Abstract
Activation of spinal astrocytes may contribute to neuropathic pain. Adjacent astrocytes can make direct communication through gap junctions formed by connexin 43 (Cx43) in the central nervous system. Yet, the role of spinal astroglial gap junctions in neuropathic pain is not fully understood. Since Cx43 is the connexin isoform expressed preferentially in astrocytes in the spinal cord, we used a small interfering RNA (siRNA) approach to examine whether suppression of spinal Cx43 expression inhibits mechanical hypersensitivity in rats after an L5 spinal nerve ligation (SNL). SNL rats were administered intrathecal Cx43 siRNA (3 μg/15 μl, twice/day) or an equal amount of mismatch siRNA (control) on days 14 through17 post-SNL. Cx43 siRNA, but not mismatch siRNA, alleviated mechanical hypersensitivity in SNL rats. Furthermore, Western blot analysis showed that the pain inhibition induced by Cx43 siRNA correlated with downregulation of Cx43 expression, but not that of Cx36 (the neuronal gap junction protein) or glial fibrillary acidic protein (GFAP, a marker for reactive astrocytes) in the spinal cord of SNL rats. Western blot analysis and immunohistochemistry also showed that SNL increased GFAP expression, but decreased Cx43 expression, in spinal cord. Our results provide direct evidence that selective suppression of spinal Cx43 after nerve injury alleviates neuropathic mechanical hypersensitivity. These findings suggest that in the spinal cord, the enhanced function of astroglial gap junctions, especially those formed by Cx43, may be important to neuropathic pain in SNL rats.
Keywords: Connexin 43, glia, gap junction, neuropathic pain
1. Introduction
Gap junctions allow second messengers, ions, and small hydrophilic molecules to pass freely and rapidly between neighboring cells in the central nervous system (CNS). They are usually formed by two hemichannels, each composed of a hexamer of connexin (Cx) protein and situated in the membrane of adjacent cells [1]. Cx43 is the connexin isoform expressed preferentially in astrocytes of the mammalian CNS [2–4]. Gap junctions composed of Cx43 may mediate the intercellular propagation of Ca2+ waves between astrocytes, enabling these cells to generate rapid electrical responses to neuronal activity [5,6].
Increasing evidence suggests that gap junctions in astrocytes may play an important role in various pathologic pain conditions [4,7,8], including neuropathic pain [3,9–11]. Studies suggest that activation of astrocytes in the spinal cord may contribute to the maintenance of dorsal horn neuronal hyperexcitability that leads to exaggerated spinal nociceptive transmission after tissue and nerve injury [12,13]. For example, patients with chemotherapy-induced neuropathy exhibit increases in both astrocyte activation and Cx43 expression [4]. Blocking gap junctions by systemic or intrathecal administration of carbenoxolone (CBX), a gap junction decoupler, inhibits neuropathic pain behavior in a variety of animal models [4,7,14–16]. However, because CBX is nonselective and could have other effects on neuronal excitability [17,18], the specificity of drug action toward glial gap junctions in previous pharmacologic studies is uncertain. Consequently, the roles of spinal astroglial gap junctions in the maintenance of neuropathic pain are not fully clear.
In this study, our goal was to examine whether spinal Cx43 contributes to the manifestation of neuropathic pain in rats subjected to L5 spinal nerve ligation (SNL). Because selective glial gap junction blockers for animal studies are lacking, we knocked down gap junction proteins using double-stranded small interfering RNA (siRNA), which causes degradation of the cognate messenger RNA.
2. Materials and Methods
2.1. Animals
Experiments were carried out in adult, male Sprague-Dawley rats (250–350 g, Harlan Laboratories, Inc., Indianapolis, IN). Efforts were made to minimize the number of animals used and their suffering. All experimental procedures were conducted in strict accordance with the guidelines established by the Nanjing University and Johns Hopkins University Animal Care and Use Committee as consistent with the National Institutes of Health Guide for the Use of Experimental Animals.
2.2. L5 SNL
The SNL model was produced as described previously [19]. In brief, the rats were anesthetized with isoflurane (2%, Abbott Laboratories, North Chicago, IL), and the left L5 spinal nerve was ligated with a 6–0 silk suture and cut distally under aseptic conditions. In the sham surgery group, the surgical procedure was identical, except that the left L5 spinal nerve was not ligated.
