ABSTRACT
Over the past decades, studies using zebrafish have significantly advanced our understanding of the cellular basis for development and human diseases. Zebrafish have rapidly developing transparent embryos that allow comprehensive imaging of embryogenesis combined with powerful genetic approaches. However, forward genetic screens in zebrafish have generated unanticipated findings that are mirrored by human genetic studies: disruption of genes implicated in basic cellular processes, such as protein secretion or cytoskeletal dynamics, causes discrete developmental or disease phenotypes. This is surprising because many processes that were assumed to be fundamental to the function and survival of all cell types appear instead to be regulated by cell-specific mechanisms. Such discoveries are facilitated by experiments in whole animals, where zebrafish provides an ideal model for visualization and manipulation of organelles and cellular processes in a live vertebrate. Here, we review well-characterized mutants and newly developed tools that underscore this notion. We focus on the secretory pathway and microtubule-based trafficking as illustrative examples of how studying cell biology in vivo using zebrafish has broadened our understanding of the role fundamental cellular processes play in embryogenesis and disease.
KEY WORDS: Zebrafish, Protein secretion, Vesicular transport, Microtubule transport
Introduction
Classical cell biologists studied cells in organisms abundantly available in their environments, such as algae, plants and marine animals. As the field evolved, cultured cells became de rigueur, and thus in vitro studies have generated much of the modern cell biology lexicon. The genetic advantages and expanding tools for studying subcellular structures in the small transparent zebrafish embryo offer the opportunity for cell biologists to return to our roots and to address fundamental cell biological questions in the context of a whole organism.
Forward genetic screens to identify genes underlying developmental events are a mainstay of zebrafish research. These screens have generated an extensive repertoire of mutants where a gene implicated in a basic cell biological process has been disrupted. Surprisingly, many of these mutants have specific phenotypes that involve only a few tissues or cell types. This is reminiscent of human genetic disorders such as mitochondriopathies (Schapira, 2006), ciliopathies (Hildebrandt et al., 2011) and diseases of protein trafficking (De Matteis and Luini, 2011) where a defect in a protein involved in a fundamental organelle function results in a discrete clinical syndrome. Similarly, researchers were surprised by the finding that many genes thought to be essential for the function or survival of cells in culture, and that were assumed to be ubiquitously expressed, were instead revealed to have spatio-temporally restricted expression patterns during zebrafish development. These findings led to the hypothesis that fundamental cellular processes are regulated by cell-type-specific mechanisms.
The use of zebrafish and other animals – both invertebrate and vertebrate – (see Box 1) provides valuable insights into how basic cellular processes are regulated during development and how disrupting these processes can impact on embryogenesis. This is elegantly illustrated by a recent investigation into the formation of the notochord, which forms the embryonic axial skeleton (Ellis et al., 2013). Notochord cells appear hollow owing to a large cytoplasmic vacuole found to be a lysosomal-derived organelle generated by the endosomal trafficking machinery. Rab32a is a GTPase which, in cultured cells, has been found to be involved in mitochondrial dynamics (Alto et al., 2002; Bui et al., 2010), trafficking to autophagosomes (Hirota and Tanaka, 2009) and other lysosome-related functions (Bultema et al., 2012; Wasmeier et al., 2006), and, in zebrafish, Rab32a has been found to be essential for vacuole formation and hence for notochord development. This exemplifies how pairing traditional cell biological approaches with advances in microscopy, genetics and pharmacology in zebrafish leads to unprecedented understanding of how basic cellular processes drive specific developmental events.
Box 1. Comparative analysis of common model organisms.
Zebrafish are the most widely used non-mammalian vertebrate organism, with thousands of laboratories devoted to zebrafish research worldwide. What distinguishes zebrafish from the fly (Drosophila melanogaster) and worm (Caenorhabditis elegans) – commonly used invertebrate organisms – is that, as a vertebrate, it shares properties with most organs found in mammals. Although zebrafish development is slower than that of invertebrates, it is considerably faster (5 days) than mice (19 days). As zebrafish development is external, like invertebrates, live embryos can be observed and manipulated; however, these same features make this system less than ideal for studies focused on in utero development or the transition from pre- to post-natal development. Finally, the high fecundity provides sufficient sample sizes to enable large-scale screening, and drug screens are facilitated by the ability to add compounds directly to their culture water.
The zebrafish genome is diploid and has been fully sequenced, with annotation being underway on Ensembl, and the Sanger Center and other genome browsers, providing advantages over the tetraploid Xenopus genome. Genome duplication occurred during teleost development so that ∼30% of zebrafish genes are duplicated (Howe et al., 2013; Woods et al., 2000; Woods et al., 2005). The advantage of this is that orthologs might have diverged in function and allow for emergence of specific phenotypes when one is targeted; however, there is the potential for duplicated genes that act redundantly, and hence phenotypes might not emerge unless both genes are targeted (Roest Crollius and Weissenbach, 2005).
The transparency and accessibility of zebrafish embryos is a significant advantage for cell biology, allowing visualization of fluorescent proteins in vivo, as in worms. A low-power microscope is sufficient for observing organotypic defects or living embryos at a single-cell resolution. Thus, zebrafish provide an excellent system for live imaging in a whole vertebrate and are comparable to Xenopus in the ease of morpholino knockdown. However, the rapidity of the system and the repertoire of tools for manipulating gene function and expression have not yet achieved the same level that is available for flies and worms. Thus, there is value in each animal model and the strengths of each can be leveraged in comparative approaches to fully understand gene function.
Here, we review the advantages of using zebrafish to study the intersection between cell biology, vertebrate development and pathology, and highlight some well-established imaging and genetic tools. We focus on the protein secretory pathway and microtubule-directed vesicular traffic to exemplify how merging cell biology, genetics and embryology has provided unique insight into how cells work in the context of tissues and organs in a living organism.
The zebrafish toolbox – microscopes, genetics and drugs
Zebrafish overview
Zebrafish produce large numbers of externally fertilized eggs, which rapidly develop into transparent embryos and progress to free-swimming feeding larvae within 5 days post fertilization (dpf). Gastrulation is complete within hours of fertilization and by 1 dpf, embryonic axes are established and neural development is underway. An exquisite map of cell division and movement during these early developmental events has been achieved by tracking individually labeled nuclei using advanced microscopy (Keller et al., 2008; Schmid et al., 2013). By the end of 2 dpf, organogenesis is underway throughout the embryo and, by 5 dpf, most organs carry out specialized functions. Although zebrafish have traditionally been used to study embryogenesis, areas such as behavior, pathology, infectious diseases and drug screening are actively investigated. Here, we focus on zebrafish tools used to study cell biology in vivo, with the aim to understand development and disease.
Labeling subcellular structures in zebrafish
High-resolution studies of embryonic development and disease models require analysis of the behaviors of individual cells, subcellular structures and proteins in the context of an intact tissue or organism. Fluorescent proteins have revolutionized cell biology. However, the relative small size and dynamic nature of subcellular components present challenges for imaging. This is exacerbated by the cellular complexity of whole animals. Thus, in contrast to the elegant studies describing organelle dynamics in cultured cells or yeast, relatively little is known about these processes in vertebrates.
Developing a zebrafish toolkit that labels, tracks and measures intracellular structures comparable to that available in mammals is a work in progress. A recent and concerted effort by the zebrafish community and several companies has improved the library of antibodies recognizing zebrafish proteins, which are cataloged in the zebrafish model organism database (http://zfin.org). However, specific antibodies for many structures remain unavailable, and antibody staining does not allow live imaging, a strength of the zebrafish system. Developing transgenics (Kawakami, 2005; Kwan et al., 2007) and vital dyes provides a good alternative. Well-characterized fluorescent markers relevant to topics discussed here are listed in Table 1 and illustrated in Fig. 1F. Fig. 1 shows two examples of such transgenics: Tg(bact:GalT-GFP) fish express a portion of Galactose-1-phosphate uridyl transferase (GALT) fused to GFP under the semi-ubiquitous β-actin promoter (Gerhart et al., 2012) to enable imaging of the trans-Golgi in the developing embryo using both a low-resolution fluorescence stereomicroscope (Fig. 1A–C) and confocal imaging (Fig. 1D). The second, Tg(ef1a:dclk-GFP) line (Tran et al., 2012), labels microtubules with GFP and, when used in combination with the vesicle marker clathrin fused to dsRed (M.S. and M.M., unpublished data; Fig. 1E), it provides a real-time view of microtubule-based vesicular transport.
Table 1. List of markers and tools of intracellular and extracellular structures used in vivo in zebrafish.

This regulates GFP translation to reflect endogenous Chop levels and monitor the ER stress response. GFP is found in neural tissues during ER and thermal stress.
Fig. 1.
