Abstract
Serotonergic neurons in the dorsal raphe nucleus (DR) are organized in anatomically distinct subregions that form connections with specific brain structures to modulate diverse behaviors, including anxiety-like behavior. It is unclear if the functional heterogeneity of these neurons is coupled to their developmental heterogeneity, and if abnormal development of specific DR serotonergic subregions can permanently impact anxiety circuits and behavior. The goal of this study was to examine if deficiencies in different components of fibroblast growth factor (Fgf) signaling could preferentially impact the development of specific populations of DR serotonergic neurons to alter anxiety-like behavior in adulthood. Wild-type and heterozygous male mice globally hypomorphic for Fgf8, Fgfr1, or both (Fgfr1/Fgf8) were tested in an anxiety-related behavioral battery. Both Fgf8- and Fgfr1/Fgf8-deficient mice display increased anxiety-like behavior as measured in the elevated plus-maze and the open-field tests. Immunohistochemical staining of a serotonergic marker, tryptophan hydroxylase (Tph), revealed reductions in specific populations of serotonergic neurons in the ventral, interfascicular, and ventrolateral/ventrolateral periaqueductal gray subregions of the DR in all Fgf-deficient mice, suggesting a neuroanatomical basis for increased anxiety-like behavior. Overall, this study suggests Fgf signaling selectively modulates the development of different serotonergic neuron subpopulations. Further, it suggests anxiety-like behavior may stem from developmental disruption of these neurons, and individuals with inactivating mutations in Fgf signaling genes may be predisposed to anxiety disorders.
Keywords: Fibroblast growth factor, Serotonin, Dorsal raphe nucleus, Anxiety
1. Introduction
Serotonergic neurons in the dorsal raphe nucleus (DR) modulate diverse physiological and behavioral outputs, including anxiety-like behavior [1]. DR serotonergic neurons are functionally heterogeneous and are organized into five functional topographically organized subregions (dorsal (DRD), ventral (DRV), ventrolateral DR/ventrolateral periaqueductal gray (DRVL/VLPAG), interfascicular (DRI), and caudal (DRC)) with distinct anatomical locations, afferent inputs, efferent targets, and physiological properties [1–4]. Two different serotonergic subsystems that modulate anxiety-like states emerge from this functional topography [5, 6]. One facilitates anxiety-like responses and includes subpopulations of serotonergic neurons in the DRD and DRC. Another system that includes DRVL/VLPAG, DRV, and DRI are co-activated in conditions associated with the inhibition of panic-like responses and thought to promote stress-resistance. Therefore, loss of and/or failure to activate subpopulations of these panic-reducing serotonergic cells can lead to increased vulnerability to panic- and anxiety-like responses [5, 6]. Thus, data suggest that while some subpopulations of DR serotonergic neurons facilitate anxiety-like responses, others inhibit anxiety- or panic-like responses.
The genesis and organization of the DR serotonergic neuronal populations are orchestrated by a number of signaling molecules and transcriptional networks during development [7–9]. Of these, fibroblast growth factor 8 (Fgf8) and one of its cognate receptors, Fgf receptor 1 (Fgfr1), represent morphogenic signals most critical to the early genesis and organization of the DR serotonergic neurons [10]. During development, Fgf8 is expressed in a temporally and spatially restricted fashion [11–13], and the secreted Fgf8 protein creates a diffusion gradient essential for the anterior-posterior patterning of the developing hindbrain region and specification of serotonergic cell fate [13–16]. In this regard, developmental deficiencies of Fgf8 and Fgfr1 may lead to abnormally formed DR serotonergic neuron populations and impact anxiety-related behaviors modulated by these neurons.
A complication associated with the study of DR serotonergic neurons is their heterogeneity. Not only are these neurons functionally heterogeneous [2], they are also developmentally heterogeneous [17, 18]. For example, in the hindbrain, the transcription factor Pet-1 is found exclusively in serotonergic neurons and is critical for the differentiation, maturation and maintenance of serotonergic neuronal phenotype [19]. Despite this critical role, about 20–30% of serotonergic neurons do not require Pet-1 for differentiation [19, 20]. Further analysis revealed that all DR serotonergic neurons in this Pet-1-independent population project to the same functionally related forebrain regions that modulate affective behavior [20], suggesting DR serotonergic neurons with similar developmental requirements are also similar in function. Although previous studies have reported malformations of the developing DR in association with Fgf signaling deficiencies [21–24], the differential impacts of Fgf signaling disruption on serotonergic neurons in DR subregions have not been described in detail and lack topographical resolution. The behavioral outcome of these differential impacts has also not been examined.
The goal of the present study is to use transgenic mouse models deficient in Fgf8, Fgfr1, or both to understand the differential impact of these deficiencies on the topographically organized DR serotonergic neurons and anxiety-related behavior. These mouse models may also provide clinically useful insights into the phenotypic manifestations, including any anxiety disorders, in humans harboring loss-of-function mutations on Fgfr1 and Fgf8 genes [25, 26]. Our results suggest that serotonergic neurons in some DR subregions are more dependent on Fgf signaling than others, and their disruption was associated with increased anxiety-like behavior. Overall, these data expand our knowledge on developmental heterogeneity of serotonergic neurons and correlate the disruption of specific DR serotonergic subpopulations to specific behavioral outcomes.
2. Materials and Methods
2.1 Animals
All experiments were conducted using 8–10 week-old offspring from crosses of Fgfr1 (129sv/CD-1; Canadian Mutant Mouse Repository, Toronto, ON) and Fgf8 heterozygous hypomorphic mice (129p2/OlaHsd* CD-1; obtained from Mouse Regional Resource Centers, Davis, CA) [27, 28]. Fgfr1 and Fgf8 hypomorphic mice contain a neomycin-resistance element inserted into non-coding regions of the Fgfr1 or Fgf8 genes. This element contains false splice sites which lead to about a 66–80% and 55% reduction in functional Fgfr1 and Fgf8 transcript levels, respectively [27, 28], under homozygous condition. Both Fgfr1 and Fgf8 homozygous hypomorphic mice die within 24 h of birth but heterozygous (HET) mice survive normally and have no obvious health problems. The four offspring genotypes used in these studies were: wild-type (WT), Fgfr1 HET, Fgf8 HET, and Fgfr1/Fgf8 double HET (Fgfr1/Fgf8 HET). Male mice were housed in same-sex littermate groups of 2–5 at weaning and genotyped using DNA isolated from tail clips and polymerase chain reaction. All mice were bred at the University of Colorado Boulder in the Integrative Physiology department animal facility under a 12L:12D photoperiod with free access to water and rodent chow. All animal procedures complied with the protocols approved by the Institutional Animal Care and Use Committee at the University of Colorado Boulder.
