Abstract
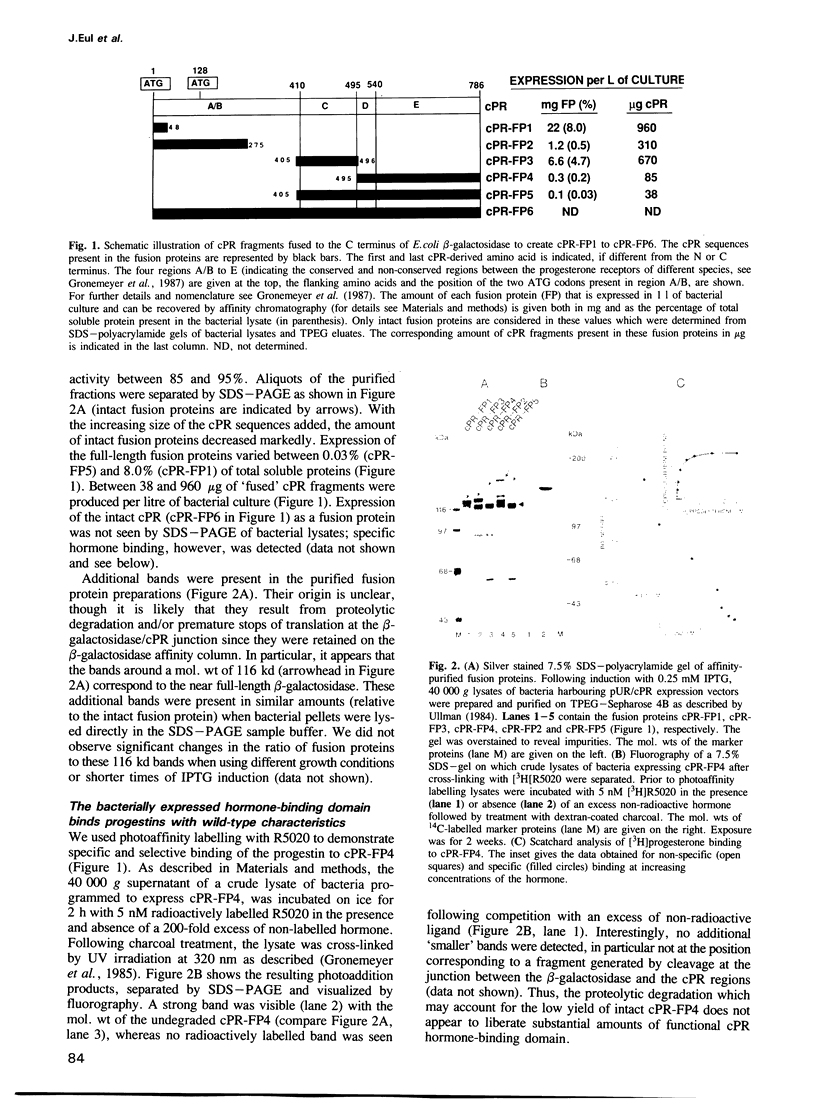
Bacterially-expressed fusion proteins containing the DNA-(region C) or hormone-binding (region E) domains of the chicken progesterone receptor (cPR) fused to the C terminus of Escherichia coli beta-galactosidase were analysed for the specificity of interaction with natural and synthetic hormone-responsive elements (HREs) and progestins, respectively. The purified fusion protein containing the progestin-binding domain bound progesterone with an apparent Kd of 1.0-1.5 nM and was specifically photocross-linked with the synthetic progestin R5020 in crude bacterial lysates. Labelling of intact bacterial cells with [3H]R5020 revealed that the majority, if not all, of the bacterially produced hormone-binding domain was active. No differences in the binding to a synthetic palindromic glucocorticoid/progestin-responsive element (GRE/PRE) were found when the bacterially produced cPR DNA-binding domain was compared in methylation interference assays with the full-length chicken progesterone receptor form A expressed in eukaryotic cells. The study of dissociation kinetics, however, revealed differences in the half-life of the complexes formed between the palindromic GRE/PRE and either the receptor form A or the fusion protein containing the cPR DNA-binding domain. DNase I protection experiments demonstrated that the bacterially produced region C of the cPR generated specific 'footprints' on the mouse mammary tumour virus long terminal repeat (MMTV-LTR) which were nearly identical to those previously reported for the rat glucocorticoid receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arriza J. L., Weinberger C., Cerelli G., Glaser T. M., Handelin B. L., Housman D. E., Evans R. M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987 Jul 17;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Migliaccio A., Di Domenico M., Nola E. Oestradiol stimulates tyrosine phosphorylation and hormone binding activity of its own receptor in a cell-free system. EMBO J. 1987 Oct;6(10):2923–2929. doi: 10.1002/j.1460-2075.1987.tb02596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkenstam A., Glaumann H., Gustafsson J. A. Unspecific and sequence-specific deoxyribonucleic acid binding of the partially purified human progesterone receptor. Mol Endocrinol. 1988 Jun;2(6):571–578. doi: 10.1210/mend-2-6-571. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M., Schrader W. T., O'Malley B. W. Photoaffinity labeling of the chick progesterone receptor proteins. Similar hormone binding domains detected after removal of proteolytic interference. J Biol Chem. 1983 Feb 10;258(3):1637–1644. [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Carson M. A., Tsai M. J., Conneely O. M., Maxwell B. L., Clark J. H., Dobson A. D., Elbrecht A., Toft D. O., Schrader W. T., O'Malley B. W. Structure-function properties of the chicken progesterone receptor A synthesized from complementary deoxyribonucleic acid. Mol Endocrinol. 1987 Nov;1(11):791–801. doi: 10.1210/mend-1-11-791. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Henderson D., Ponta H. The hormone response element of the mouse mammary tumour virus DNA mediates the progestin and androgen induction of transcription in the proviral long terminal repeat region. EMBO J. 1987 Feb;6(2):363–368. doi: 10.1002/j.1460-2075.1987.tb04763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Skroch P., Weinmann J., Butkeraitis P., Ponta H. DNA sequences outside the receptor-binding sites differently modulate the responsiveness of the mouse mammary tumour virus promoter to various steroid hormones. EMBO J. 1988 May;7(5):1403–1410. doi: 10.1002/j.1460-2075.1988.tb02957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalepakis G., Arnemann J., Slater E., Brüller H. J., Gross B., Beato M. Differential gene activation by glucocorticoids and progestins through the hormone regulatory element of mouse mammary tumor virus. Cell. 1988 May 6;53(3):371–382. doi: 10.1016/0092-8674(88)90157-2. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988 Apr 15;240(4850):324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Danielsen M., Northrop J. P., Ringold G. M. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986 Oct;5(10):2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Rusconi S., Miesfeld R., Yamamoto K. R. Glucocorticoid receptor mutants that are constitutive activators of transcriptional enhancement. Nature. 1987 Jan 22;325(6102):365–368. doi: 10.1038/325365a0. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. A superfamily of potentially oncogenic hormone receptors. Nature. 1986 Dec 18;324(6098):615–617. doi: 10.1038/324615a0. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Oestradiol induction of a glucocorticoid-responsive gene by a chimaeric receptor. Nature. 1987 Jan 1;325(6099):75–78. doi: 10.1038/325075a0. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Govindan M. V., Chambon P. Immunological similarity between the chick oviduct progesterone receptor forms A and B. J Biol Chem. 1985 Jun 10;260(11):6916–6925. [PubMed] [Google Scholar]
- Gronemeyer H., Turcotte B., Quirin-Stricker C., Bocquel M. T., Meyer M. E., Krozowski Z., Jeltsch J. M., Lerouge T., Garnier J. M., Chambon P. The chicken progesterone receptor: sequence, expression and functional analysis. EMBO J. 1987 Dec 20;6(13):3985–3994. doi: 10.1002/j.1460-2075.1987.tb02741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham J., Thomson A., Needham M., Webb P., Parker M. Characterization of response elements for androgens, glucocorticoids and progestins in mouse mammary tumour virus. Nucleic Acids Res. 1988 Jun 24;16(12):5263–5276. doi: 10.1093/nar/16.12.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg S. M., Giguere V., Segui P., Evans R. M. Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell. 1987 Apr 10;49(1):39–46. doi: 10.1016/0092-8674(87)90753-7. [DOI] [PubMed] [Google Scholar]