Fluorescent protein markers of subcellular structures in zebrafish. (A–C) Live 2 dpf Tg(bact:GalT-GFP) embryo highlighting the trans-Golgi complex. The boxed region in B is magnified in the C. (D) Confocal projection through a cryosection of the liver from 5 dpf Tg(bact:GalT-GFP) larva. Nuclei are stained with DAPI (gray). (E) Muscle in a live 1 dpf Tg(ef1a:dclk-GFP) embryo with GFP-tagged microtubule-associated protein (DCK) and vesicles marked with clathrin-DS-Red (magenta). (F) Zebrafish fluorescent-protein-tagged organelle markers that can be expressed with transgenics. PM, plasma membrane; LE, late endosomes; EE, early endosomes; RE, recycling endosomes.
Fluorescent vital dyes also allow for imaging subcellular structures in vivo. BODIPY, Alizarin Red, quantum dots and dyes, such as MitoTracker or LysoTracker, are versatile counterstains to visualize dynamic cellular processes in live fish (Cooper et al., 2005; He et al., 2009; Köster and Fraser, 2006; Rieger et al., 2005; Zhang et al., 2008). In combination, transgenics and dyes provide a microscopic view of intracellular processes and a macroscopic view of how disrupting basic cellular processes interrupt development or cause disease.
Imaging
Many zebrafish imaging studies are carried out using microscopes that are available to most researchers, whereas an in-depth analysis of dynamic movements or morphometric analyses of subcellular structures requires the advanced microscopy techniques limited to researchers who have specialized training and access to facilities with these capabilities.
Low-resolution epifluorescent and stereomicroscopes can be used to detect gross changes in localization or intensity of many markers, and standard confocal microscopy can image cells that are close to the surface of live embryos but imaging deeper cells requires histological sections. Unprecedented views of how cells move individually and in concert in a developing embryo have been provided using light-sheet microscopy to track individual nuclei labeled with fluorescently-tagged histones (Huisken and Stainier, 2009; Keller et al., 2008). Selective plane illumination microscopy has provided a three-dimensional view of endodermal cell movement in older live embryos (Schmid et al., 2013). Multiphoton imaging has been used to investigate how neuronal circuits assemble during retinal development by tracking dynamic changes in cell structure and connectivity (Williams et al., 2013), and super-resolution microscopy has been used to image a single receptor molecule uncovering the role for receptor clustering in the antiviral immune response (Gabor et al., 2013). Many more spectacular zebrafish images are being generated as imaging technologies continue to improve.
Genetics
The zebrafish genome has been sequenced and annotated, and most zebrafish genes are highly conserved in mammals, with a zebrafish ortholog identified for ∼70% of human genes (Howe et al., 2013). Transgenesis and forward genetic screens are important widely used methods in zebrafish, and recent advances in reverse genetic approaches are having a major impact on the field. Box 2 details methods of generating transgenics and targeted mutagenesis using TALENs and the Crispr/Cas systems.
Box 2. Genetic approaches – a summary.
Transgenics are a common means to overexpress genes in zebrafish and, now, both transient knockdown by morpholinos and gene targeting are routine methods to induce gene loss. Random targeting of a transgene and promoter in the genome is facilitated using the tol2 transposon (Suster et al., 2009). Cell-specific promoters are available for many cell types to direct transgene expression and bacterial artificial chromosome recombineering can recapitulate endogenous gene expression patterns and levels; the heat shock promoter (hsp70) is also a widely used inducible system (Halloran et al., 2000). Multiple lines of fish expressing the same transgene in different tissues can be quickly generated by crossing fish in which the transgene is under the UAS promoter, to lines that express the Gal4 transcriptional activator under a tissue-specific promoter (Distel et al., 2009; Halpern et al., 2008).
Chemical and gene-breaking transposons (http://zfishbook.org/) have generated a large library of non-targeted mutants. Homologous recombination to generate targeted gene mutations has eluded the zebrafish community for years. However, use of TALENs (transcription activator-like effector nucleases) (Bedell et al., 2012; Huang et al., 2011; Sander et al., 2011) and the type II prokaryotic CRISPR/Cas (clustered regularly interspaced short palindromic repeats/CRISPR-associated) system (Hwang et al., 2013; Jao et al., 2013; Xiao et al., 2013) has recently been successful in specific gene targeting to generate germ-line mutants. These systems use chimeric nucleases composed of programmable sequence-specific DNA-binding modules linked to nonspecific DNA nucleases to induce double-stranded DNA breaks that are inefficiently repaired by error-prone non-homologous end-joining or homology-directed repair (Gaj et al., 2013). TALEN pairs that have been engineered by a zebrafish consortium are commercially available from http://www.addgene.com for many genes. Resources for TALEN design include https://groups.google.com/group/talengineering, http://www.addgene.org/talengineering/TALENzebrafish/ and http://www.TALengineering.org The CRISPR/Cas system introduces single nucleotide substitutions, insertions and deletions ranging from 1 to 20 bp. The relative ease of synthesis makes the CRISPR/Cas systems an attractive approach for genome editing (see Hwang et al., 2013; http://www.addgene.org; http://www.crispr-cas.org). It is anticipated that within the near future, nearly all protein-coding genes in zebrafish will have been disrupted by either forward genetic screens or one of these reverse genetic approaches.
Forward genetic screens allow the unbiased discovery of genes that contribute to a specific phenotype and have generated thousands of mutants in the zebrafish genome. One drawback, however, is that generating and maintaining stable lines can be laborious and another is that the genome duplication that occurred during teleost evolution (Postlethwait et al., 1998) can generate genes that act redundantly, necessitating that both are targeted before revealing a phenotype. In addition, maternal stores of mRNA and proteins can delay the emergence of mutant phenotypes until after these are exhausted, and thus mutant phenotypes often reflect the impact of gene loss on later developmental stages.
Morpholinos provide a complementary approach, whereby oligonucleotides that are injected into the fertilized egg block target mRNA translation or splicing. Although the use of morpholinos is plagued by fears of off-target effects and transient effects, translation-blocking morpholinos deplete proteins derived from both maternal and zygotic mRNA, so that the effects of target gene loss on early embryonic events can be studied. Additionally, by titrating the amount of morpholino injected, the degree of knockdown can be finely tuned. This has proved particularly useful for our work to model one type of congenital disorder of glycosylation (CDG), which, in humans is caused by a hypomorphic mutation in one of the genes required for N-linked protein glycosylation (Freeze, 2007). Homozygous null mutations of these genes are lethal in mammals (Thiel and Körner, 2011) and injecting high morpholino concentrations causes severe embryonic phenotypes and high mortality in zebrafish (Chu et al., 2013; Cline et al., 2012). However, fine-tuning of the knockdown was facilitated by injecting lower morpholino concentrations so that residual enzyme activity in the zebrafish morphants matched that measured in samples from patients, improving survival and revealing novel phenotypes (Chu et al., 2013; Cline et al., 2012). Although the breadth and speed of zebrafish genetic approaches do not match those available in invertebrates, they are accomplished at a fraction of the costs of, and with sample sizes exceeding, typical rodent experiments (see Box 1).
Drugs
Zebrafish have a rich history in toxicology research, as compounds can be simply added to their water. For example, zebrafish are proving useful for alcohol research (Jang et al., 2012; Monk et al., 2013; North et al., 2010; Passeri et al., 2009; Tsedensodnom et al., 2013; Yin et al., 2012). By using transgenic zebrafish expressing a GFP-tagged secreted glycoprotein in hepatocytes (Howarth et al., 2013; Tsedensodnom et al., 2013; Xie et al., 2010), and zebrafish that express fluorescent protein markers of the hepatocyte secretory organelles and of other cells in the liver (Yin et al., 2012), the mechanisms by which alcohol and other drugs cause organ-specific and organelle-mediated toxicity can be uniquely addressed. Moreover, large-scale drug screens exploit the ease of treating zebrafish with drugs (Peterson and Macrae, 2012) and have identified compounds modulating processes ranging from metabolism (Nath et al., 2013) to sleep (Rihel and Schier, 2012).