2.3 Behavioral tests
Hypersensitivity of rats to punctuate mechanical stimulation with von Frey filaments (0.38, 0.57, 1.23, 1.83, 3.66, 5.93, 9.13, 13.1 g) was determined with the up-down method as described previously [20]. The paw withdrawal threshold (PWT) was determined according to the method and formula provided by Dixon [21]. To minimize experimenter bias, the investigator who performed the behavioral tests was blinded to the drug treatment conditions.
2.4. Western blotting
Ipsilateral and contralateral lumbar spinal segments (including L4 and L5 segments) of SNL and sham-operated rats were separated and homogenized for immunoblotting. The tissues were lysed in ice-cold RIPA buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 10% glycerol, 0.1% Triton X-100, 0.5 mg/ml BSA). After centrifugation of the homogenates, the protein concentration was determined by using a detergent-compatible protein assay with a bovine serum albumin standard. Samples were separated on a 7.5% (w/v) sodium dodecyl sulfatepolyacrylamide gel and transferred onto a nitrocellulose membrane (Amersham, Pittsburgh, PA) with a Trans-Blot Transfer Cell system (Bio-Rad, Hercules, CA). Membranes were incubated with the indicated primary antibody overnight at 4°C, and immunoreactivity was detected by enhanced chemiluminescence (ECL, Amersham). We used antibodies against Cx43 (1:8000, Sigma-Aldrich, St. Louis, MO, Cat. #: C6219), Cx36 (1:1000, Life technologies, Grand Island, NY, Cat. #: 51-6200), glial fibrillary acidic protein (GFAP; 1:2000, Millipore, Billerica, MA, Cat. # MAB360), and β-actin (1:10,000, Chemicon, Temecula, CA). We then quantified the intensity of immunoreactive bands of interest on the autoradiograms using Image J 1.46r software (NIH, Bethesda, MD). Actin staining was used as an internal control for protein loading.
2.5. Immunofluorescence
Animals were anesthetized with pentobarbital and perfused with 20 ml of 0.1 M phosphate-buffered saline (PBS; pH 7.4, 4°C) followed by 25 ml of fixative (4% formaldehyde and 14% [v/v] saturated picric acid in PBS; 4°C). Lumbar spinal cord tissues were cryoprotected in 20% sucrose (24 h) before being serially cut into 15-μm sections and placed onto slides. The slides were pre-incubated in blocking solution (5 % normal donkey serum, 0.2% Triton X-100 in PBS) for 1 h at room temperature and then incubated overnight with the primary antibody at 4°C. The primary antibody was diluted to its final working concentration in PBS [pH 7.4] with 1% BSA, 0.01% sodium azide, and 0.3% Triton X-100. The slides were then incubated in secondary antibody at room temperature for 45 min and covered with Fluoroshield histology mounting medium (Sigma-Aldrich). The primary antibodies used were rabbit anti-Cx43 (1:2000, Sigma-Aldrich, Cat. #: C6219), mouse anti-GFAP (1:5000, Millipore, Cat. #: MAB360), and mouse anti-OX42 (1:2000, Millipore, Cat. # CBL1512). The secondary antibodies used were Rhodamine Red-X–conjugated AffiniPure donkey anti-rabbit IgG(H+L) (1:100, Jackson ImmunoResearch, West Grove, PA, Cat. #: 711-295-152) and Fluorescein-conjugated AffiniPure donkey anti-mouse IgG(H+L) (1:100, Jackson ImmunoResearch, Cat. #: 711-095-151).
Digitized images were captured using Olympus BX51 microscope system (Melville, NY). We then quantified the integrated optical density (IOD) of immunostaining from the dorsal horn of lumbar spinal cord slice (3–4 sections/animal) using Image J 1.46r software (NIH, Bethesda, MD). Similar areas were examined in different animals. The background signal in each slice was subtracted. The mean IOD of sham-operated rats was calculated, and the ratio of IOD in SNL rats was then normalized to the mean value of sham-operated rats and illustrated as fold of sham for the purpose of comparison. Data were analyzed by an investigator blinded to experimental group.
