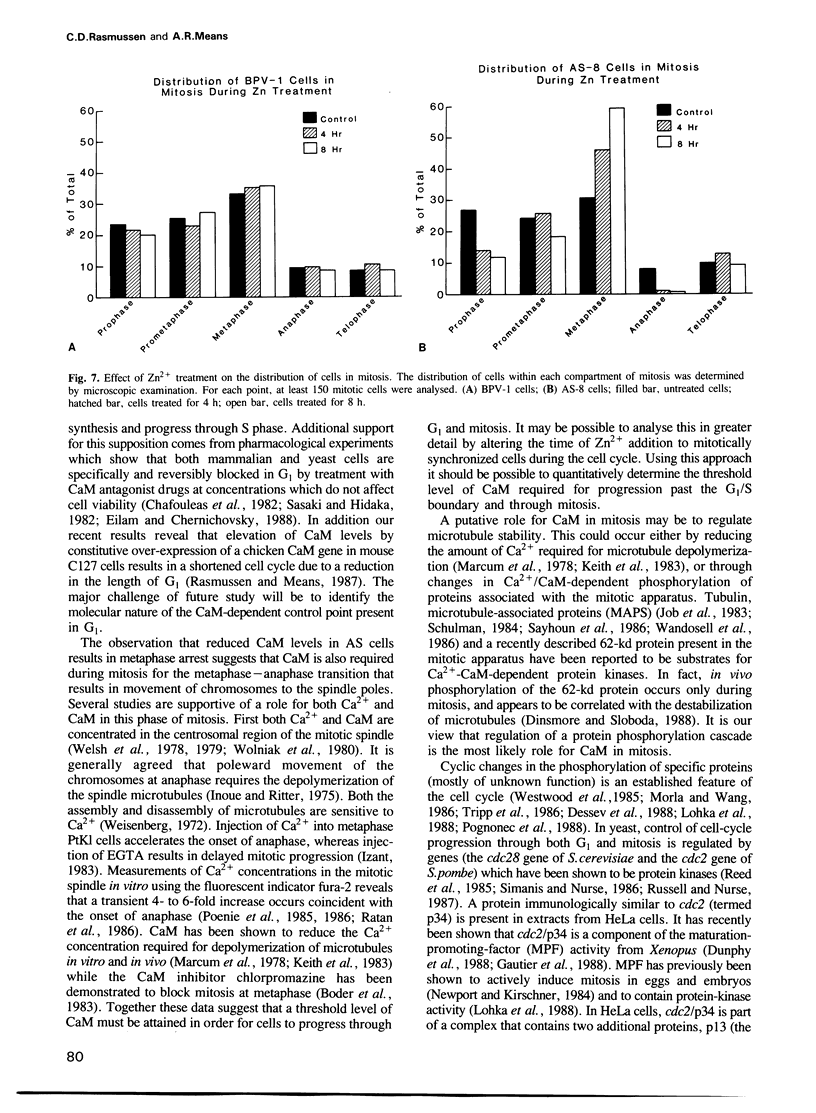
Abstract
In order to examine the consequences of a transient increase or decrease in intracellular calmodulin (CaM) levels, two bovine-papilloma-virus (BPV)-based expression vectors capable of inducibly synthesizing CaM sense (BPV-MCM) or anti-sense (BPV-CaMAS) RNA have been constructed and used to stably transform mouse C127 cells. Upon addition of Zn2+, cells containing the BPV-MCM vector have transiently increased CaM mRNA and protein levels. Cells carrying the BPV-CaMAS vector transiently produce CaM anti-sense RNA resulting in a significant decrease in intracellular CaM concentration. Increased CaM caused a transient acceleration of proliferation, while the anti-sense RNA induced decrease in CaM caused a transient cell cycle arrest. Flow cytometric analysis showed that progression through G1 and mitosis was affected by changes in CaM levels. These data indicate that CaM levels may limit the rate of cell-cycle progression under normal conditions of growth.