The protein secretory pathway
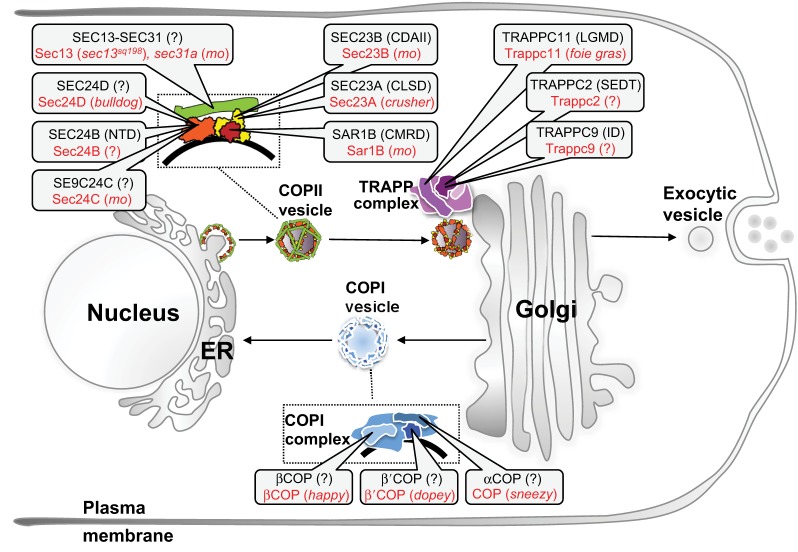
The secretory pathway generates, trafficks and processes proteins destined for the extracellular space or the plasma membrane. It comprises the endoplasmic reticulum (ER), the ER-Golgi intermediate compartment (ERGIC), the Golgi complex and the vesicles that carry cargo between them (Fig. 2). Protein synthesis and glycosylation occur in the ER and Golgi, respectively. The coat protein II (COPII) complex facilitates cargo selection, vesicle formation and anterograde trafficking from the ER to the Golgi, whereas retrograde transport occurs in COPI vesicles (Fig. 1F; Fig. 2). Diverse protein complexes function at each step of this pathway to recruit Rab GTPases and SNARE proteins, which direct and tether vesicles to target organelles and facilitate membrane fusion.
Fig. 2.
Secretory pathway components studied in zebrafish and implicated in human diseases. Secretory pathway proteins implicated in human disease are shown in black with corresponding zebrafish tools to study these in red. NTD, neural tube defects; CLSD, cranio-lenticular-sutural dysplasia; CDAII: congenital dyserythropoietic anemia II; CMRD, chylomicron retention disease; LGMD, limb girdle muscular dystrophy; SEDL, X-linked spondyloepiphyseal dysplasia tarda; ID, intellectual disability.
Work in unicellular organisms and cultured cells created an assumption that protein secretion is uniformly regulated across cell types. Studies in human, zebrafish and other vertebrates, however, revealed that this pathway is regulated in a spatio-temporal and paralog-specific manner (Melville and Knapik, 2011; Unlu et al., 2013). Although all cells secrete proteins, some – including B-cells, chondrocytes, hepatocytes and the endocrine and exocrine pancreatic cells – are considered ‘professional’ secretory cells and these cells were assumed to be particularly sensitive to secretory pathway disruption. Zebrafish mutants in secretory pathway genes both supported and refuted this hypothesis: some mutants show phenotypes in most highly secretory cells, whereas others have phenotypes that are restricted to only a subset of cells.
The emerging view is that the secretory machinery is integral to morphogenesis and organ function in a cell-specific fashion. The availability of zebrafish genetic mutants and fluorescent in vivo reporters provides novel insight into organismal functions of the secretory pathway. The effects of secretory pathway disruption relevant to development and disease are discussed below.
Developmental consequences of secretory pathway disruption
The COPII complex comprises the Sar1 GTPase, Sec23–Sec24 dimers of the inner coat, and Sec13–Sec31 heterotetramers of the outer coat (Fig. 2; Kaiser and Schekman, 1990; Novick et al., 1980). Zebrafish mutants of sec23a (crusher) and sec24d (bulldog) develop craniofacial dysmorphology, kinked pectoral fins and short body length (Lang et al., 2006; Sarmah et al., 2010). These are attributed to a failure of extracellular matrix (ECM) secretion during chondrocyte differentiation. Sec23A- and Sec24D-deficient animals fail to export collagen and other N-glycosylated proteins from the chondrocyte ER, arresting differentiation and ultimately causing cell death (Lang et al., 2006; Sarmah et al., 2010; Unlu et al., 2013), whereas collagen secretion and skeletal development are intact upon depletion of the close paralog Sec24C (Sarmah et al., 2010). Sec23B mutations in humans and zebrafish disrupt erythropoesis (Bianchi et al., 2009; Schwarz et al., 2009), a different phenotype from the chondrocyte defects observed in crusher and bulldog mutants. These COPII phenotypes are distinct from the dwarf mutants sneezy, happy and dopey, which disrupt genes that encode the α, β and β′ subunits of the COPI complex, respectively (Fig. 2), which are characterized by defects in notochord and melanosome formation (Coutinho et al., 2004). These data suggest that although COPI and COPII are required for efficient secretion and membrane recycling in all cells, loss of specific members of each complex have profound and disparate effects on a subset of cells. Mutations in individual COPII components cause an array of phenotypes in highly secretory cell types in organs such as cartilage, notochord, eye and gut (Niu et al., 2012; Schmidt et al., 2013; Townley et al., 2008; Townley et al., 2012), and in erythrocytes (Bianchi et al., 2009; Schwarz et al., 2009; Unlu et al., 2013), whereas cells that depend on vacuole formation are most sensitive to COPI depletion.
So how do transcriptional regulatory mechanisms direct the secretory pathway to assure a timely availability of cargo-specific coats? A large-scale screen in zebrafish identified the feelgood mutant, which carries a missense variant in the creb3L2 gene (Driever et al., 1996; Knapik, 2000; Neuhauss et al., 1996) – the first known transcription factor that regulates availability of the COPII components sec24d and sec23a, but not sec24c (Melville et al., 2011). Similarities between feelgood, crusher and bulldog mutant phenotypes suggest that a ‘secretory module’ consisting of Creb3L2–Sec23A–Sec24D specializes in procollagen secretion. Given that zebrafish depleted of Sec24C do not manifest skeletal dysmorphology and the gene is not a target of Creb3L2, it is likely that other cargo-specific secretory modules regulate sec24c and other genes in this pathway. Future studies in zebrafish and other animal models will be needed to crack the code of physiologically relevant, cargo-specific secretory networks.
Diseases caused by secretory pathway disruption in zebrafish and humans
Several human syndromes are associated with secretory pathway defects (De Matteis and Luini, 2011), some of which are recapitulated in mutations in orthologous zebrafish genes (Fig. 2). The concurrent identification of crusher/sec23a mutants in zebrafish and patients with SEC23A/cranio-lenticulo-sutural dysplasia (CLSD) variants (Boyadjiev et al., 2006; Lang et al., 2006) provides an excellent example of convergence between human genetics and zebrafish developmental biology to uncover physiological ramifications caused by disrupting basic cell biological processes. Both crusher mutants and CLSD patients present with craniofacial dysmorphology and axial skeleton defects attributed to backlog of ECM proteins in the ER (Boyadjiev et al., 2006; Lang et al., 2006). The closely related SEC23B gene is mutated in congenital dyserythropoietic anemia type II patients who have multinucleated erythroblasts in bone marrow, a phenotype recapitulated in zebrafish sec23b morphants (Bianchi et al., 2009; Schwarz et al., 2009).
It is unclear why mutations in SEC23A and SEC23B paralogs, which differ only by an 18 amino acid stretch, cause such different phenotypes. One possibility is that spatio-temporal differences in expression confer cell-specific functions of some COPII complex genes. However, as sar1a and sar1b are ubiquitously expressed early in development and become enriched in distinct tissues later on (E.W.K., unpublished observations), it is unlikely that their gene expression pattern is solely responsible for the divergent phenotypes observed in these mutants.
The craniofacial phenotypes of COPII mutants suggest that chondrocytes are highly sensitive to ECM secretory defects. This predicts that other manipulations that block the secretory pathway would also cause craniofacial dysmorphology. This, however, is not supported by data from zebrafish or humans when factors functioning at other steps in the secretory pathway are depleted. The transport protein particle (TRAPP) complex tethers ER-derived vesicles to the cis-Golgi membrane (Fig. 2 and Sacher et al., 2008). Fibroblasts from patients with TRAPPC11 or TRAPPC2 mutations (Bögershausen et al., 2013; Scrivens et al., 2009), and cultured cells depleted of TRAPPC11 (Scrivens et al., 2011; Wendler et al., 2010) display Golgi fragmentation and secretory protein retention. However, when these proteins are depleted in whole organisms their cell-specific roles are uncovered: patients with TRAPPC2 mutation develop spondyloepiphyseal dysplasia tarda (SEDT) distinguished by skeletal defects, short stature and microcephaly (Gedeon et al., 1999; Huson et al., 1993). This is recapitulated in trappc2 zebrafish morphants, which have a short trunk and microcephaly (A. M. Vacaru and K. C. Sadler, unpublished). The short stature and/or trunk phenotype might reflect a defect in chondrocyte formation or in ECM deposition, as in COPII mutants, but patients and zebrafish with TRAPPC11 mutation present very differently. TRAPPC11 mutation in humans causes myopathy, intellectual impairment and hyperkinetic movements (Bögershausen et al., 2013). Interstingly, zebrafish foie gras (foigr) mutants carrying a mutagenic viral insertion in the trappc11 gene display a different phenotype from other models of TRAPP complex disruption: they develop fatty liver, hepatomegaly, smaller gut and jaw, and fin defects (Cinaroglu et al., 2011; Sadler et al., 2005). The foigr/trappc11 fatty liver phenotype is partially attributed to activation of the unfolded protein response (Cinaroglu et al., 2011); however, neither mammalian cultured cells that are depleted of TRAPPC11 nor trappc2 morphants induce this response (A.M.V. and K.C.S., unpublished observations), highlighting the utility of in vivo cell biology studies in vertebrates.