2.6. siRNA
ON-TARGET plus SMARTpool Cx43 siRNA and mismatch siRNA (control) were purchased from Thermo Scientific (Pittsburgh, PA). Single deprotected strands were resuspended with an isotonic buffer (100 mM potassium acetate, 30 mM HEPES-KOH, 2 mM magnesium acetate [pH 7.4 at 37°C]; 26 mM NaCl was added to produce a non-irritating, isotonic solution) to a concentration of 1 μg/μl. The strands were incubated at 90°C for 5 min and then at 37°C for 1 h. siRNAs were prepared immediately before administration by mixing the RNA solution (1 μg/μl in annealing buffer) with the transfection reagent i-Fect (v/v:1/4; Neuromics, Edina, MN) to a final siRNA/lipid complex concentration of 0.2 μg/μl. Cx43 siRNA or mismatch siRNA was infused into SNL rats (15 μl/injection, twice daily) through an implanted intrathecal catheter (PE-10 tubing) for 4 consecutive days.
2.7. Statistics
The methods for statistical comparisons in each study are given in the figure legends. Representative immunohistochemistry data are from experiments that were replicated biologically at least three times with similar results. STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK) was used to conduct all statistical analyses. The Tukey honestly significant difference (HSD) post-hoc test was used to compare specific data points in ANOVA. Bonferroni correction was applied for multiple comparisons. Two-tailed tests were performed, and data are expressed as mean ± SEM; P<0.05 was considered significant in all tests.
3. Results
3.1. Intrathecal Cx43 siRNA attenuates mechanical hypersensitivity in SNL rats
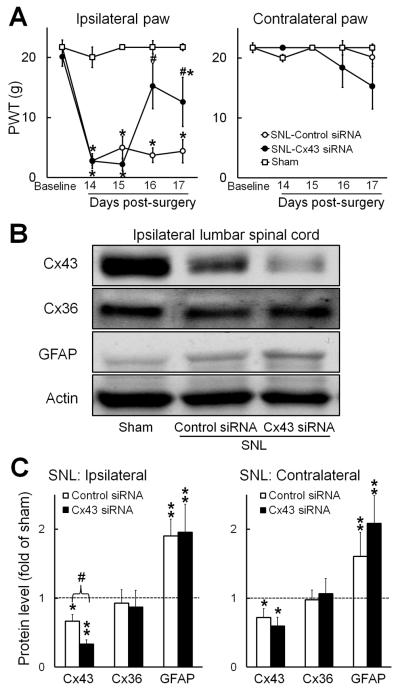
Our primary goal was to examine whether intrathecal administration of Cx43 siRNA affects neuropathic mechanical hypersensitivity. Accordingly, SNL rats were administered intrathecal Cx43 siRNA (3 μg/15 μl, twice/day, n=5) or an equal amount of mismatch siRNA (control, n=5) on days 14 through 17 post-SNL. Sham-operated rats that did not receive siRNA treatment (n=4) were used as a surgical control. PWT in sham-operated rats did not significant change from baseline during the testing period. On day 14 post-SNL, ipsilateral PWT was significantly decreased, as compared to that in sham-operated rats. However, PWT increased significantly from this level (i.e., day 14 pre-siRNA treatment) on the third and fourth days of Cx43 siRNA treatment (Fig. 1A). Intrathecal mismatch siRNA did not alter ipsilateral PWT in SNL rats, and neither Cx43 nor control siRNA significantly altered PWT on the side contralateral to SNL. Animals received siRNA treatments appeared healthy and behaved normally.
Fig. 1. Effects of repetitive intrathecal Cx43 siRNA infusions on mechanical hypersensitivity and expression of Cx43, Cx36, and GFAP in rats after spinal nerve injury.