2.2 Battery of behavioral tests
2.2.1 General procedures
Two cohorts of male mice (Cohort 1: n = 3 WT, n = 4 Fgfr1 HET, n = 10 Fgf8 HET, n = 4 Fgfr1/Fgf8 HET; Cohort 2: n = 12 WT, n = 12 Fgfr1 HET, n = 12 Fgf8 HET, n = 15 Fgfr1/Fgf8 HET) were used to test anxiety-related behavior in a test battery. Both cohorts of mice experienced the exact same behavioral testing procedures, except the second cohort of mice were also tested for motor ability following the completion of the behavioral battery. Other than the handling associated with cage changes, mice were not handled prior to behavioral testing. Behavioral testing commenced 2 h and was completed within 6 h of light phase onset. The interval between different anxiety-related behavioral tests in the test battery was 2 days [29] and was conducted in the following order: (1) elevated plus-maze, (2) open-field, and (3) light-dark exploration. Despite the anxiogenic nature of the elevated plus-maze test, it was performed first as it has been shown to be sensitive to prior testing experience [30, 31]. Due to this design, we cannot rule out the possibility that exposure to the elevated plus-maze test interacted with Fgf deficiencies to influence behavior on subsequent tests. Two additional motor tasks were performed in the second cohort of mice immediately after the light-dark exploration test: vertical pole, and wire grip tests. Table 1 outlines the testing order and interval for the mice. Room lighting was approximately 480 lux. Behavioral testing equipment was cleaned with 70% ethanol before testing and in between each test subject. A video camera was mounted above the behavioral test apparatus and behavior was recorded for later scoring by an observer blinded to the genotypes. For each behavioral test, the entries or total duration within an area began when all four paws crossed into the area of interest.
Table 1.
Sequence of anxiety-related behavioral and motor tests
| Day 1 | Day 3 | Day 5 |
|---|---|---|
| Elevated plus-maze | Open-field | Light-dark exploration Vertical pole (Cohort 2) Wire grip (Cohort 2) |
2.2.2 Elevated plus-maze (EPM)
The brown acrylic EPM consisted of a center area (5.5 cm × 5.5 cm) from which two opposing open arms (30 cm × 5.5 cm) and two opposing closed arms with the same dimensions and walls (15 cm high) were extended. The maze was elevated 60 cm off the ground. Mice were placed in the center area of the EPM facing an open arm to start the 5 min test [30, 32]. Mice that fell off the maze were excluded from analysis (n = 1 WT, n = 1 Fgfr1 HET, n = 5 Fgf8 HET, n = 5 Fgfr1/Fgf8 HET). The time spent in the open, closed and center areas and number of entries into each arm were scored manually. For analysis, the time spent on the arms and number of entries were expressed as a percentage of the total test duration and number of arm entries, respectively.
2.2.3 Open-field (OF)
The OF test measures both locomotion and anxiety-related behaviors. Mice were placed in the center of a white 40 cm × 40 cm × 30 cm-high white acrylic box with an open top and recorded for 15 min [32, 33]. Sixteen 10 cm × 10 cm squares were drawn onto the OF floor to visually divide the box into an outer perimeter zone surrounding an inner zone (20 cm × 20 cm) for analysis by EthoVision XT software (version 6.0; Noldus Information Technologies). The time spent (expressed as percent time for analysis) in each zone and total distance traveled (locomotor activity) were scored.
2.2.4 Light-dark exploration (LD)
The final anxiety-related behavioral test in the battery was the LD test [34, 35]. An acrylic box was divided into two unequal-sized compartments. The larger “light” compartment was white with an open top (25 cm × 20 cm × 30 cm) and was connected to the “dark” smaller enclosed black compartment (15 cm × 20 cm × 30 cm) by a floor-level 7.5 cm × 7.5 cm opening centered in the partition separating the two compartments. Mice were placed in the middle of the light compartment facing away from the dark compartment, and the time spent (expressed as percent time for analysis) and total distance traveled in the light compartment during the 10 min test were scored using EthoVision XT software (version 6.0; Noldus Information Technologies). The latency to enter the dark compartment and total number of transitions were scored manually.
2.2.5 Vertical pole and wire grip tests
The vertical pole and wire grip tests were included in Cohort 2 to measure motor coordination, balance, and strength [36]. Both tests were performed as described by [36] immediately after the LD test. Briefly, the vertical pole test consisted of placing a mouse on the center of a wooden dowel (2 cm × 40 cm) wrapped in masking tape that is elevated above a cage filled with bedding. The dowel is lifted from a horizontal to vertical position over the course of 45 s. Mice that remained on the pole throughout the test were considered to have passed the test. The wire grip test was performed 15–30 s after the vertical pole test. As described [36], mice were placed on a wire cage top that was tapped three times to cause the mouse to grip and subsequently turned upside down. The cage top was held about 20 cm above a cage filled with bedding for 60 s. The latency to fall was recorded. Mice that fell while the cage top was being inverted were excluded from analysis (n = 5 WT, n = 4 Fgfr1 HET, n = 4 Fgf8 HET, n = 1 Fgfr1/Fgf8 HET).
2.3 Tissue collection and preparation
For immunohistochemistry, behaviorally naive male mice (n = 8 WT, n = 8 Fgfr1 HET, n = 7 Fgf8 HET, n = 10 Fgfr1/Fgf8 HET) were terminally anesthetized with pentobarbital sodium and perfused transcardially with 15 mL of heparinized saline and 50 mL of 4% paraformaldehyde in 0.1 M sodium phosphate buffer. Brains were removed and post-fixed in 4% paraformaldehyde for 24 h at 4°C then cryoprotected in 30% sucrose until sectioning. Before sectioning, brains were blocked at the caudal border of the mammillary body using a mouse brain matrix (RBM 2000C, ASI Instruments). The tissue block posterior to the mammillary body containing the midbrain raphe complex was immediately sectioned using a cryostat into 30 µm frozen coronal floating sections that were collected into a series of six microcentrifuge tubes filled with a cryoprotectant (30% sucrose, 30% ethylene glycol, 1% polyvinylpyrolidone in 0.2 M sodium phosphate buffer).