- Jeltsch J. M., Krozowski Z., Quirin-Stricker C., Gronemeyer H., Simpson R. J., Garnier J. M., Krust A., Jacob F., Chambon P. Cloning of the chicken progesterone receptor. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5424–5428. doi: 10.1073/pnas.83.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klock G., Strähle U., Schütz G. Oestrogen and glucocorticoid responsive elements are closely related but distinct. Nature. 1987 Oct 22;329(6141):734–736. doi: 10.1038/329734a0. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Staub A., Chambon P. Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. EMBO J. 1986 Sep;5(9):2231–2236. doi: 10.1002/j.1460-2075.1986.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loosfelt H., Atger M., Misrahi M., Guiochon-Mantel A., Meriel C., Logeat F., Benarous R., Milgrom E. Cloning and sequence analysis of rabbit progesterone-receptor complementary DNA. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9045–9049. doi: 10.1073/pnas.83.23.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sullivan P. M., Willard H. F., French F. S., Wilson E. M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988 Apr 15;240(4850):327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Misrahi M., Atger M., d'Auriol L., Loosfelt H., Meriel C., Fridlansky F., Guiochon-Mantel A., Galibert F., Milgrom E. Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophys Res Commun. 1987 Mar 13;143(2):740–748. doi: 10.1016/0006-291x(87)91416-1. [DOI] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Yamamoto K. R. Functional dissection of the hormone and DNA binding activities of the glucocorticoid receptor. EMBO J. 1987 May;6(5):1309–1315. doi: 10.1002/j.1460-2075.1987.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader W. T., Birnbaumer M. E., Hughes M. R., Weigel N. L., Grody W. W., O'Malley B. W. Studies on the structure and function of the chicken progesterone receptor. Recent Prog Horm Res. 1981;37:583–633. doi: 10.1016/b978-0-12-571137-1.50017-7. [DOI] [PubMed] [Google Scholar]
- Schüle R., Muller M., Otsuka-Murakami H., Renkawitz R. Cooperativity of the glucocorticoid receptor and the CACCC-box binding factor. Nature. 1988 Mar 3;332(6159):87–90. doi: 10.1038/332087a0. [DOI] [PubMed] [Google Scholar]
- Strähle U., Klock G., Schütz G. A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7871–7875. doi: 10.1073/pnas.84.22.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L., Gaub M. P., Mader S., Dierich A., Bellard M., Chambon P. Cell-specific activity of a GGTCA half-palindromic oestrogen-responsive element in the chicken ovalbumin gene promoter. EMBO J. 1988 Dec 1;7(12):3771–3778. doi: 10.1002/j.1460-2075.1988.tb03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L., Gronemeyer H., Turcotte B., Gaub M. P., Chambon P. The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature. 1988 May 12;333(6169):185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- Trapman J., Klaassen P., Kuiper G. G., van der Korput J. A., Faber P. W., van Rooij H. C., Geurts van Kessel A., Voorhorst M. M., Mulder E., Brinkmann A. O. Cloning, structure and expression of a cDNA encoding the human androgen receptor. Biochem Biophys Res Commun. 1988 May 31;153(1):241–248. doi: 10.1016/s0006-291x(88)81214-2. [DOI] [PubMed] [Google Scholar]
- Ullmann A. One-step purification of hybrid proteins which have beta-galactosidase activity. Gene. 1984 Jul-Aug;29(1-2):27–31. doi: 10.1016/0378-1119(84)90162-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]