(A) Rats received twice-daily intrathecal infusions of Cx43 siRNA (3 μg/15 μl, n=5) or mismatch siRNA (control, n=5) on days 14 through 17 after spinal nerve ligation (SNL). Paw withdrawal thresholds (PWT) were measured before SNL and before siRNA injection on days 14 through 17 post-SNL. Left: In SNL rats, PWT in response to mechanical stimuli increased in the ipsilateral hind paw after 2 days of intrathecal Cx43 siRNA, but not after mismatch siRNA. PWT in sham-operated rats did not significant change from baseline during the testing period. Right: The contralateral PWT was not significantly changed after siRNA treatment. *P < 0.05 versus sham-operated rats, # P < 0.05 versus day 14 post-SNL, two-way mixed model ANOVA. (B) Representative immunoblots show Cx43, Cx36, and GFAP expression in the ipsilateral lumbar spinal cord of sham-operated rats (n=4) and SNL rats administered intrathecal injections of mismatch or Cx43 siRNA. (C) Quantification of Cx43, Cx36, and GFAP protein levels in the ipsilateral and contralateral lumbar spinal cord of the different groups. *P < 0.05, **P < 0.01 versus sham-operated rats, #P < 0.05 versus control siRNA, student t-test. Data are expressed as mean+ SEM.
3.2. Intrathecal Cx43 siRNA reduces spinal Cx43 expression
We next examined how SNL affects Cx43 expression in the spinal cord and whether intrathecal Cx43 siRNA decreases Cx43 levels in SNL rats. Lumbar spinal cord (L4–L5) tissue was collected for Western blot analysis at 2–3 h after the last siRNA injection on day 17 post-SNL. Compared to that in sham-operated rats, Cx43 expression was significantly decreased in both ipsilateral (0.66±0.09 fold of sham) and contralateral (0.71±0.13 fold of sham) spinal cord of SNL rats (Fig.1B,C). Cx43 siRNA treatment further reduced Cx43 expression in the ipsilateral (0.33±0.06 fold of sham, p<0.05) and contralateral (0.59±0.13 fold of sham) spinal cord of SNL rats (Fig. 1C), but mismatch (control) siRNA did not. The level of neuronal gap junction protein Cx36 did not change significantly in the spinal cord after SNL. Importantly, neither Cx43 siRNA nor control siRNA altered the expression of Cx36 in SNL rats. GFAP level was significantly greater in the ipsilateral (1.90±0.24 fold of sham) and contralateral (1.60±0.35 fold of sham) spinal cord of SNL rats that received control siRNA than in the corresponding spinal cord of sham-operated rats (Fig. 1C). The GFAP level remained significantly higher than that in sham controls on both the ipsilateral (1.95±0.40 fold of sham) and contralateral (2.08±0.43 fold of sham) sides after Cx43 siRNA treatment. Thus, intrathecal Cx43 siRNA suppressed Cx43 expression, but not Cx36 or GFAP expression, in the spinal cord of SNL rats.
3.3. SNL increases GFAP but decreases Cx43 immunoreactivity in the spinal cord
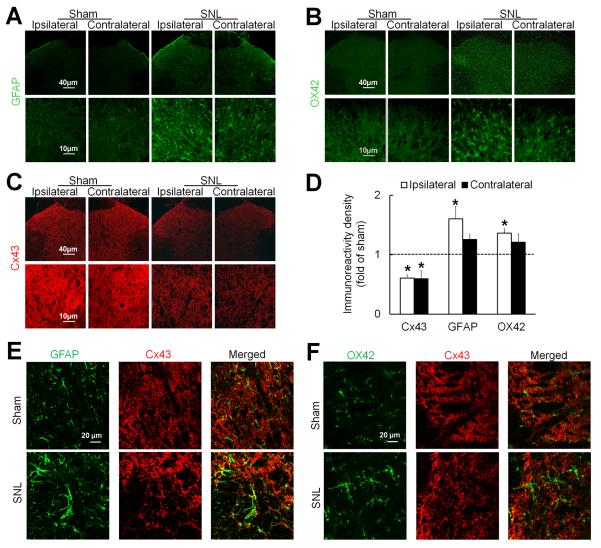
To further determine the effects of SNL on spinal Cx43 expression and glial cell activation, we also examined immunoreactivity of Cx43 and markers of reactive astrocytes (GFAP) and microglia (OX42) in rat spinal cord. GFAP and OX42 were both clearly visible in the lumbar spinal cord of rats at day 14 post-SNL (Fig. 2A, B). At that time the activated astrocytes and microglia were hypertrophic and had enlarged, densely stained cell bodies. Consistent with the Western blot study, Cx43 immunoreactivity was decreased bilaterally in lumbar spinal cord after SNL (n=3, Fig. 2C,D), as compared to that after sham surgery (n=3). The immunoreactivity of GFAP and OX42 was greater in lumbar spinal cord of SNL rats than in lumbar spinal cord of sham-operated rats, especially on the side ipsilateral to the injury (Fig. 2A, B, D). The Cx43 immunoreactivity overlapped with that of GFAP (Fig. 2E), but not OX42 (Fig. 2F), in SNL rats. These findings suggest that SNL increases the activation of microglia and astrocytes but paradoxically reduces the expression of Cx43 in the spinal cord.