The unique phenotypes that differentiate TRAPP complex disruption from COPII or COPI mutations indicate that a global block in protein secretion is not the only mechanism that underlies their associated phenotypes. Moreover, although depleting individual TRAPP or COP complex factors has similar effects in isolated cells, the physiological consequences could not be predicted without use of whole animals. These findings point to cell- and developmental-specific roles for each gene involved in protein secretion and underscore the need for comparative whole animal models to decipher the cellular and physiological functions of this pathway.
Microtubule highways in vesicular transport
Microtubules serve as tracks for organelles and vesicle movement (Figs 1, 3), an especially crucial task for the long axons of nerve cells. Microtubules are formed by α-tubulin–β-tubulin heterodimers that generate a polarized structure – the primary determinant of motor protein directional movement. The fast growing plus-end (β-tubulin capped) and a degrading minus-end (α-tubulin capped) have centrosomes that serve as microtubule-organizing centers from which microtubules undergo assembly and disassembly (Wade, 2007).
Fig. 3.
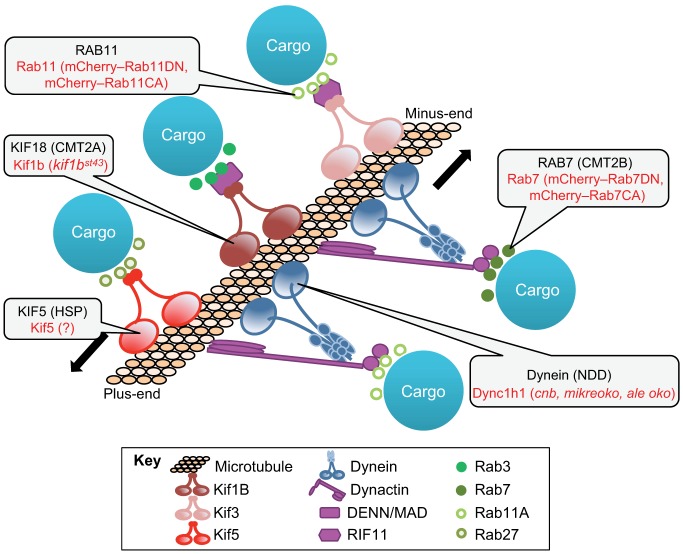
Microtubules, motor proteins and motor- and microtubule-binding proteins studied in zebrafish and involved in human diseases. Microtubule associated proteins implicated in human disease are shown in black with corresponding zebrafish tools to study these are in red. HSP, hereditary spastic paraplegia; NDD, neurodegenerative disease.
Zebrafish tools to study microtubules
Several transgenic zebrafish lines have been developed to image microtubules in vivo (Table 1; Figs 1, 3). A GAL4–UAS system has been used to spatially and temporally express GFP-tagged α-tubulin (Asakawa and Kawakami, 2010). GFP-tagged doublecortin-like kinase (York et al., 2012), a ubiquitously expressed microtubule-associated protein, uncovered the dynamics and polarity of microtubules in early-stage embryos (Tran et al., 2012). Markers of the growing plus-end of microtubules, including GFP-tagged Tau (Yanicostas et al., 2009; Yoshida et al., 2002) and the plus-end microtubule components Eb1 and Eb3 (Tran et al., 2012), have been combined with other lines that express markers of specific vesicle populations (Fig. 1C) for live imaging of vesicular transport along microtubules in whole animals.
Cargos are transported along microtubules by members of two motor protein superfamilies, the kinesins (Kifs) and the dyneins (Fig. 3). Although Kifs are cargo-specific, only cytoplasmic dynein1 (Dync1) is involved in directed microtubule transport of cargos (Roberts et al., 2013). Dync1 is a multi-protein complex that achieves specificity and versatility through distinct subunits and interaction with regulatory proteins, including dynactin (Karki and Holzbaur, 1999; King and Schroer, 2000; King, 2000).
Like vesicle trafficking in the secretory pathway, cargo–motor interactions are regulated by Rab GTPases which serve to regulate, connect and attach cargo to microtubule motors. Rab3A connects vesicles to Kif1Bβ and Kif1A in neuronal cells (Niwa et al., 2008), whereas Rab11A regulates endosomal trafficking events through interaction with Kif3B (Schonteich et al., 2008) and Rab7 controls late endosomal transport (Jordens et al., 2001). The available markers for Rabs and their dominant-negative versions in zebrafish are included in Table 1. GFP-tagged Rabs in zebrafish colocalize to vesicles with lipophilic dye FM4-64 (Fischer-Parton et al., 2000), and experiments using this system have shown that different Rabs associate with different types of vesicles (Clark et al., 2011). This analysis was aided by an automated tracking program (https://pantherfile.uwm.edu/cohena/www/rabtools.html), which enabled the in-depth analysis of recycling endosomes and their intracellular dynamics, an unprecedented feat in whole organisms.
Collectively, work on microtubule-based transport in zebrafish has led to several important conclusions: (1) microtubule-based cargo transport shapes embryonic development from fertilization onwards; (2) different motor proteins control transport in different cell compartments – for instance, Kif17 in the photoreceptor outer segment and Kif3 in the cytoplasm; and (3) zebrafish Rab homologs recapitulate the behavior of their counterparts in mammalian cells. Thus, the expanding set of tools in zebrafish is enabling organism-wide functional analysis of proteins involved in directed vesicular transport.
Off-track – microtubule-based diseases in humans and zebrafish
Although most cells rely on microtubules for transporting their cargo, disruption of this process profoundly affects the central and peripheral nervous system due to the long-distance that cargos travel from cell bodies to axonal tips. Alzheimer, Huntington disease (HD) and Charcot–Marie–Tooth disease (CMT) (De Vos et al., 2008) are some neurodegenerative diseases (NDDs) caused by defects in vesicular transport. Fig. 3 illustrates the orthologous genes underlying NDDs in humans and mutant phenotypes in zebrafish.
CMT type 2A is an axonal sensorimotor neuropathy characterized by severe peripheral muscle weakness and atrophy induced by loss of function mutations in the kinesin-3 family member, Kif1B (Zhao et al., 2001). In zebrafish, the point mutation kif1bst43 affects the microtubule interaction site and has been used to study kif1b functions in the developing central nervous system. The use of chimeric embryos, generated by transplanting kif1b-deficient neuronal cells into wild-type hosts (and vice versa), has revealed that there is a reduced growth potential in mutant neuronal axons (Lyons et al., 2009). Thus, kif1b is required in a cell autonomous manner for axonal development in the central and peripheral nervous system.
Shprintzen–Goldberg syndrome is characterized by central and enteric nervous system defects and caused by homozygous mutations of the Kif1-binding protein Kbp (Brooks et al., 2005). The role of kbp in axonal outgrowth and maintenance, microtubule organization and localization of axonal mitochondria and vesicles was elucidated using kbpst23 zebrafish line that expresses a mutated version of kbp (Lyons et al., 2008). These studies uncovered a reduced number of axons in the enteric nervous system of kbpst23 mutants, providing a new animal model for this syndrome.
Several mutations in cytoplasmic dynein 1 heavy chain 1 (DYNC1H1) are linked to hereditary motor neuropathies including CMT and spinal muscular atrophy with lower extremity predominance (Harms et al., 2012; Weedon et al., 2011). Mutations in axonemal dynein or cytoplasmic dynein 2 have been linked to a large number of ciliopathies (Leigh et al., 2009; Huber and Cormier-Daire, 2012). Zebrafish morphants for axonemal dyneins are used to study left–right asymmetry (Kawakami et al., 2005); (Essner et al., 2005), and morphants of cytoplasmic dynein 2 subunits revealed ciliary abnormalities in zebrafish kidney, eye and nose (Krock et al., 2009).
Although the mechanism of pathogenesis caused by dync1h1 mutations remains unclear, experiments in mice have suggested that there are defects in axonal transport of mitochondria (Eschbach et al., 2013). This is also reflected in the zebrafish dync1h1 (cannonball) mutant, which displays abnormal organelle positioning and accumulation of Golgi-associated vesicles in the inner segment of the retina (Insinna et al., 2010) and Schwann cell deficient myelination (Langworthy and Appel, 2012), a symptom common to NDDs. Additionally, dynactin mutations have been found in patients with amyotrophic lateral sclerosis and Perry syndrome (Stockmann et al., 2013). Death of sensory neurons and axonal degeneration is also reported in zebrafish with morpholino knockdown of dctn1a, dctn1b and dctn2a (Insinna et al., 2010) and the mutants dctn1a (mikre oko) (Tsujikawa et al., 2007) and dctn2 (ale oko) (Jing and Malicki, 2009). This suggests that depletion of Dync1 or Dync2 has a dose-dependent effect on photoreceptor cell organization and on vesicle transport.