2.4 Immunohistochemistry (IHC)
The IHC used tryptophan hydroxylase (Tph; the rate-limiting enzyme for serotonin biosynthesis) as a marker of serotonergic neurons. Briefly, one third of the sections were taken through a series of rinses and sequential incubations on an orbital shaker with a sheep anti-tryptophan hydroxylase antibody that has been previously characterized and has been shown to bind specifically to both isoforms of Tph [37, 38] (T8575, Sigma-Aldrich), a biotinylated donkey anti-sheep secondary antibody (713-065-147, Jackson ImmunoResearch Laboratories), avidin-biotin complex (ABC; NeutrAvidin® biotin-binding protein, A2666, Life Technologies; Peroxidase-biotinamidocaproyl conjugate, P-9568, Sigma-Aldrich), and reacted with 3,3′-diaminobenzidine (DAB; D5637, Sigma-Aldrich) for color detection [37–39]. After the color reaction, sections were rinsed, mounted on gelatin-coated glass slides, dehydrated through increasing concentrations of ethanol (70– 100%), cleared in Histo-Clear (National Diagnostics), and coverslipped with Permount (Fisher Scientific).
2.5 Quantification of Tph neurons
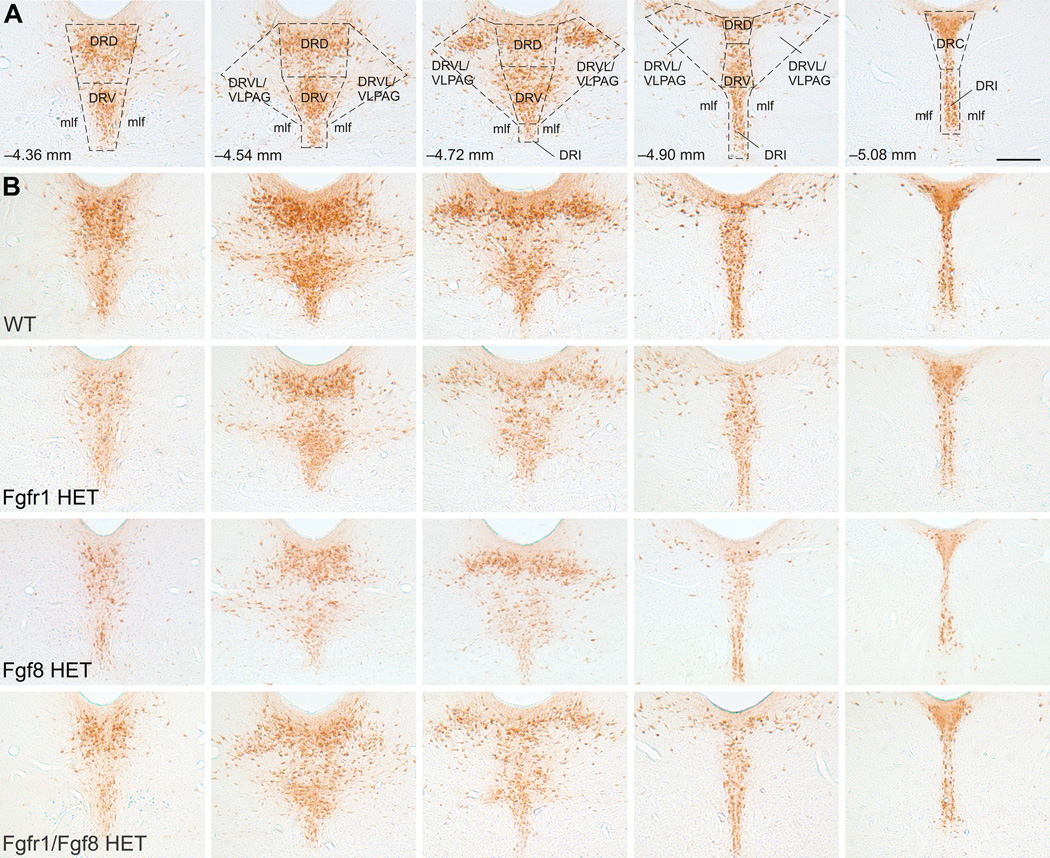
The numbers of Tph-immunoreactive (ir) neurons were counted by an investigator blind to the treatment groups at five rostrocaudal levels (−4.36, −4.54, −4.72, −4.90, and −5.08 mm bregma, Fig. 1A) under a brightfield microscope. Tph-ir neurons were quantified in the dorsal (DRD; −4.36, −4.54, −4.72, and −4.90 mm bregma), ventral (DRV; −4.36, −4.54, −4.72, and −4.90 mm bregma), ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG; −4.54, −4.72, and −4.90 mm bregma), interfascicular (DRI; −4.72, −4.90, and −5.08 bregma), and caudal (DRC; −5.08 mm bregma) subregions of the DR. Representative photomicrographs for each genotype at each rostrocaudal level of the DR are shown in Fig. 1B.
Fig. 1.
(A) Schematic overlay outlining each DR subregion where Tph-ir neurons were quantified at five rostrocaudal levels. Each column represents an anatomical level organized from rostral (left) to caudal (right). Distance from bregma (mm) is indicated in lower left hand corner. Scale bar, 250 µm for all images in figure. (B) Representative photomicrographs for each genotype (organized by row) at each rostrocaudal level. Abbreviations: DRD, dorsal raphe nucleus, dorsal part; DRC, dorsal raphe nucleus, caudal part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; DRI, dorsal raphe nucleus, interfascicular part; mlf, medial longitudinal fasciculus.
2.6 Statistical analysis
All statistical analyses were completed using SPSS Statistics (version 21.0 for Mac; IBM). Cohorts 1 and 2 were combined for the anxiety-related behavioral test analyses. The behavioral and wire grip tests were analyzed using one-way ANOVA with Welsh’s correction for unequal variance when necessary, followed by planned pairwise contrasts corrected for unequal variance when appropriate. All mice passed the vertical pole test; hence no data analysis was performed. Data for the number of Tph-ir neurons were analyzed using a linear mixed model analysis using genotype as the between-subjects factor and subregion as the repeated-measure. Planned pairwise contrasts corrected for unequal variance when appropriate were applied for each of the five subregions of the DR to reveal subregion-specific genotype effects on Tph-ir neuron number. Statistical outliers were determined using the Grubbs’ test and were removed [40]. For the EPM, 2 out of 72 data points for percent time in open arms were excluded (2.8% of total data), and 1 out of 72 data points for each the percent time in closed arms and center area were excluded (1.4% of total data for each); for the OF, 1 out of 72 data points for percent time in outer zone were excluded (1.4% of total data) and 4 out of 72 data points for percent time in inner zone were excluded (5.6% of total data). There were no outliers for the LD. Values are shown as the mean ± the standard error of the mean (SEM). Data were significant when p < 0.05.