Fig. 2. Spinal nerve injury increases glial activation but decreases Cx43 immunoreactivity in the spinal cord.
(A) In rats on day 14 after spinal nerve ligation (SNL), astrocytes in the spinal cord exhibited increased immunoreactivity of GFAP, especially on the side ipsilateral (left) to nerve injury. (B) Meanwhile, microglia exhibited increased immunoreactivity of OX42. Compared to spinal astrocytes and microglia in sham-operated rats, those in SNL rats were hypertrophic. (C) In contrast, Cx43 immunoreactivity was decreased on both sides of the spinal cord in SNL rats compared to that in sham-operated rats. (D) Quantification of Cx43, GFAP, and OX42 immunoreactivity in the ipsilateral and contralateral dorsal horn. The immunostaining density of SNL rats (3–4 sections/rat, n=3) were normalized to that of sham-operated rats (n=3). *P < 0.05 versus sham-operated rats, student t-test. Data are expressed as mean+ SEM. (E) The overlap (yellow) of GFAP (green) and Cx43 (red) immunoreactivity was increased after SNL. (F) OX42 (green) and Cx43 (red) did not colocalize.
4. Discussion
Preclinical studies suggest that spinal glial cells, especially astrocytes, may play an important role in neuropathic pain [8,22,23]. Yet, the roles of glial gap junctions in neuropathic pain are not fully understood. Because there is no selective blocker of glial gap junctions, conventional pharmacologic studies have not yet provided direct evidence that pain inhibition from drug treatment (e.g., CBX) occurs by blockade of glial gap junctions. Cx43 is the gap junction protein that is preferentially expressed in astrocytes of the CNS [24,25]. In our study, knockdown of Cx43 by intrathecal administration of Cx43 siRNA alleviated mechanical hypersensitivity in SNL rats. Further, the pain inhibition correlated with downregulation of spinal Cx43, but not the neuronal gap junction protein Cx36 or reactive astrocyte marker GFAP. These results may represent direct evidence that selective suppression of spinal astroglial gap junction can alleviate neuropathic mechanical hypersensitivity.
Previous studies have shown that Cx43 is upregulated in the trigeminal ganglia after trigeminal nerve injury and in trigeminal nucleus after tooth pulp inflammation [26–28]. It was postulated that upregulation of Cx43 may increase the formation of new gap junction connections and hence increase membrane permeability. In this regard, it is surprising that our Western blot and immunohistochemistry studies showed that spinal Cx43 was downregulated in rats after SNL. The mechanism for the decreased spinal Cx43 expression after SNL is unclear. It is possible that different insults (e.g., nerve injury versus tissue inflammation) and different types of nerve injury (e.g., SNL versus chronic constriction injury of trigeminal nerve) may differentially regulate spinal Cx43 expression. Intriguingly, lipopolysaccharide-activated microglia, medium harvested from activated microglia, and mixed cytokines (TNF-α and IL-1β) each downregulated Cx43 expression when added to cultured astrocytes [29]. Previous studies showed that activation of spinal microglia is prominent after nerve injury [9,30,31]. Likewise, our immunohistochemistry study showed that immunoreactivity of OX42, a marker of reactive microglia, was increased in the spinal cord of SNL rats. Accordingly, future studies should examine whether reactive microglia also release pro-inflammatory cytokines in spinal cord that lead to decreased Cx43 expression, as observed in vitro [29]. Although Cx43 expression was decreased in the spinal cord after SNL, activation of spinal astrocytes was increased (i.e., increased GFAP level). This finding may imply a functional enhancement of astroglial gap junctions after nerve injury. Intriguingly, a previous study showed that addition of activated microglia or mixed cytokines downregulated Cx43 expression but increased the open probability of hemichannels in cultured astrocytes [29]. This finding may partially explain why further suppression of Cx43 expression by siRNA in SNL rats led to inhibition of mechanical hypersensitivity. Our findings may imply that enhanced function, rather than increased expression, of glial gap junctions formed by Cx43 may contribute to neuropathic pain in SNL rats, presumably by increasing the release of active molecules.