Mutations in Rab GTPases can also lead to NDDs. It has been shown that Rab11-dependent vesicle formation and transport is influenced by the huntingtin (Htt) protein, which causes HD (Li et al., 2009a; Li et al., 2009b). Zebrafish expressing a GFP-tagged Htt mutant protein (Williams et al., 2008) are a valuable tool to identify the Rabs and other factors that are required for Htt trafficking. The transgenics described above with dominant-negative and constitutively active versions of fluorescently tagged Rab5, Rab7 and Rab11 proteins (Clark et al., 2011; Ellis et al., 2013) will be valuable systems for dissecting specific contributions of Dync1, dynactin and Rab GTPases in NDDs.
All cells are not created equal – conclusions and future directions
Identifying novel genes that regulate embryogenesis is a common aim of zebrafish forward genetic screens, which continue to supply libraries of mutants with disease-related phenotypes. Historically, researchers have focused on those mutants caused by disrupting genes encoding transcription factors or signaling molecules that are thought to serve key roles in directing specific developmental processes. However, similar phenotypes result from mutations in genes that serve basic cellular functions. These have been largely overlooked. The vacuolar sorting protein 18 (vps18a) mutant serves to illustrate this point: VPS18 is required for endosomal and lysosomal trafficking in yeast (Poupon et al., 2003) but in zebrafish, vps18a mutation causes specific defects in the hepatobiliary system, notochord and melanocytes (Ellis et al., 2013; Maldonado et al., 2006; Sadler et al., 2005). This recurring theme – whereby a gene is assumed to serve a universal and essential cellular function based on studies in yeast or isolated cells but instead is revealed to play cell-specific roles during development or in a pathology – is an important and largely underexplored topic in cell biology, and can be addressed by using zebrafish.
Other lines of evidence that challenge the widely held assumption that genes that carry out basic cellular functions must be ubiquitously expressed and function equivalently in all cells include an in situ screen to document the expression patterns of the zebrafish transcriptome during development, with many such genes found to be expressed in a spatio-temporally specific fashion. For instance, the zebrafish orthologs of members of the heterotrimeric Sec61 translocon complex – sec61al1, sec61al2, sec61b and sec61 – which threads newly synthesized proteins from the ribosome to ER lumen, all have distinct in situ expression patterns [Thisse, B. and Thisse, C. (2004) Fast Release Clones: A High Throughput Expression Analysis. ZFIN Direct Data Submission (http://zfin.org/)]. Interestingly, mutation in sec61al1 causes defects in the jaw (Nissen et al., 2006), liver and gut (K.C.S., unpublished observations) and brain ventricle laterality (Doll et al., 2011), corresponding to the organs where sec61al1 is highly expressed, indicating tissue-specific roles for this translocon component.
Screens typically yield more mutants than can be examined in detail. Although much has been learned from mutants that have obvious ties to well-studied transcriptional or signaling networks, we propose that focusing instead on those mutants resulting from defects in genes that have been previously classified as fundamental to cellular homeostasis are equally valuable because such mutants can clarify how cells are integrated into the function and formation of the organism.
In summary, in vivo cell biology in zebrafish is uncovering tissue- and organ-specific functions for genes that previously have been assumed to serve the same function across cell types. The multicolor imaging tools and multiple genetic approaches allow zebrafish researchers to address both developmental and disease-related questions pertinent to cell biology. Because of these advances, we are coming full circle back to the topics that were of interest to the cell biology founders who pioneered studies of cells in situ.
Acknowledgments
We are grateful to Jaime Chu and Michel Bagnat for critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
Funding
The work of our laboratories is supported by the Association for International Cancer Research (to M.M.); the National Institutes of Health (to K.C.S. and E.W.K.); the Zebrafish Initiative of the Vanderbilt University Academic Venture Capital Fund (to E.W.K.); and the Vanderbilt International Scholar Program (to G.U.). Deposited in PMC for release after 12 months.
References
- Alto N. M., Soderling J., Scott J. D. (2002). Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J. Cell Biol. 158, 659–668 10.1083/jcb.200204081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa K., Kawakami K. (2010). A transgenic zebrafish for monitoring in vivo microtubule structures. Dev. Dyn. 239, 2695–2699 [DOI] [PubMed] [Google Scholar]
- Bedell V. M., Wang Y., Campbell J. M., Poshusta T. L., Starker C. G., Krug R. G. I. I., Tan W., Penheiter S. G., Ma A. C., Leung A. Y. et al. (2012). In vivo genome editing using a high-efficiency TALEN system. Nature 491, 114–118 10.1038/nature11537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi P., Fermo E., Vercellati C., Boschetti C., Barcellini W., Iurlo A., Marcello A. P., Righetti P. G., Zanella A. (2009). Congenital dyserythropoietic anemia type II (CDAII) is caused by mutations in the SEC23B gene. Hum. Mutat. 30, 1292–1298 10.1002/humu.21077 [DOI] [PubMed] [Google Scholar]
- Bögershausen N., Shahrzad N., Chong J. X., von Kleist-Retzow J. C., Stanga D., Li Y., Bernier F. P., Loucks C. M., Wirth R., Puffenberger E. G. et al. (2013). Recessive TRAPPC11 mutations cause a disease spectrum of limb girdle muscular dystrophy and myopathy with movement disorder and intellectual disability. Am. J. Hum. Genet. 93, 181–190 10.1016/j.ajhg.2013.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjiev S. A., Fromme J. C., Ben J., Chong S. S., Nauta C., Hur D. J., Zhang G., Hamamoto S., Schekman R., Ravazzola M. et al. (2006). Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat. Genet. 38, 1192–1197 10.1038/ng1876 [DOI] [PubMed] [Google Scholar]
- Brooks A. S., Bertoli-Avella A. M., Burzynski G. M., Breedveld G. J., Osinga J., Boven L. G., Hurst J. A., Mancini G. M., Lequin M. H., de Coo R. F. et al. (2005). Homozygous nonsense mutations in KIAA1279 are associated with malformations of the central and enteric nervous systems. Am. J. Hum. Genet. 77, 120–126 10.1086/431244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui M., Gilady S. Y., Fitzsimmons R. E., Benson M. D., Lynes E. M., Gesson K., Alto N. M., Strack S., Scott J. D., Simmen T. (2010). Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J. Biol. Chem. 285, 31590–31602 10.1074/jbc.M110.101584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultema J. J., Ambrosio A. L., Burek C. L., Di Pietro S. M. (2012). BLOC-2, AP-3, and AP-1 proteins function in concert with Rab38 and Rab32 proteins to mediate protein trafficking to lysosome-related organelles. J. Biol. Chem. 287, 19550–19563 10.1074/jbc.M112.351908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J., Mir A., Gao N., Rosa S., Monson C., Sharma V., Steet R., Freeze H. H., Lehrman M. A., Sadler K. C. (2013). A zebrafish model of congenital disorders of glycosylation with phosphomannose isomerase deficiency reveals an early opportunity for corrective mannose supplementation. Dis. Model. Mech. 6, 95–105 10.1242/dmm.010116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinaroglu A., Gao C., Imrie D., Sadler K. C. (2011). Activating transcription factor 6 plays protective and pathological roles in steatosis due to endoplasmic reticulum stress in zebrafish. Hepatology 54, 495–508 10.1002/hep.24396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. S., Winter M., Cohen A. R., Link B. A. (2011). Generation of Rab-based transgenic lines for in vivo studies of endosome biology in zebrafish. Dev. Dyn. 240, 2452–2465 10.1002/dvdy.22758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline A., Gao N., Flanagan-Steet H., Sharma V., Rosa S., Sonon R., Azadi P., Sadler K. C., Freeze H. H., Lehrman M. A. et al. (2012). A zebrafish model of PMM2-CDG reveals altered neurogenesis and a substrate-accumulation mechanism for N-linked glycosylation deficiency. Mol. Biol. Cell 23, 4175–4187 10.1091/mbc.E12-05-0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. S., Szeto D. P., Sommers-Herivel G., Topczewski J., Solnica-Krezel L., Kang H. C., Johnson I., Kimelman D. (2005). Visualizing morphogenesis in transgenic zebrafish embryos using BODIPY TR methyl ester dye as a vital counterstain for GFP. Dev. Dyn. 232, 359–368 10.1002/dvdy.20252 [DOI] [PubMed] [Google Scholar]