3. Results
3.1 EPM
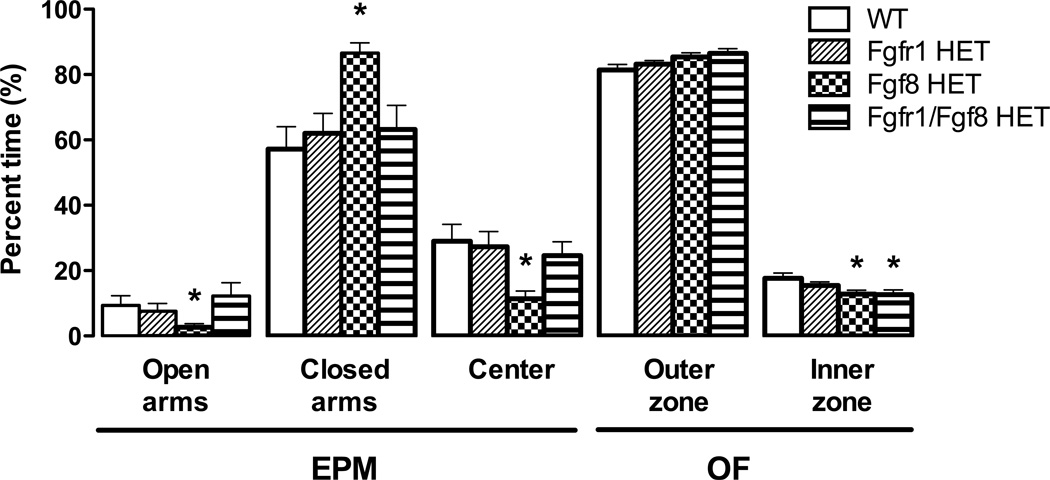
As shown in Fig. 2, there was a statistically significant genotype effect on the percentage of time spent in the open [Welsh’s F(3, 24.56) = 3.45, p = 0.032], closed [Welsh’s F(3, 26.97) = 8.57, p = 0.001], and center area [Welsh’s F(3, 27.66) = 5.92, p = 0.003] of the EPM. Post hoc planned contrasts revealed that Fgf8 HET mice spent significantly less time on the open arms [t(18.37) = 3.89, p = 0.001] and center area [t(18.57) = −3.11 p = 0.006] and more time in the closed arms [t(15.43) = −2.16, p = 0.047] than WT mice. There were no significant genotype differences in the total number of closed arm entries (a measure of exploratory behavior and motor function; data not shown) or percentage of open or closed entries (Table 2).
Fig. 2.
The elevated plus-maze (EPM) and open-field (OF) tests were used to detect anxiety-like behavior in Fgf-deficient mice. Only Fgf8 HET mice exhibited increased anxiety-like behavior in the EPM compared to WT controls as measured by a lower percentage of time spent in the open arms (n = 13–14 WT, n = 14–15 Fgfr1 HET, n = 16–17 Fgf8 HET, n = 13 Fgfr1/Fgf8 HET). However, both Fgf8 HET and Fgfr1/Fgf8 HET mice spent significantly less time in the inner zone of the OF (an indication of increased anxiety-like behavior) compared to WT controls (n = 15 WT, n = 14–15 Fgfr1 HET, n = 21–22 Fgf8 HET, n = 18–19 Fgfr1/Fgf8 HET). *p < 0.05 vs. WT; bars represent the mean ± SEM.
Table 2.
Summary of behavioral and motor tests
| WT | Fgfr1 HET | Fgf8 HET | Fgfr1/Fgf8 HET | ||
|---|---|---|---|---|---|
| EPM | % Open entries | 19.93 ± 5.82 | 15.51 ± 4.06 | 8.77 ± 3.61 | 24.80 ± 7.33 |
| % Closed entries | 80.07 ± 5.82 | 81.78 ± 4.65 | 91.23 ± 3.61 | 75.20 ± 7.33 | |
| OF | Total distance traveled (cm) | 4982 ± 185 | 4756 ± 284 | 4691 ± 179 | 4467 ± 178 |
| LD | Total distance traveled in light compartment (cm) | 1886 ± 92 | 1787 ± 107 | 1684 ± 117 | 1763 ± 119 |
| Latency to enter dark compartment (s) | 9.00 ± 1.68 | 7.19 ± 1.24 | 5.90 ± 0.81 | 8.33 ± 1.40 | |
| Total number transitions (#) | 28.8 ± 1.17 | 25.44 ± 1.87 | 25.18 ± 1.28 | 27.68 ± 1.46 | |
| % Time in light compartment | 52.71 ± 3.55 | 48.31 ± 3.62 | 47.18 ± 3.11 | 49.09 ± 2.98 | |
| Grip Test | Latency to fall (s) | 54.29 ± 3.48 | 49.25 ± 5.47 | 58.75 ± 0.996 | 52.00 ± 3.36 |
No significant differences between genotypes were found for any behavior or motor task listed in this table. EPM, OF, LD (n = 14–15 WT, n = 14–16 Fgfr1 HET, n = 16–22 Fgf8 HET, n = 13–19 Fgfr1/Fgf8 HET). Grip test (n = 7 WT, n = 8 Fgfr1 HET, n = 8 Fgf8 HET, n = 14 Fgfr1/Fgf8 HET). Values represent the mean ± SEM.
3.2 OF
Locomotor activity and anxiety-like behavior were measured during the OF test. There were no genotype differences in the total distance traveled (Table 2), indicating that motor function is not impacted by Fgf deficiency. There was a significant effect of genotype on the percentage of time spent in the inner zone of the OF [F(3, 64) = 3.04, p = 0.035] and a corresponding trend towards differences in time spent in the outer zone that did not reach statistical significance [F(3, 67) = 2.53, p = 0.065]. Post hoc planned contrasts revealed that both Fgf8 HET [t(64) = −2.55 p = 0.013] and Fgfr1/Fgf8 HET mice [t(64) = −2.58 p = 0.012] displayed increased anxiety-like behavior by spending significantly less time in the inner zone compared to WT controls (Fig. 2).
3.3 LD, vertical pole, and wire grip tests
There was no significant genotype effect on any LD behavior or latency to fall during the wire grip motor task (Table 2). All mice passed the vertical pole test.