In a study by Ohara et al., the pain inhibition produced by Cx43 siRNA treatment in the trigeminal ganglia was associated with decreased GFAP in the ganglia [26]. Yet, in our study, intrathecal Cx43 siRNA alleviated mechanical hypersensitivity without normalizing the upregulated GFAP in the spinal cord of SNL rats. The reason for this discrepancy is unclear, but it should be noted that the functional status of astrocytes may change after injury. For example, whereas inhibiting Cx43 expression in the trigeminal ganglion after injury attenuated trigeminal neuropathic pain [26], the same treatment elicited pain hypersensitivity in normal animals [27,32]. Glial activation could also be beneficial, depending upon the stage of activation [12,33,34]. One possibility is that GFAP is a marker of reactive astrocytes but is not indicative of whether the astrocytes are releasing pro- or anti-inflammatory cytokines. Hence, as with microglia, astrocyte reactivity may not always correlate with neuropathic pain manifestations [35–37]. Finally, the Cx43 expressed in satellite glial cells in dorsal root ganglia and trigeminal ganglia increases after nerve injury and contributes to pain hypersensitivity [16,38]. Inhibiting Cx43 expression in the trigeminal ganglion was shown to attenuate trigeminal neuropathic pain [26]. Accordingly, we can not exclude the possibility that the pain inhibition induced by intrathecal injection of Cx43 siRNA may also involve inhibition of Cx43 expression in dorsal root ganglia, owing to diffusion of siRNA. Future study may determine the time when the suppression of spinal Cx43 expression by siRNA may diminish after the last treatment, and whether it is associated with pain behavioral changes in SNL rats.
In summary, our study showed that selective downregulation of astroglial gap junction protein by intrathecal Cx43 siRNA ameliorates neuropathic mechanical hypersensitivity in SNL rats. This finding suggests that enhanced activity of astroglial gap junctions in the spinal cord might play an essential role in the maintenance of neuropathic pain.
Highlights
Intrathecal Cx43 siRNA attenuated neuropathic mechanical hypersensitivity in rats.
Pain inhibition by Cx43 siRNA was correlated with downregulation of spinal Cx43 expression.
Cx43 siRNA treatment did not decrease protein levels of Cx36 and GFAP in the spinal cord.
The enhanced function of spinal astroglial gap junctions may be important to neuropathic pain.
Acknowledgements
The authors thank Claire F. Levine, MS (Scientific Editor, Department of Anesthesiology/CCM, the Johns Hopkins University) for editing the manuscript. This study was supported by a research grant to Q.X. from the China Scholarship Council (Beijing, China) and a grant to Y.G. from the National Institutes of Health (NS70814, Bethesda, Maryland, USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Herve JC, Derangeon M. Gap-junction-mediated cell-to-cell communication. Cell Tissue Res. 2013;352:21–31. doi: 10.1007/s00441-012-1485-6. [DOI] [PubMed] [Google Scholar]
- [2].Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res. Brain Res. Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- [3].Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012;60:1660–1670. doi: 10.1002/glia.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yoon SY, Robinson CR, Zhang H, Dougherty PM. Spinal astrocyte gap junctions contribute to oxaliplatin-induced mechanical hypersensitivity. J. Pain. 2013;14:205–214. doi: 10.1016/j.jpain.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burra S, Jiang JX. Regulation of cellular function by connexin hemichannels. Int. J. Biochem. Mol. Biol. 2011;2:119–128. [PMC free article] [PubMed] [Google Scholar]
- [6].Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- [7].Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR. Spinal gap junctions: potential involvement in pain facilitation. J. Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- [8].Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- [10].Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10:167–184. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- [11].Wu XF, Liu WT, Liu YP, Huang ZJ, Zhang YK, Song XJ. Reopening of ATP-sensitive potassium channels reduces neuropathic pain and regulates astroglial gap junctions in the rat spinal cord. Pain. 2011;152:2605–2615. doi: 10.1016/j.pain.2011.08.003. [DOI] [PubMed] [Google Scholar]