- Coutinho P., Parsons M. J., Thomas K. A., Hirst E. M., Saúde L., Campos I., Williams P. H., Stemple D. L. (2004). Differential requirements for COPI transport during vertebrate early development. Dev. Cell 7, 547–558 10.1016/j.devcel.2004.07.020 [DOI] [PubMed] [Google Scholar]
- De Matteis M. A., Luini A. (2011). Mendelian disorders of membrane trafficking. N. Engl. J. Med. 365, 927–938 10.1056/NEJMra0910494 [DOI] [PubMed] [Google Scholar]
- De Vos K. J., Grierson A. J., Ackerley S., Miller C. C. (2008). Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 31, 151–173 10.1146/annurev.neuro.31.061307.090711 [DOI] [PubMed] [Google Scholar]
- Distel M., Wullimann M. F., Köster R. W. (2009). Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc. Natl. Acad. Sci. USA 106, 13365–13370 10.1073/pnas.0903060106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll C. A., Burkart J. T., Hope K. D., Halpern M. E., Gamse J. T. (2011). Subnuclear development of the zebrafish habenular nuclei requires ER translocon function. Dev. Biol. 360, 44–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W., Solnica-Krezel L., Schier A. F., Neuhauss S. C., Malicki J., Stemple D. L., Stainier D. Y., Zwartkruis F., Abdelilah S., Rangini Z. et al. (1996). A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123, 37–46 [DOI] [PubMed] [Google Scholar]
- Ellis K., Bagwell J., Bagnat M. (2013). Notochord vacuoles are lysosome-related organelles that function in axis and spine morphogenesis. J. Cell Biol. 200, 667–679 10.1083/jcb.201212095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschbach J., Sinniger J., Bouitbir J., Fergani A., Schlagowski A. I., Zoll J., Geny B., René F., Larmet Y., Marion V. et al. (2013). Dynein mutations associated with hereditary motor neuropathies impair mitochondrial morphology and function with age. Neurobiol. Dis. 58, 220–230 10.1016/j.nbd.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner J. J., Amack J. D., Nyholm M. K., Harris E. B., Yost H. J. (2005). Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 132, 1247–1260 10.1242/dev.01663 [DOI] [PubMed] [Google Scholar]
- Fischer-Parton S., Parton R. M., Hickey P. C., Dijksterhuis J., Atkinson H. A., Read N. D. (2000). Confocal microscopy of FM4-64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J. Microsc. 198, 246–259 10.1046/j.1365-2818.2000.00708.x [DOI] [PubMed] [Google Scholar]
- Freeze H. H. (2007). Congenital disorders of glycosylation: CDG-I, CDG-II, and beyond. Curr. Mol. Med. 7, 389–396 10.2174/156652407780831548 [DOI] [PubMed] [Google Scholar]
- Gabor K. A., Stevens C. R., Pietraszewski M. J., Gould T. J., Shim J., Yoder J. A., Lam S. H., Gong Z., Hess S. T., Kim C. H. (2013). Super resolution microscopy reveals that caveolin-1 is required for spatial organization of CRFB1 and subsequent antiviral signaling in zebrafish. PLoS ONE 8, e68759 10.1371/journal.pone.0068759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T., Gersbach C. A., Barbas C. F., III (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 10.1016/j.tibtech.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedeon A. K., Colley A., Jamieson R., Thompson E. M., Rogers J., Sillence D., Tiller G. E., Mulley J. C., Gécz J. (1999). Identification of the gene (SEDL) causing X-linked spondyloepiphyseal dysplasia tarda. Nat. Genet. 22, 400–404 10.1038/11976 [DOI] [PubMed] [Google Scholar]
- Gerhart S. V., Eble D. M., Burger R. M., Oline S. N., Vacaru A., Sadler K. C., Jefferis R., Iovine M. K. (2012). The Cx43-like connexin protein Cx40.8 is differentially localized during fin ontogeny and fin regeneration. PLoS ONE 7, e31364 10.1371/journal.pone.0031364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran M. C., Sato-Maeda M., Warren J. T., Su F., Lele Z., Krone P. H., Kuwada J. Y., Shoji W. (2000). Laser-induced gene expression in specific cells of transgenic zebrafish. Development 127, 1953–1960 [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Rhee J., Goll M. G., Akitake C. M., Parsons M., Leach S. D. (2008). Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish 5, 97–110 10.1089/zeb.2008.0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M. B., Ori-McKenney K. M., Scoto M., Tuck E. P., Bell S., Ma D., Masi S., Allred P., Al-Lozi M., Reilly M. M. et al. (2012). Mutations in the tail domain of DYNC1H1 cause dominant spinal muscular atrophy. Neurology 78, 1714–1720 10.1212/WNL.0b013e3182556c05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Bartholomew C. R., Zhou W., Klionsky D. J. (2009). Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy 5, 520–526 10.4161/auto.5.4.7768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F., Benzing T., Katsanis N. (2011). Ciliopathies. N. Engl. J. Med. 364, 1533–1543 10.1056/NEJMra1010172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Tanaka Y. (2009). A small GTPase, human Rab32, is required for the formation of autophagic vacuoles under basal conditions. Cell. Mol. Life Sci. 66, 2913–2932 10.1007/s00018-009-0080-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth D. L., Yin C., Yeh K., Sadler K. C. (2013). Defining hepatic dysfunction parameters in two models of Fatty liver disease in zebrafish larvae. Zebrafish 10, 199–210 10.1089/zeb.2012.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K., Clark M. D., Torroja C. F., Torrance J., Berthelot C., Muffato M., Collins J. E., Humphray S., McLaren K., Matthews L. et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503 10.1038/nature12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Xiao A., Zhou M., Zhu Z., Lin S., Zhang B. (2011). Heritable gene targeting in zebrafish using customized TALENs. Nat. Biotechnol. 29, 699–700 10.1038/nbt.1939 [DOI] [PubMed] [Google Scholar]
- Huber C., Cormier-Daire V. (2012). Ciliary disorder of the skeleton. Am. J. Med. Genet. C. Semin. Med. Genet. 160C, 165–174 10.1002/ajmg.c.31336 [DOI] [PubMed] [Google Scholar]
- Huisken J., Stainier D. Y. (2009). Selective plane illumination microscopy techniques in developmental biology. Development 136, 1963–1975 10.1242/dev.022426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson S. M., Crowley S., Hall C. M., Supramaniam G., Winter R. M. (1993). Previously unrecognized form of familial spondyloepiphyseal dysplasia tarda with characteristic facies. Clin. Dysmorphol. 2, 20–27 10.1097/00019605-199301000-00002 [DOI] [PubMed] [Google Scholar]
- Hwang W. Y., Fu Y., Reyon D., Maeder M. L., Tsai S. Q., Sander J. D., Peterson R. T., Yeh J. R., Joung J. K. (2013). Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 10.1038/nbt.2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C., Baye L. M., Amsterdam A., Besharse J. C., Link B. A. (2010). Analysis of a zebrafish dync1h1 mutant reveals multiple functions for cytoplasmic dynein 1 during retinal photoreceptor development. Neural Dev. 5, 12 10.1186/1749-8104-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Z. H., Chung H. C., Ahn Y. G., Kwon Y. K., Kim J. S., Ryu J. H., Ryu H., Kim C. H., Hwang G. S. (2012). Metabolic profiling of an alcoholic fatty liver in zebrafish (Danio rerio). Mol. Biosyst. 8, 2001–2009 10.1039/c2mb25073j [DOI] [PubMed] [Google Scholar]
- Jao L. E., Wente S. R., Chen W. (2013). Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 110, 13904–13909 10.1073/pnas.1308335110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X., Malicki J. (2009). Zebrafish ale oko, an essential determinant of sensory neuron survival and the polarity of retinal radial glia, encodes the p50 subunit of dynactin. Development 136, 2955–2964 10.1242/dev.037739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens I., Fernandez-Borja M., Marsman M., Dusseljee S., Janssen L., Calafat J., Janssen H., Wubbolts R., Neefjes J. (2001). The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680–1685 [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Schekman R. (1990). Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 61, 723–733 10.1016/0092-8674(90)90483-U [DOI] [PubMed] [Google Scholar]
- Karki S., Holzbaur E. L. (1999). Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 11, 45–53 10.1016/S0955-0674(99)80006-4 [DOI] [PubMed] [Google Scholar]
- Kawakami K. (2005). Transposon tools and methods in zebrafish. Dev. Dyn. 234, 244–254 10.1002/dvdy.20516 [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Raya A., Raya R. M., Rodríguez-Esteban C., Izpisúa Belmonte J. C. (2005). Retinoic acid signalling links left-right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature 435, 165–171 10.1038/nature03512 [DOI] [PubMed] [Google Scholar]