3.4 Tph-ir neuron counts
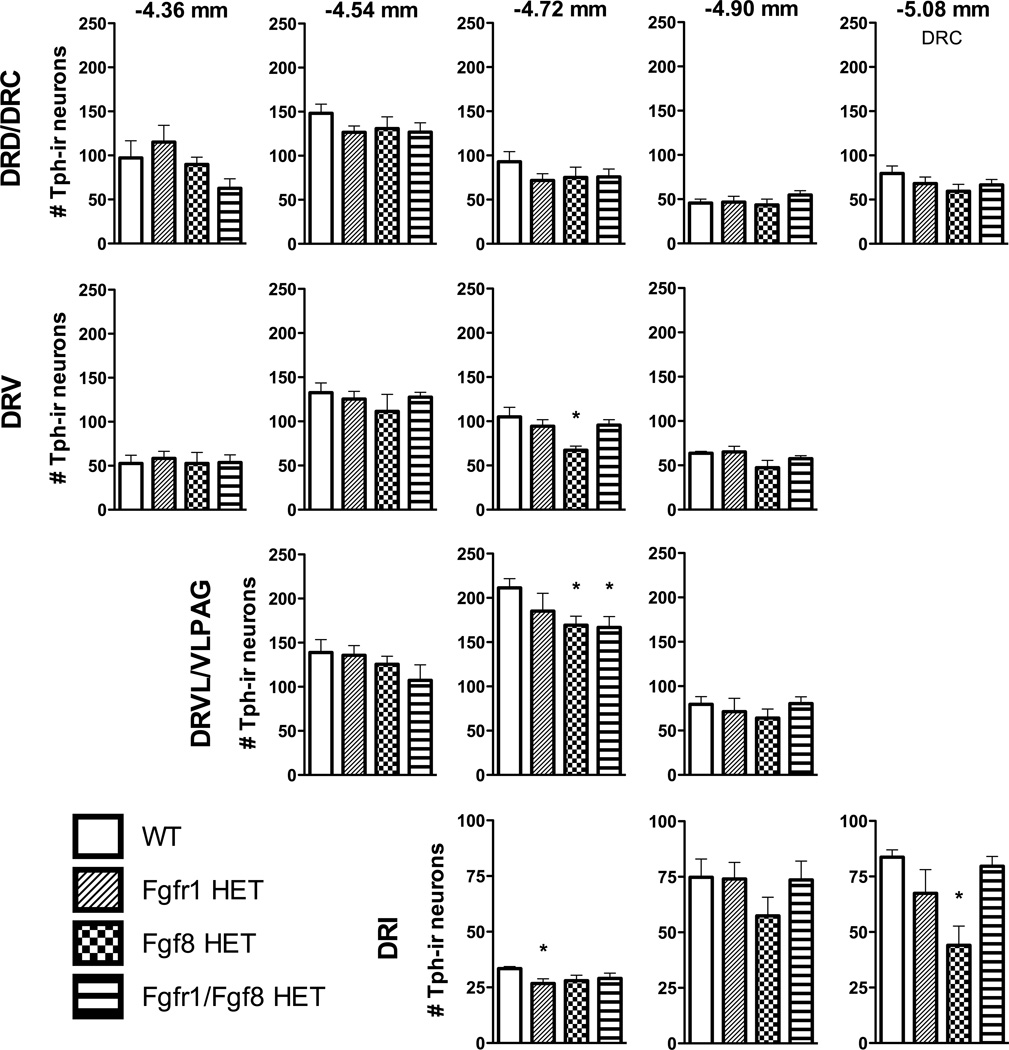
Linear mixed model analysis of the number of Tph-ir neurons within specific subregions of the DR revealed a significant interaction between subregion and genotype [F(42, 29.12) = 1.84 p = 0.044]. As Fig. 3 illustrates, post hoc planned contrasts revealed significant reductions in the number of Tph-ir neurons mainly in the mid- to caudal DR between WT and Fgf8 HET in the DRV and DRVL [−4.72 mm bregma; t(28) = −3.35, p = 0.002, t(11.99) = −2.89, p = 0.014, respectively] and DRI [−5.08 mm bregma; t(7.70) = −4.29, p = 0.003], between WT and Fgfr1 HET in the DRI [−4.72 mm bregma; t(9.73) = −2.93, p = 0.016], and between WT and Fgfr1/Fgf8 HET in the DRVL [−4.72 mm bregma; t(14.97) = −2.76, p = 0.015]. There were also significant main effects for both genotype [F(3, 28.12) = 4.01, p = 0.017] and DR subregion F(14, 27.87) = 172.96, p = 0.001]. Post hoc analyses indicated that Fgf8 HET [t(22) = −3.79, p = 0.001] and Fgfr1/Fgf8 HET [t(22) = −2.68, p = 0.014] mice had significantly fewer total DR Tph-ir neurons than WT controls (1166 ± 68.74, 1264 ± 53.36, 1479 ± 56.65, mean ± SEM for Fgf8 HET, Fgfr1/Fgf8 HET, and WT, respectively).
Fig. 3.
The number of Tph-ir neurons for each genotype within different subregions of the DR at five rostrocaudal levels. DR subregions are organized by row, and anatomical levels are organized in columns from rostral (left) to caudal (right). Distance from bregma is indicated above each column in millimeters. N = 7–8 WT, n = 6–8 Fgfr1 HET, n = 7 Fgf8 HET, n = 9–10 Fgfr1/Fgf8 HET; *p < 0.05 vs. WT; bars represent the mean ± SEM. Abbreviations: DRD, dorsal raphe nucleus, dorsal part; DRC, dorsal raphe nucleus, caudal part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; DRI, dorsal raphe nucleus, interfascicular part.
4. Discussion
Fgf signaling deficiencies differentially reduced subpopulations of DR serotonergic neurons, and these reductions were associated with elevated anxiety-like behavior as measured by the EPM and OF tests in adult male mice. Decreases in serotonergic neurons were restricted to specific subregions within the DR. Specifically, Fgf8 deficiency increased anxiety-like behavior and decreased serotonergic cell numbers in the DRVL/VLPAG, caudal DRV, and DRI. Due to the unique projections to and from these serotonergic cell groups, they have collectively been implicated in multiple animal models of chronic anxiety-like states and increased susceptibility to panic- and anxiety-like behaviors, including models of early life adverse experience [2, 6]. The effects of compound Fgfr1 and Fgf8 deficiencies were somewhat similar to Fgf8 deficiency alone but less severe. These data support the documented necessity of Fgf signaling in the formation of DR serotonergic neurons [16, 21, 24, 41]. Importantly, they highlight the subregional specificity of Fgf signaling in the developing DR and behavioral consequences associated with Fgf8 or Fgfr1 deficits.