Full text
PDF
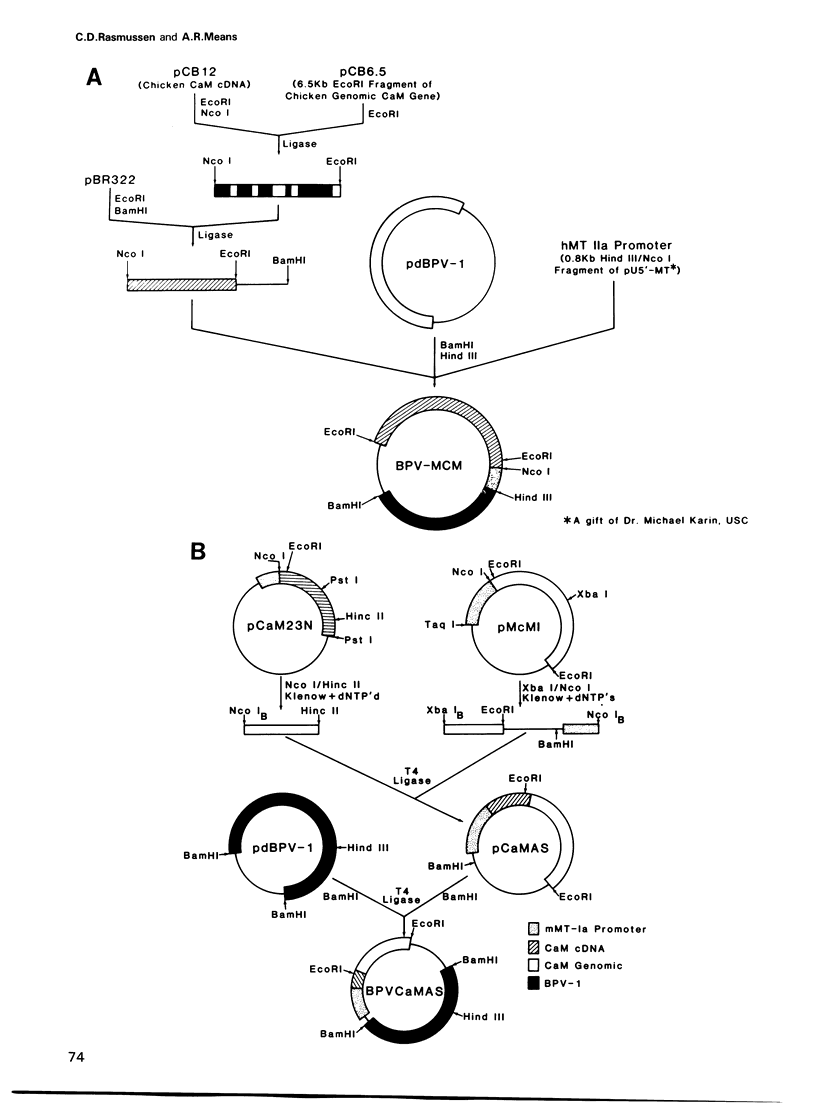

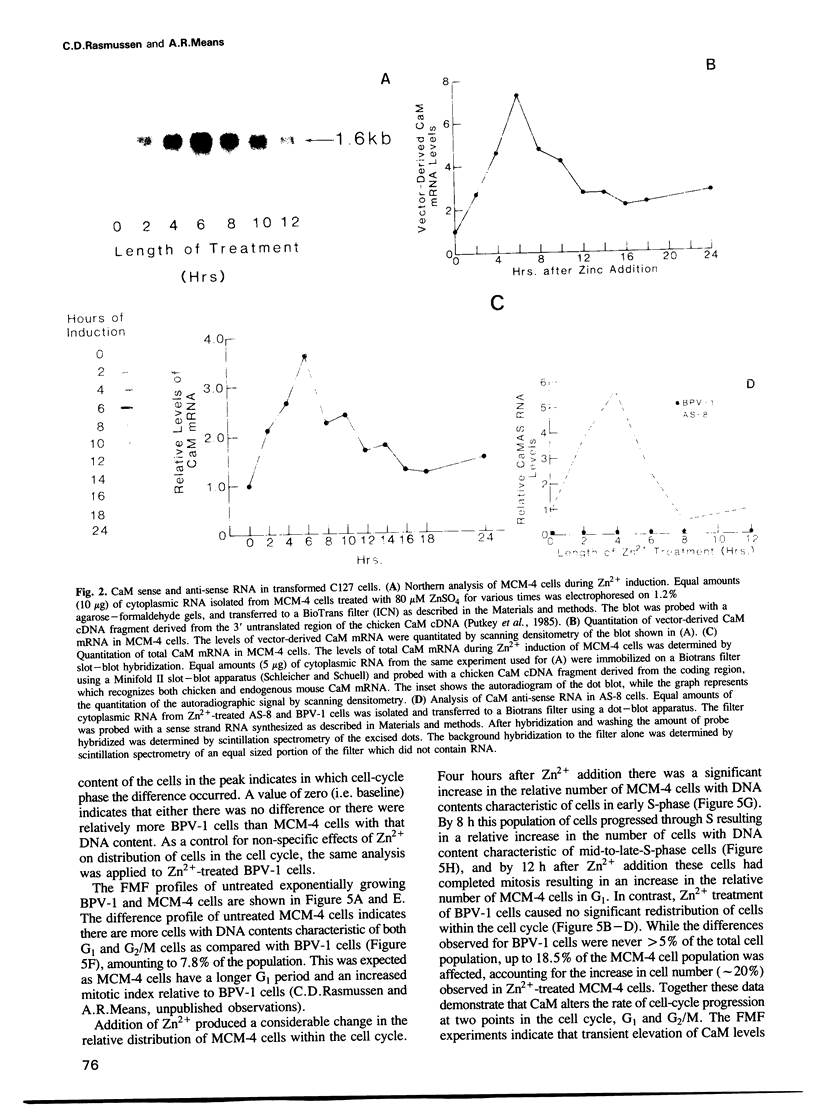
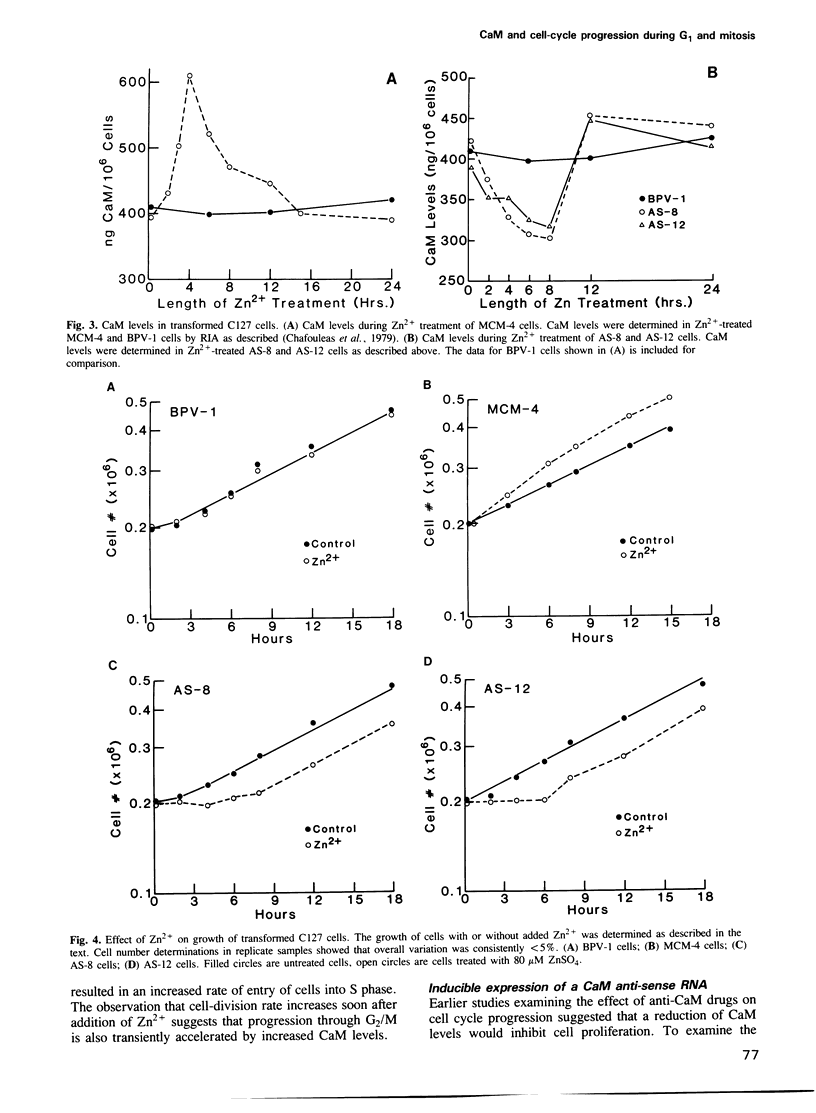
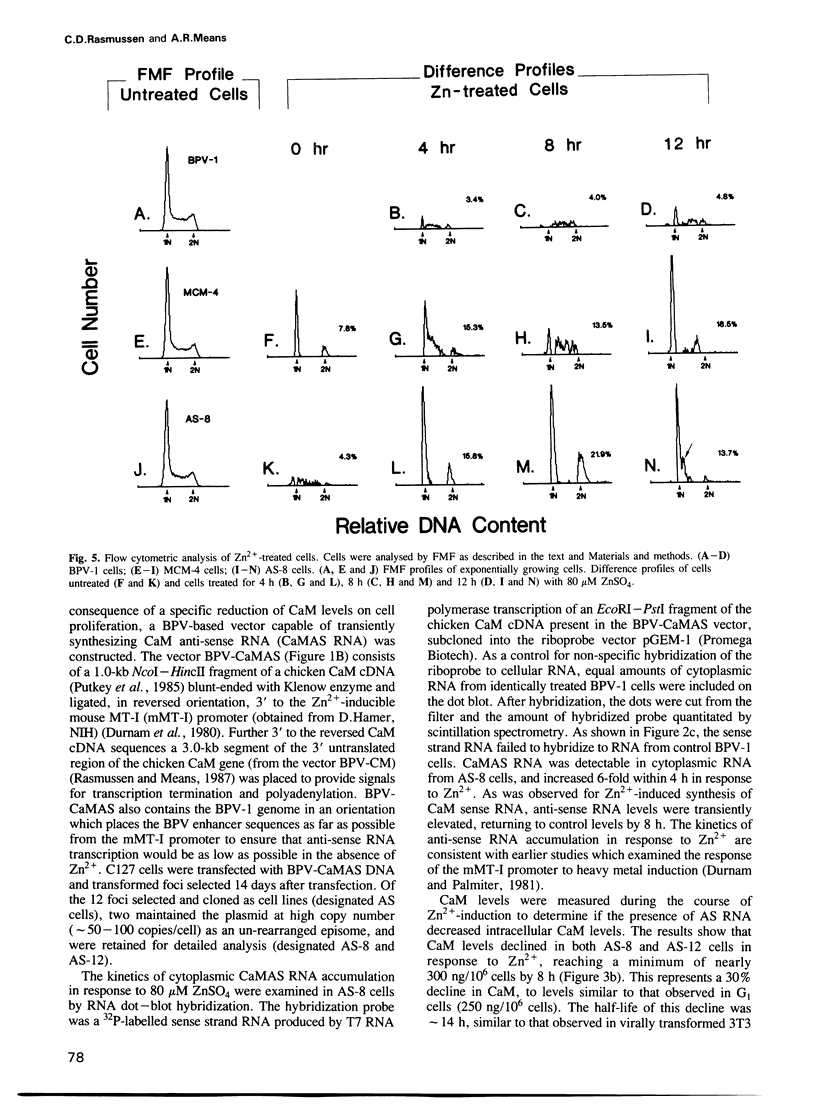


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boder G. B., Paul D. C., Williams D. C. Chlorpromazine inhibits mitosis of mammalian cells. Eur J Cell Biol. 1983 Sep;31(2):349–353. [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Hidaka H., Boyd A. E., 3rd, Means A. R. Calmodulin and the cell cycle: involvement in regulation of cell-cycle progression. Cell. 1982 Jan;28(1):41–50. doi: 10.1016/0092-8674(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Chafouleas J. G., Lagacé L., Bolton W. E., Boyd A. E., 3rd, Means A. R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell. 1984 Jan;36(1):73–81. doi: 10.1016/0092-8674(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Pardue R. L., Brinkley B. R., Dedman J. R., Means A. R. Regulation of intracellular levels of calmodulin and tubulin in normal and transformed cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):996–1000. doi: 10.1073/pnas.78.2.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. N., Urdea M. S., Masiarz F. R., Thorner J. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 1986 Nov 7;47(3):423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- Dessev G., Iovcheva C., Tasheva B., Goldman R. Protein kinase activity associated with the nuclear lamina. Proc Natl Acad Sci U S A. 1988 May;85(9):2994–2998. doi: 10.1073/pnas.85.9.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsmore J. H., Sloboda R. D. Calcium and calmodulin-dependent phosphorylation of a 62 kd protein induces microtubule depolymerization in sea urchin mitotic apparatuses. Cell. 1988 Jun 3;53(5):769–780. doi: 10.1016/0092-8674(88)90094-3. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Draetta G., Brizuela L., Potashkin J., Beach D. Identification of p34 and p13, human homologs of the cell cycle regulators of fission yeast encoded by cdc2+ and suc1+. Cell. 1987 Jul 17;50(2):319–325. doi: 10.1016/0092-8674(87)90227-3. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam Y., Chernichovsky D. Low concentrations of trifluoperazine arrest the cell division cycle of Saccharomyces cerevisiae at two specific stages. J Gen Microbiol. 1988 Apr;134(4):1063–1069. doi: 10.1099/00221287-134-4-1063. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Hazelton B., Mitchell B., Tupper J. Calcium, magnesium, and growth control in the WI-38 human fibroblast cell. J Cell Biol. 1979 Nov;83(2 Pt 1):487–498. doi: 10.1083/jcb.83.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S., Ritter H., Jr Dynamics of mitotic spindle organization and function. Soc Gen Physiol Ser. 1975;30:3–30. [PubMed] [Google Scholar]
- Izant J. G. The role of calcium ions during mitosis. Calcium participates in the anaphase trigger. Chromosoma. 1983;88(1):1–10. doi: 10.1007/BF00329497. [DOI] [PubMed] [Google Scholar]
- Job D., Rauch C. T., Fischer E. H., Margolis R. L. Regulation of microtubule cold stability by calmodulin-dependent and -independent phosphorylation. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3894–3898. doi: 10.1073/pnas.80.13.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Keith C., DiPaola M., Maxfield F. R., Shelanski M. L. Microinjection of Ca++-calmodulin causes a localized depolymerization of microtubules. J Cell Biol. 1983 Dec;97(6):1918–1924. doi: 10.1083/jcb.97.6.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J., Hayes M. K., Maller J. L. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc Natl Acad Sci U S A. 1988 May;85(9):3009–3013. doi: 10.1073/pnas.85.