- [12].Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013 doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roh DH, Yoon SY, Seo HS, Kang SY, Han HJ, Beitz AJ, Lee JH. Intrathecal injection of carbenoxolone, a gap junction decoupler, attenuates the induction of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2010;224:123–132. doi: 10.1016/j.expneurol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- [15].Wang H, Cao Y, Chiang CY, Dostrovsky JO, Sessle BJ. The gap junction blocker carbenoxolone attenuates nociceptive behavior and medullary dorsal horn central sensitization induced by partial infraorbital nerve transection in rats. Pain. 2013 doi: 10.1016/j.pain.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Warwick RA, Hanani M. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur. J. Pain. 2012 doi: 10.1002/j.1532-2149.2012.00219.x. [DOI] [PubMed] [Google Scholar]
- [17].Tovar KR, Maher BJ, Westbrook GL. Direct actions of carbenoxolone on synaptic transmission and neuronal membrane properties. J. Neurophysiol. 2009;102:974–978. doi: 10.1152/jn.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chepkova AN, Sergeeva OA, Haas HL. Carbenoxolone impairs LTP and blocks NMDA receptors in murine hippocampus. Neuropharmacology. 2008;55:139–147. doi: 10.1016/j.neuropharm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- [19].Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, Meyer RA, Raja SN. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;138:318–329. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- [21].Dixon WJ. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- [22].Ji XT, Qian NS, Zhang T, Li JM, Li XK, Wang P, Zhao DS, Huang G, Zhang L, Fei Z, Jia D, Niu L. Spinal astrocytic activation contributes to mechanical allodynia in a rat chemotherapy-induced neuropathic pain model. PLoS. One. 2013;8:e60733. doi: 10.1371/journal.pone.0060733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- [24].Orellana JA, Saez PJ, Shoji KF, Schalper KA, Palacios-Prado N, Velarde V, Giaume C, Bennett MV, Saez JC. Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid. Redox. Signal. 2009;11:369–399. doi: 10.1089/ars.2008.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system, Brain Res. Brain Res. Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- [26].Ohara PT, Vit JP, Bhargava A, Jasmin L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J. Neurophysiol. 2008;100:3064–3073. doi: 10.1152/jn.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vit JP, Jasmin L, Bhargava A, Ohara PT. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2006;2:247–257. doi: 10.1017/s1740925x07000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain 86 709. J. Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Meme W, Calvo CF, Froger N, Ezan P, Amigou E, Koulakoff A, Giaume C. Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by beta-amyloid. FASEB J. 2006;20:494–496. doi: 10.1096/fj.05-4297fje. [DOI] [PubMed] [Google Scholar]
- [30].Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–269. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, Tsuboi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J. Neurosci. 2009;29:11161–11171. doi: 10.1523/JNEUROSCI.3365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Donegan M, Kernisant M, Cua C, Jasmin L, Ohara PT. Satellite glial cell proliferation in the trigeminal ganglia after chronic constriction injury of the infraorbital nerve. Glia. 2013 doi: 10.1002/glia.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Friend DM, Keefe KA. Glial reactivity in resistance to methamphetamine-induced neurotoxicity. J. Neurochem. 2013;125:566–574. doi: 10.1111/jnc.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Williams A, Piaton G, Lubetzki C. Astrocytes--friends or foes in multiple sclerosis? Glia. 2007;55:1300–1312. doi: 10.1002/glia.20546. [DOI] [PubMed] [Google Scholar]
- [35].Winkelstein BA, DeLeo JA. Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: considerations for persistent pain. Brain Res. 2002;956:294–301. doi: 10.1016/s0006-8993(02)03560-6. [DOI] [PubMed] [Google Scholar]
- [36].Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J. Neuroimmunol. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- [37].Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- [38].Zhang H, Mei X, Zhang P, Ma C, White FA, Donnelly DF, Lamotte RH. Altered functional properties of satellite glial cells in compressed spinal ganglia. Glia. 2009;57:1588–1599. doi: 10.1002/glia.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]