Serotonin-modulated anxiety-like behaviors depend on the unique afferent and efferent connections between the DR and selective brain structures involved in emotional regulation. There are two DR serotonergic subsystems that modulate anxiety-like states: the anxiety-promoting DRD/DRC and the anxiety-reducing DRVL/VLPAG, caudal DRV, and DRI systems (for in depth reviews see [5, 6]). DRD/DRC connect with forebrain structures involved in emotional regulation and anxiety-related behavior such as the infralimbic and prelimbic cortices, lateral habenula, central and basolateral nucleus of the amygdala, and bed nucleus of the stria terminalis [2, 5]. Together these circuits facilitate anxiety-like responses to anxiogenic drugs, inescapable shock, and behavioral tests such as social defeat and fear-potentiated startle [42–45]. On the other hand, the DRVL/VLPAG connect with brain structures involved in both the autonomic and behavioral components of emotional states including the rostral ventrolateral medulla, dorsal periaqueductal gray, lateral hypothalamus, lateral parabrachial nucleus, nucleus of the solitary tract, central nucleus of the amygdala, lateral and perifornical hypothalamic nuclei, median preoptic area, and the infralimbic cortex [2, 5]. The DRI has afferent and efferent connections with several forebrain structures involved in emotional control including the infralimbic and prelimbic cortices, dorsal and ventral hippocampus, median preoptic nucleus and lateral parabrachial nucleus and is thought to be co-activated with the DRVL/VLPAG and caudal DRV to inhibit panic-like responses and promote stress resistance [5, 6]. Our data suggest Fgf8 deficiency disrupts a subpopulation of “stress-resistant” serotonergic neurons and possibly the associated connectivity, leading to elevated anxiety-like behavior. Indeed, reduced activity in these anxiolytic serotonergic subregions has been implicated in multiple animal models of chronic anxiety-like states and increased susceptibility to panic- and anxiety-like behaviors, including models of early life adverse experience [2, 5].
Fgf signaling deficiency was associated with decreased serotonergic cell numbers in specific subregions of the DR, including the DRVL/VLPAG (Fgf8 HET and Fgfr1/Fgf8 HET), caudal DRV (Fgf8 HET), and DRI (Fgf8 and Fgfr1 HET). Based on the modulatory roles of serotonin in these circuitries, these affected DR subregions have been implicated in rodent models of panic- and anxiety-like behavior. These models include amygdala priming, adolescent social isolation, and disinhibition of the dorsomedial hypothalamus [46–48]. For example, adolescent social isolation in rats, which led to increased vigilance behaviors following treatment with an anxiogenic drug, was associated with lower baseline tph expression in the DRVL/VLPAG and caudal DRV. Similarly, in a model of panic-like anxiety, serotonergic neurons in the DRVL/VLPAG and caudal DRV and DRI became dysregulated in panic-prone rats and could not be activated by sodium lactate (a panicogenic agent) [46]. Given their role in reducing panic-like responses, loss of neurons in these subregions may lead to increased panic-vulnerability. Together these data suggest that loss of function of subsets of serotonergic neurons in the DRVL/VLPAG, caudal DRV, and DRI may contribute to increased vulnerability to panic- and anxiety-like behaviors. Despite this evidence in postnatal models, detailed subregional analyses of DR have not been described in animal models where serotonergic neurons are disrupted prenatally [19–21, 24, 49, 50]. To our knowledge, this study is the first to demonstrate developmental disruption of specific serotonergic subregions and a correlation between neuroanatomical and behavioral disruptions.
The mechanisms underlying the topographical specificity of Fgf8 deficiency on serotonergic neuron development are unclear. One possibility is related to the spatial pattern of Fgf8 distribution during development. Peak Fgf8 expression in the developing hindbrain occurs around embryonic day (E) 9–9.5 and is restricted to a tight band in the rostral-most portion of the anterior hindbrain known as the isthmus [51]. This peak expression coincides with the birth of DR serotonergic neurons (E9.5–12.5) [7]. At E12.5, isthmic Fgf8 expression is nearly gone, but serotonergic neurons continue to differentiate [9, 52]. Between E9–12.5, secreted Fgf8 peptide forms a diffusion gradient that diminishes in strength as it diffuses further away from the isthmus [53, 54]. DR serotonergic neurons arise from the entire rostral to caudal extent of the anterior portion of the developing hindbrain, an area known as rhombomere 1 [55]. Hence, serotonergic neurons that are derived further away from the isthmus may be more vulnerable to loss of Fgf8 and fail to develop properly when there is inadequate Fgf8. This may explain the selective reduction of the mid- to caudal serotonergic neurons in the Fgf8-deficient mice. In addition to a spatial element, the temporal pattern of Fgf8 production may also contribute to the selective reduction of the more ventral and lateral serotonergic neuron populations. For example, serotonergic neurons that arise earlier generally form the more ventral and lateral DR subregions (i.e. DRV, DRI, DRVL) [56, 57], whereas the more dorsal DRD is composed of cells that arise slightly later [57]. The late-arising population may be more resilient to loss of Fgf8 because those neurons normally form during a time when Fgf8 signaling is diminished.
A surprising outcome is that compound deficiencies of Fgfr1 and Fgf8 do not result in a more severe phenotype than either Fgfr1 or Fgf8 deficiency alone. In fact, the compound hypomorphy abrogates the serotonergic neuron phenotype seen in Fgf8 HET. We believe that the redundancy in Fgf signaling may contribute to this phenomenon. Although Fgfr1 is the only Fgf receptor that continuously overlaps Fgf8 expression in the isthmus during the time when serotonergic neurons are forming [58], Fgfr2 has also been implicated in the development of this region. Supporting this notion is that conditional Fgfr2 deletion, when compounded with Fgfr1 deletion, led to a more deleterious impact on the DR than Fgfr1 loss alone [21, 24, 59]. This suggests that the Fgf8 signal can be conveyed through redundant Fgfrs in rhombomere 1 [21]. Further, Fgfrs form both hetero- and homodimers upon ligand binding [58, 60], thus reductions in Fgfr1 may force it to heterodimerize in a configuration that is more favorable to Fgf8 binding, thereby preventing neuronal loss when compared to Fgf8 deficiency alone. In fact, it has been shown that Fgf8 binds with higher affinity to both Fgfr2 and Fgfr3 than to Fgfr1 [61, 62], which are dynamically expressed in rhombomere 1 and are also found in E12.5 serotonergic neuronal cells [63]. There may also be functional redundancy with other isthmic Fgf ligands such as Fgf17 and Fgf18 [52] during this period. In sum, compensatory changes in other Fgf ligands and receptors may occur in compound hypomorphs to lessen their phenotype.