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Wang J. Y. Protein tyrosine phosphorylation in the cell cycle of BALB/c 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8191–8195. doi: 10.1073/pnas.83.21.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J. W., Kirschner M. W. Regulation of the cell cycle during early Xenopus development. Cell. 1984 Jul;37(3):731–742. doi: 10.1016/0092-8674(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Pognonec P., Boulukos K. E., Gesquière J. C., Stéhelin D., Ghysdael J. Mitogenic stimulation of thymocytes results in the calcium-dependent phosphorylation of c-ets-1 proteins. EMBO J. 1988 Apr;7(4):977–983. doi: 10.1002/j.1460-2075.1988.tb02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkey J. A., Donnelly P. V., Means A. R. Bacterial expression vectors for calmodulin. Methods Enzymol. 1987;139:303–317. doi: 10.1016/0076-6879(87)39094-9. [DOI] [PubMed] [Google Scholar]
- Putkey J. A., Slaughter G. R., Means A. R. Bacterial expression and characterization of proteins derived from the chicken calmodulin cDNA and a calmodulin processed gene. J Biol Chem. 1985 Apr 25;260(8):4704–4712. [PubMed] [Google Scholar]
- Rasmussen C. D., Means A. R. Calmodulin is involved in regulation of cell proliferation. EMBO J. 1987 Dec 20;6(13):3961–3968. doi: 10.1002/j.1460-2075.1987.tb02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C. D., Simmen R. C., MacDougall E. A., Means A. R. Methods for analyzing bovine papilloma virus-based calmodulin expression vectors. Methods Enzymol. 1987;139:642–654. doi: 10.1016/0076-6879(87)39117-7. [DOI] [PubMed] [Google Scholar]
- Ratan R. R., Shelanski M. L., Maxfield F. R. Transition from metaphase to anaphase is accompanied by local changes in cytoplasmic free calcium in Pt K2 kidney epithelial cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5136–5140. doi: 10.1073/pnas.83.14.