- Keller P. J., Schmidt A. D., Wittbrodt J., Stelzer E. H. (2008). Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069 10.1126/science.1162493 [DOI] [PubMed] [Google Scholar]
- King S. M. (2000). The dynein microtubule motor. Biochim. Biophys. Acta 1496, 60–75 10.1016/S0167-4889(00)00009-4 [DOI] [PubMed] [Google Scholar]
- King S. J., Schroer T. A. (2000). Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2, 20–24 10.1038/71338 [DOI] [PubMed] [Google Scholar]
- Knapik E. W. (2000). ENU mutagenesis in zebrafish – from genes to complex diseases. Mamm. Genome 11, 511–519 10.1007/s003350010098 [DOI] [PubMed] [Google Scholar]
- Köster R. W., Fraser S. E. (2006). FGF signaling mediates regeneration of the differentiating cerebellum through repatterning of the anterior hindbrain and reinitiation of neuronal migration. J. Neurosci. 26, 7293–7304 10.1523/JNEUROSCI.0095-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krock B. L., Mills-Henry I., Perkins B. D. (2009). Retrograde intraflagellar transport by cytoplasmic dynein-2 is required for outer segment extension in vertebrate photoreceptors but not arrestin translocation. Invest. Ophthalmol. Vis. Sci. 50, 5463–5471 10.1167/iovs.09-3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 10.1002/dvdy.21343 [DOI] [PubMed] [Google Scholar]
- Lang M. R., Lapierre L. A., Frotscher M., Goldenring J. R., Knapik E. W. (2006). Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation. Nat. Genet. 38, 1198–1203 10.1038/ng1880 [DOI] [PubMed] [Google Scholar]
- Langworthy M. M., Appel B. (2012). Schwann cell myelination requires Dynein function. Neural Dev. 7, 37 10.1186/1749-8104-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh M. W., Pittman J. E., Carson J. L., Ferkol T. W., Dell S. D., Davis S. D., Knowles M. R., Zariwala M. A. (2009). Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet. Med. 11, 473–487 10.1097/GIM.0b013e3181a53562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Sapp E., Chase K., Comer-Tierney L. A., Masso N., Alexander J., Reeves P., Kegel K. B., Valencia A., Esteves M. et al. (2009a). Disruption of Rab11 activity in a knock-in mouse model of Huntington's disease. Neurobiol. Dis. 36, 374–383 10.1016/j.nbd.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Standley C., Sapp E., Valencia A., Qin Z. H., Kegel K. B., Yoder J., Comer-Tierney L. A., Esteves M., Chase K. et al. (2009b). Mutant huntingtin impairs vesicle formation from recycling endosomes by interfering with Rab11 activity. Mol. Cell. Biol. 29, 6106–6116 10.1128/MCB.00420-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D. A., Naylor S. G., Mercurio S., Dominguez C., Talbot W. S. (2008). KBP is essential for axonal structure, outgrowth and maintenance in zebrafish, providing insight into the cellular basis of Goldberg-Shprintzen syndrome. Development 135, 599–608 10.1242/dev.012377 [DOI] [PubMed] [Google Scholar]
- Lyons D. A., Naylor S. G., Scholze A., Talbot W. S. (2009). Kif1b is essential for mRNA localization in oligodendrocytes and development of myelinated axons. Nat. Genet. 41, 854–858 10.1038/ng.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado E., Hernandez F., Lozano C., Castro M. E., Navarro R. E. (2006). The zebrafish mutant vps18 as a model for vesicle-traffic related hypopigmentation diseases. Pigment Cell Res. 19, 315–326 10.1111/j.1600-0749.2006.00320.x [DOI] [PubMed] [Google Scholar]
- Melville D. B., Knapik E. W. (2011). Traffic jams in fish bones: ER-to-Golgi protein transport during zebrafish development. Cell Adh. Migr. 5, 114–118 10.4161/cam.5.2.14377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville D. B., Montero-Balaguer M., Levic D. S., Bradley K., Smith J. R., Hatzopoulos A. K., Knapik E. W. (2011). The feelgood mutation in zebrafish dysregulates COPII-dependent secretion of select extracellular matrix proteins in skeletal morphogenesis. Dis. Model. Mech. 4, 763–776 10.1242/dmm.007625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk K. R., Voas M. G., Franzini-Armstrong C., Hakkinen I. S., Talbot W. S. (2013). Mutation of sec63 in zebrafish causes defects in myelinated axons and liver pathology. Dis. Model. Mech. 6, 135–145 10.1242/dmm.009217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. K., Roberts L. D., Liu Y., Mahon S. B., Kim S., Ryu J. H., Werdich A., Januzzi J. L., Boss G. R., Rockwood G. A. et al. (2013). Chemical and metabolomic screens identify novel biomarkers and antidotes for cyanide exposure. FASEB J. 27, 1928–1938 10.1096/fj.12-225037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauss S. C., Solnica-Krezel L., Schier A. F., Zwartkruis F., Stemple D. L., Malicki J., Abdelilah S., Stainier D. Y., Driever W. (1996). Mutations affecting craniofacial development in zebrafish. Development 123, 357–367 [DOI] [PubMed] [Google Scholar]
- Nissen R. M., Amsterdam A., Hopkins N. (2006). A zebrafish screen for craniofacial mutants identifies wdr68 as a highly conserved gene required for endothelin-1 expression. BMC Dev. Biol. 6, 28 10.1186/1471-213X-6-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X., Gao C., Jan Lo L., Luo Y., Meng C., Hong J., Hong W., Peng J. (2012). Sec13 safeguards the integrity of the endoplasmic reticulum and organogenesis of the digestive system in zebrafish. Dev. Biol. 367, 197–207 10.1016/j.ydbio.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Niwa S., Tanaka Y., Hirokawa N. (2008). KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat. Cell Biol. 10, 1269–1279 10.1038/ncb1785 [DOI] [PubMed] [Google Scholar]
- North T. E., Babu I. R., Vedder L. M., Lord A. M., Wishnok J. S., Tannenbaum S. R., Zon L. I., Goessling W. (2010). PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc. Natl. Acad. Sci. USA 107, 17315–17320 10.1073/pnas.1008209107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. (1980). Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 10.1016/0092-8674(80)90128-2 [DOI] [PubMed] [Google Scholar]
- Paquet D., Bhat R., Sydow A., Mandelkow E. M., Berg S., Hellberg S., Fälting J., Distel M., Köster R. W., Schmid B. et al. (2009). A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J. Clin. Invest. 119, 1382–1395 10.1172/JCI37537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passeri M. J., Cinaroglu A., Gao C., Sadler K. C. (2009). Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology 49, 443–452 10.1002/hep.22667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. T., Macrae C. A. (2012). Systematic approaches to toxicology in the zebrafish. Annu. Rev. Pharmacol. Toxicol. 52, 433–453 10.1146/annurev-pharmtox-010611-134751 [DOI] [PubMed] [Google Scholar]
- Postlethwait J. H., Yan Y. L., Gates M. A., Horne S., Amores A., Brownlie A., Donovan A., Egan E. S., Force A., Gong Z. et al. (1998). Vertebrate genome evolution and the zebrafish gene map. Nat. Genet. 18, 345–349 10.1038/ng0498-345 [DOI] [PubMed] [Google Scholar]
- Poupon V., Stewart A., Gray S. R., Piper R. C., Luzio J. P. (2003). The role of mVps18p in clustering, fusion, and intracellular localization of late endocytic organelles. Mol. Biol. Cell 14, 4015–4027 10.1091/mbc.E03-01-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger S., Kulkarni R. P., Darcy D., Fraser S. E., Köster R. W. (2005). Quantum dots are powerful multipurpose vital labeling agents in zebrafish embryos. Dev. Dyn. 234, 670–681 10.1002/dvdy.20524 [DOI] [PubMed] [Google Scholar]
- Rihel J., Schier A. F. (2012). Behavioral screening for neuroactive drugs in zebrafish. Dev. Neurobiol. 72, 373–385 10.1002/dneu.20910 [DOI] [PubMed] [Google Scholar]
- Roberts A. J., Kon T., Knight P. J., Sutoh K., Burgess S. A. (2013). Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 14, 713–726 10.1038/nrm3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roest Crollius H., Weissenbach J. (2005). Fish genomics and biology. Genome Res. 15, 1675–1682 10.1101/gr.3735805 [DOI] [PubMed] [Google Scholar]
- Sacher M., Kim Y. G., Lavie A., Oh B. H., Segev N. (2008). The TRAPP complex: insights into its architecture and function. Traffic 9, 2032–2042 10.1111/j.1600-0854.2008.00833.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler K. C., Amsterdam A., Soroka C., Boyer J., Hopkins N. (2005). A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development 132, 3561–3572 10.1242/dev.01918 [DOI] [PubMed] [Google Scholar]