Two caveats are associated with our data interpretation. First, we cannot exclude the possibility that loss of Fgf signaling in other brain structures involved in emotional regulation contribute to the observed behavioral deficits. For example, loss of Fgf8 signaling results in cortical patterning defects, whereas deletion of Fgfr1 in the cortex and hippocampus results in dysgenesis of the corpus callosum and hippocampal atrophy [64, 65]. Conditional knockout of Fgfr1 in dopamine neurons results in fewer dopamine neurons and decreased social interaction [66]. Hence, it is possible that Fgf deficits in brain structures other than the DR may contribute to the behavioral phenotype observed in this study. Additional studies are needed to explore the interdependence of Fgf-related anatomical abnormalities and the anxiety-like behavior identified in this study. Second, because we used only three behavioral tests of anxiety, there may be missed opportunity for detecting additional anxiety-like behaviors. That said, we would not anticipate behavioral changes in tests that specifically activate the DRD/DRC system, like social defeat or learned helplessness, because the DRD and DRC are intact in our mice [43, 44]. In contrast, we would expect increased panic-susceptibility and anxiety-like behavior following manipulations like adolescent social isolation or administration of panicogenic agents (i.e. sodium lactate) that specifically involve the DRVL/VLPAG, caudal DRV and DRI [46, 48].
In this study, we show that reduced Fgf signaling, particularly Fgf8, is correlated with increased anxiety-like behavior and specific reductions in serotonergic neuron numbers in the DRVL/VLPAG, caudal DRV, and DRI. Although the mechanisms underlying the regional specificity of serotonergic neuronal loss and how this manifests as anxiety-related behavior are unclear, it is likely that the dynamic spatio-temporal expression patterns of Fgf signaling components in the developing midbrain/hindbrain region contribute to this selectivity. Unraveling these mechanisms and exploring functional changes in serotonergic neurons associated with Fgf signaling defects will be important future objectives. Overall, this study adds to our understanding of the developmental heterogeneity of serotonergic neurons and how disruptions to this developmental programming can ultimately impact the manifestation of anxiety-related behavior.
Fgf signaling disruption is associated with increased anxiety-like behavior.
Serotonergic neurons are reduced in subregions of the DR in Fgf-deficient mice.
Fgf signaling is important for the formation of anxiety-related DR subregions.
Acknowledgements
We thank Drs. WC Chung, SI Kavanaugh, and BN Greenwood for helpful suggestions and Dr. MB McQueen for statistical consultation. This work was supported by NIH grant R01 HD042634 to PS Tsai and NIH grant R01 MH086539 to CA Lowry. CL Enix was supported by an Undergraduate Research Opportunities Program (UROP)/Howard Hughes Medical Institute (HHMI) Individual Grant and a UROP Assistantship grant. SC Rich and JA Magno were supported by UROP Assistantship grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- 2.Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology. 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- 3.Calizo LH, Akanwa A, Ma X, Pan YZ, Lemos JC, Craige C, et al. Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology. 2011;61:524–543. doi: 10.1016/j.neuropharm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiological reviews. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 5.Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cellular and molecular neurobiology. 2012;32:695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul ED, Lowry CA. Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. Journal of psychopharmacology. 2013 doi: 10.1177/0269881113490328. [DOI] [PubMed] [Google Scholar]
- 7.Cordes SP. Molecular genetics of the early development of hindbrain serotonergic neurons. Clin Genet. 2005;68:487–494. doi: 10.1111/j.1399-0004.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 8.Kiyasova V, Gaspar P. Development of raphe serotonin neurons from specification to guidance. The European journal of neuroscience. 2011;34:1553–1562. doi: 10.1111/j.1460-9568.2011.07910.x. [DOI] [PubMed] [Google Scholar]
- 9.Deneris ES, Wyler SC. Serotonergic transcriptional networks and potential importance to mental health. Nature neuroscience. 2012;15:519–527. doi: 10.1038/nn.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partanen J. FGF signalling pathways in development of the midbrain and anterior hindbrain. J Neurochem. 2007;101:1185–1193. doi: 10.1111/j.1471-4159.2007.04463.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu A, Joyner AL. EN and GBX2 play essential roles downstream of FGF8 in patterning the mouse mid/hindbrain region. Development. 2001;128:181–191. doi: 10.1242/dev.128.2.181. [DOI] [PubMed] [Google Scholar]
- 12.Martinez S, Crossley PH, Cobos I, Rubenstein JL, Martin GR. FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development. 1999;126:1189–1200. doi: 10.1242/dev.126.6.1189. [DOI] [PubMed] [Google Scholar]
- 13.Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nature reviews Neuroscience. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- 14.Liu A, Joyner AL. Early anterior/posterior patterning of the midbrain and cerebellum. Annual review of neuroscience. 2001;24:869–896. doi: 10.1146/annurev.neuro.24.1.869. [DOI] [PubMed] [Google Scholar]
- 15.Echevarria D, Vieira C, Gimeno L, Martinez S. Neuroepithelial secondary organizers and cell fate specification in the developing brain. Brain Res Brain Res Rev. 2003;43:179–191. doi: 10.1016/j.brainresrev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- 17.Gaspar P, Lillesaar C. Probing the diversity of serotonin neurons. Philos Trans R Soc Lond B Biol Sci. 2012;367:2382–2394. doi: 10.1098/rstb.2011.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deneris ES. Molecular genetics of mouse serotonin neurons across the lifespan. Neuroscience. 2011;197:17–27. doi: 10.1016/j.neuroscience.2011.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxietylike and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 20.Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, et al. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saarimaki-Vire J, Peltopuro P, Lahti L, Naserke T, Blak AA, Vogt Weisenhorn DM, et al. Fibroblast growth factor receptors cooperate to regulate neural progenitor properties in the developing midbrain and hindbrain. J Neurosci. 2007;27:8581–8592. doi: 10.1523/JNEUROSCI.0192-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blak AA, Naserke T, Saarimaki-Vire J, Peltopuro P, Giraldo-Velasquez M, Vogt Weisenhorn DM, et al. Fgfr2 and Fgfr3 are not required for patterning and maintenance of the midbrain and anterior hindbrain. Dev Biol. 2007;303:231–243. doi: 10.1016/j.ydbio.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Sato T, Joyner AL. The duration of Fgf8 isthmic organizer expression is key to patterning different tectal-isthmo-cerebellum structures. Development. 