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. The mitotic inducer nim1+ functions in a regulatory network of protein kinase homologs controlling the initiation of mitosis. Cell. 1987 May 22;49(4):569–576. doi: 10.1016/0092-8674(87)90459-4. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., LeVine H., 3rd, McDonald O. B., Cuatrecasas P. Specific postsynaptic density proteins bind tubulin and calmodulin-dependent protein kinase type II. J Biol Chem. 1986 Sep 15;261(26):12339–12344. [PubMed] [Google Scholar]
- Sarver N., Gruss P., Law M. F., Khoury G., Howley P. M. Bovine papilloma virus deoxyribonucleic acid: a novel eucaryotic cloning vector. Mol Cell Biol. 1981 Jun;1(6):486–496. doi: 10.1128/mcb.1.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Hidaka H. Calmodulin and cell proliferation. Biochem Biophys Res Commun. 1982 Jan 29;104(2):451–456. doi: 10.1016/0006-291x(82)90658-1. [DOI] [PubMed] [Google Scholar]
- Schatzman R. C., Raynor R. L., Kuo J. F. N-(6-Aminohexyl)-5-chloro-1-naphthalenesulfonamide(W-7), a calmodulin antagonist, also inhibits phospholipid-sensitive calcium-dependent protein kinase. Biochim Biophys Acta. 1983 Jan 4;755(1):144–147. doi: 10.1016/0304-4165(83)90284-2. [DOI] [PubMed] [Google Scholar]
- Schulman H. Phosphorylation of microtubule-associated proteins by a Ca2+/calmodulin-dependent protein kinase. J Cell Biol. 1984 Jul;99(1 Pt 1):11–19. doi: 10.1083/jcb.99.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Takeda T., Yamamoto M. Analysis and in vivo disruption of the gene coding for calmodulin in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3580–3584. doi: 10.1073/pnas.84.11.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp M. L., Piñon R., Meisenhelder J., Hunter T. Identification of phosphoproteins correlated with proliferation and cell cycle arrest in Saccharomyces cerevisiae: positive and negative regulation by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5973–5977. doi: 10.1073/pnas.83.16.