- Sander J. D., Cade L., Khayter C., Reyon D., Peterson R. T., Joung J. K., Yeh J. R. (2011). Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat. Biotechnol. 29, 697–698 10.1038/nbt.1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S., Barrallo-Gimeno A., Melville D. B., Topczewski J., Solnica-Krezel L., Knapik E. W. (2010). Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis. PLoS ONE 5, e10367 10.1371/journal.pone.0010367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira A. H. (2006). Mitochondrial disease. Lancet 368, 70–82 10.1016/S0140-6736(06)68970-8 [DOI] [PubMed] [Google Scholar]
- Schmid B., Shah G., Scherf N., Weber M., Thierbach K., Campos C. P., Roeder I., Aanstad P., Huisken J. (2013). High-speed panoramic light-sheet microscopy reveals global endodermal cell dynamics. Nat. Commun. 4, 2207 10.1038/ncomms3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K., Cavodeassi F., Feng Y., Stephens D. J. (2013). Early stages of retinal development depend on Sec13 function. Biol. Open 2, 256–266 10.1242/bio.20133251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonteich E., Wilson G. M., Burden J., Hopkins C. R., Anderson K., Goldenring J. R., Prekeris R. (2008). The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J. Cell Sci. 121, 3824–3833 10.1242/jcs.032441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K., Iolascon A., Verissimo F., Trede N. S., Horsley W., Chen W., Paw B. H., Hopfner K. P., Holzmann K., Russo R. et al. (2009). Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat. Genet. 41, 936–940 10.1038/ng.405 [DOI] [PubMed] [Google Scholar]
- Scrivens P. J., Shahrzad N., Moores A., Morin A., Brunet S., Sacher M. (2009). TRAPPC2L is a novel, highly conserved TRAPP-interacting protein. Traffic 10, 724–736 10.1111/j.1600-0854.2009.00906.x [DOI] [PubMed] [Google Scholar]
- Scrivens P. J., Noueihed B., Shahrzad N., Hul S., Brunet S., Sacher M. (2011). C4orf41 and TTC-15 are mammalian TRAPP components with a role at an early stage in ER-to-Golgi trafficking. Mol. Biol. Cell 22, 2083–2093 10.1091/mbc.E10-11-0873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmann M., Meyer-Ohlendorf M., Achberger K., Putz S., Demestre M., Yin H., Hendrich C., Linta L., Heinrich J., Brunner C. et al. (2013). The dynactin p150 subunit: cell biology studies of sequence changes found in ALS/MND and Parkinsonian syndromes. J. Neural Transm. 120, 785–798 10.1007/s00702-012-0910-z [DOI] [PubMed] [Google Scholar]
- Suster M. L., Kikuta H., Urasaki A., Asakawa K., Kawakami K. (2009). Transgenesis in zebrafish with the tol2 transposon system. Methods Mol. Biol. 561, 41–63 10.1007/978-1-60327-019-9_3 [DOI] [PubMed] [Google Scholar]
- Thiel C., Körner C. (2011). Mouse models for congenital disorders of glycosylation. J. Inherit. Metab. Dis. 34, 879–889 10.1007/s10545-011-9295-7 [DOI] [PubMed] [Google Scholar]
- Townley A. K., Feng Y., Schmidt K., Carter D. A., Porter R., Verkade P., Stephens D. J. (2008). Efficient coupling of Sec23-Sec24 to Sec13-Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J. Cell Sci. 121, 3025–3034 10.1242/jcs.031070 [DOI] [PubMed] [Google Scholar]
- Townley A. K., Schmidt K., Hodgson L., Stephens D. J. (2012). Epithelial organization and cyst lumen expansion require efficient Sec13-Sec31-driven secretion. J. Cell Sci. 125, 673–684 10.1242/jcs.091355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L. D., Hino H., Quach H., Lim S., Shindo A., Mimori-Kiyosue Y., Mione M., Ueno N., Winkler C., Hibi M. et al. (2012). Dynamic microtubules at the vegetal cortex predict the embryonic axis in zebrafish. Development 139, 3644–3652 10.1242/dev.082362 [DOI] [PubMed] [Google Scholar]
- Tsedensodnom O., Vacaru A. M., Howarth D. L., Yin C., Sadler K. C. (2013). Ethanol metabolism and oxidative stress are required for unfolded protein response activation and steatosis in zebrafish with alcoholic liver disease. Dis. Model. Mech. 6, 1213–1226 10.1242/dmm.012195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M., Omori Y., Biyanwila J., Malicki J. (2007). Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proc. Natl. Acad. Sci. USA 104, 14819–14824 10.1073/pnas.0700178104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unlu G., Levic D. S., Melville D. B., Knapik E. W. (2013). Trafficking mechanisms of extracellular matrix macromolecules: Insights from vertebrate development and human diseases. Int. J. Biochem. Cell Biol 47, 57–67 10.1016/j.biocel.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade R. H. (2007). Microtubules: an overview. Methods Mol. Med. 137, 1–16 10.1007/978-1-59745-442-1_1 [DOI] [PubMed] [Google Scholar]
- Wasmeier C., Romao M., Plowright L., Bennett D. C., Raposo G., Seabra M. C. (2006). Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J. Cell Biol. 175, 271–281 10.1083/jcb.200606050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedon M. N., Hastings R., Caswell R., Xie W., Paszkiewicz K., Antoniadi T., Williams M., King C., Greenhalgh L., Newbury-Ecob R. et al. (2011). Exome sequencing identifies a DYNC1H1 mutation in a large pedigree with dominant axonal Charcot-Marie-Tooth disease. Am. J. Hum. Genet. 89, 308–312 10.1016/j.ajhg.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler F., Gillingham A. K., Sinka R., Rosa-Ferreira C., Gordon D. E., Franch-Marro X., Peden A. A., Vincent J. P., Munro S. (2010). A genome-wide RNA interference screen identifies two novel components of the metazoan secretory pathway. EMBO J. 29, 304–314 10.1038/emboj.2009.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A., Sarkar S., Cuddon P., Ttofi E. K., Saiki S., Siddiqi F. H., Jahreiss L., Fleming A., Pask D., Goldsmith P. et al. (2008). Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 4, 295–305 10.1038/nchembio.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. R., Morgan J. L., Kerschensteiner D., Wong R. O. (2013). In vivo imaging of zebrafish retina. Cold Spring Harb. Protoc. 2013, pdb.prot072652. [DOI] [PubMed] [Google Scholar]
- Woods I. G., Kelly P. D., Chu F., Ngo-Hazelett P., Yan Y. L., Huang H., Postlethwait J. H., Talbot W. S. (2000). A comparative map of the zebrafish genome. Genome Res. 10, 1903–1914 10.1101/gr.10.12.1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods I. G., Wilson C., Friedlander B., Chang P., Reyes D. K., Nix R., Kelly P. D., Chu F., Postlethwait J. H., Talbot W. S. (2005). The zebrafish gene map defines ancestral vertebrate chromosomes. Genome Res. 15, 1307–1314 10.1101/gr.4134305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A., Wang Z., Hu Y., Wu Y., Luo Z., Yang Z., Zu Y., Li W., Huang P., Tong X. et al. (2013). Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Res. 41, e141 10.1093/nar/gkt464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Farage E., Sugimoto M., Anand-Apte B. (2010). A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev. Biol. 10, 76 10.1186/1471-213X-10-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanicostas C., Herbomel E., Dipietromaria A., Soussi-Yanicostas N. (2009). Anosmin-1a is required for fasciculation and terminal targeting of olfactory sensory neuron axons in the zebrafish olfactory system. Mol. Cell. Endocrinol. 312, 53–60 10.1016/j.mce.2009.04.017 [DOI] [PubMed] [Google Scholar]
- Yin C., Evason K. J., Maher J. J., Stainier D. Y. (2012). The basic helix-loop-helix transcription factor, heart and neural crest derivatives expressed transcript 2, marks hepatic stellate cells in zebrafish: analysis of stellate cell entry into the developing liver. Hepatology 56, 1958–1970 10.1002/hep.25757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York A. G., Parekh S. H., Dalle Nogare D., Fischer R. S., Temprine K., Mione M., Chitnis A. B., Combs C. A., Shroff H. (2012). Resolution doubling in live, multicellular organisms via multifocal structured illumination microscopy. Nat. Methods 9, 749–754 10.1038/nmeth.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Ito A., Matsuda N., Mishina M. (2002). Regulation by protein kinase A switching of axonal pathfinding of zebrafish olfactory sensory neurons through the olfactory placode-olfactory bulb boundary. J. Neurosci. 22, 4964–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Z., Ouyang Y. C., Hou Y., Schatten H., Chen D. Y., Sun Q. Y. (2008). Mitochondrial behavior during oogenesis in zebrafish: a confocal microscopy analysis. Dev. Growth Differ. 50, 189–201 10.1111/j.1440-169X.2008.00988.x [DOI] [PubMed] [Google Scholar]
- Zhao C., Takita J., Tanaka Y., Setou M., Nakagawa T., Takeda S., Yang H. W., Terada S., Nakata T., Takei Y. et al. (2001). Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell 105, 587–597 10.1016/S0092-8674(01)00363-4 [DOI] [PubMed] [Google Scholar]