2009;136:3617–3626. doi: 10.1242/dev.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jukkola T, Lahti L, Naserke T, Wurst W, Partanen J. FGF regulated gene-expression and neuronal differentiation in the developing midbrain-hindbrain region. Dev Biol. 2006;297:141–157. doi: 10.1016/j.ydbio.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118:2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- 27.Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- 28.Partanen J, Schwartz L, Rossant J. Opposite phenotypes of hypomorphic and Y766 phosphorylation site mutations reveal a function for Fgfr1 in anteroposterior patterning of mouse embryos. Genes Dev. 1998;12:2332–2344. doi: 10.1101/gad.12.15.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Voikar V, Vasar E, Rauvala H. Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav. 2004;3:27–38. doi: 10.1046/j.1601-183x.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- 31.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plusmaze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 34.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology, biochemistry, and behavior. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 35.Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 36.Crawley J. What’s wrong with my mouse. New York: Wiley-Liss; 2000. [Google Scholar]
- 37.Hale MW, Shekhar A, Lowry CA. Development by environment interactions controlling tryptophan hydroxylase expression. J Chem Neuroanat. 2011;41:219–226. doi: 10.1016/j.jchemneu.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hale MW, Dady KF, Evans AK, Lowry CA. Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling. Experimental neurology. 2011;227:264–278. doi: 10.1016/j.expneurol.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Brooks LR, Chung WC, Tsai PS. Abnormal hypothalamic oxytocin system in fibroblast growth factor 8-deficient mice. Endocrine. 2010;38:174–180. doi: 10.1007/s12020-010-9366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grubbs FE. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- 41.Chi CL, Martinez S, Wurst W, Martin GR. The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development. 2003;130:2633–2644. doi: 10.1242/dev.00487. [DOI] [PubMed] [Google Scholar]
- 42.Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 43.Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF. Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 45.Spannuth BM, Hale MW, Evans AK, Lukkes JL, Campeau S, Lowry CA. Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fearpotentiated startle. Neuroscience. 2011;179:104–119. doi: 10.1016/j.neuroscience.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson P, Lowry C, Truitt W, Shekhar A. Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J Psychopharmacol. 2008;22:642–652. doi: 10.1177/0269881107082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depression and anxiety. 2012;29:307–319. doi: 10.1002/da.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukkes JL, Kopelman JM, Donner NC, Hale MW, Lowry CA. Development x environment interactions control tph2 mRNA expression. Neuroscience. 2013;237:139–150. doi: 10.1016/j.neuroscience.2013.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brodski C, Weisenhorn DM, Signore M, Sillaber I, Oesterheld M, Broccoli V, et al. Location and size of dopaminergic and serotonergic cell populations are controlled by the position of the midbrain-hindbrain organizer. J Neurosci. 2003;23:4199–4207. doi: 10.1523/JNEUROSCI.23-10-04199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blaess S, Corrales JD, Joyner AL. Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development. 2006;133:1799–1809. doi: 10.1242/dev.02339. [DOI] [PubMed] [Google Scholar]
- 51.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Liu Z, Ornitz DM. Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development. 2000;127:1833–1843. doi: 10.1242/dev.127.9.1833. [DOI] [PubMed] [Google Scholar]
- 53.Trokovic R, Jukkola T, Saarimaki J, Peltopuro P, Naserke T, Weisenhorn DM, et al. Fgfr1- dependent boundary cells between developing mid- and hindbrain. Dev Biol. 2005;278:428–439. doi: 10.1016/j.ydbio.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Vieira C, Pombero A, Garcia-Lopez R, Gimeno L, Echevarria D, Martinez S. Molecular mechanisms controlling brain development: an overview of neuroepithelial secondary organizers. The International journal of developmental biology. 2010;54:7–20. doi: 10.1387/ijdb.092853cv. [DOI] [PubMed] [Google Scholar]
- 55.Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wallace JA, Lauder JM. Development of the serotonergic system in the rat embryo: an immunocytochemical study. Brain research bulletin. 1983;10:459–479. doi: 10.1016/0361-9230(83)90144-2. [DOI] [PubMed] [Google Scholar]
- 57.Hawthorne AL, Wylie CJ, Landmesser LT, Deneris ES, Silver J. Serotonergic neurons migrate radially through the neuroepithelium by dynamin-mediated somal translocation. J Neurosci. 2010;30:420–430. doi: 10.1523/JNEUROSCI.2333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blak AA, Naserke T, Weisenhorn DM, Prakash N, Partanen J, Wurst W. Expression of Fgf receptors 1, 2, and 3 in the developing mid- and hindbrain of the mouse. Dev Dyn. 2005;233:1023–1030. doi: 10.1002/dvdy.20386. [DOI] [PubMed] [Google Scholar]
- 59.Trokovic R, Trokovic N, Hernesniemi S, Pirvola U, Vogt Weisenhorn DM, Rossant J, et al. FGFR1 is independently required in both developing mid- and hindbrain for sustained response to isthmic signals. EMBO J. 2003;22:1811–1823. doi: 10.1093/emboj/cdg169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dono R. Fibroblast growth factors as regulators of central nervous system development and function. Am J Physiol Regul Integr Comp Physiol. 2003;284:R867–R881. doi: 10.1152/ajpregu.00533.2002. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olsen SK, Li JY, Bromleigh C, Eliseenkova AV, Ibrahimi OA, Lao Z, et al. Structural basis by which alternative splicing modulates the organizer activity of FGF8 in the brain. Genes & development. 2006;20:185–198. doi: 10.1101/gad.1365406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wylie CJ, Hendricks TJ, Zhang B, Wang L, Lu P, Leahy P, et al. Distinct transcriptomes define rostral and caudal serotonin neurons. J Neurosci. 2010;30:670–684. doi: 10.1523/JNEUROSCI.4656-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- 65.Ohkubo Y, Uchida AO, Shin D, Partanen J, Vaccarino FM. Fibroblast growth factor receptor 1 is required for the proliferation of hippocampal progenitor cells and for hippocampal growth in mouse. J Neurosci. 2004;24:6057–6069. doi: 10.1523/JNEUROSCI.1140-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klejbor I, Kucinski A, Wersinger SR, Corso T, Spodnik JH, Dziewiatkowski J, et al. Serotonergic hyperinnervation and effective serotonin blockade in an FGF receptor developmental model of psychosis. Schizophr Res. 2009;113:308–321. doi: 10.1016/j.schres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]