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veigl M. L., Sedwick W. D., Niedel J., Branch M. E. Induction of myeloid differentiation of HL-60 cells with naphthalene sulfonamide calmodulin antagonists. Cancer Res. 1986 May;46(5):2300–2305. [PubMed] [Google Scholar]
- Wandosell F., Serrano L., Hernández M. A., Avila J. Phosphorylation of tubulin by a calmodulin-dependent protein kinase. J Biol Chem. 1986 Aug 5;261(22):10332–10339. [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Calcium-dependent regulator protein: localization in mitotic apparatus of eukaryotic cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1867–1871. doi: 10.1073/pnas.75.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Tubulin and calmodulin. Effects of microtubule and microfilament inhibitors on localization in the mitotic apparatus. J Cell Biol. 1979 Jun;81(3):624–634. doi: 10.1083/jcb.81.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood J. T., Church R. B., Wagenaar E. B. Changes in protein phosphorylation during the cell cycle of Chinese hamster ovary cells. J Biol Chem. 1985 Aug 25;260(18):10308–10313. [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H., Boynton A. L., Youdale T., Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro. 1976 Jan;12(1):1–18. doi: 10.1007/BF02832787. [DOI] [PubMed] [Google Scholar]
- Wolniak S. M., Hepler P. K., Jackson W. T. Detection of the membrane-calcium distribution during mitosis in Haemanthus endosperm with chlorotetracycline. J Cell Biol. 1980 Oct;87(1):23–32. doi: 10.1083/jcb.87.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagle M. K., Palmiter R. D. Coordinate regulation of mouse metallothionein I and II genes by heavy metals and glucocorticoids. Mol Cell Biol. 1985 Feb;5(2):291–294. doi: 10.1128/mcb.5.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]




