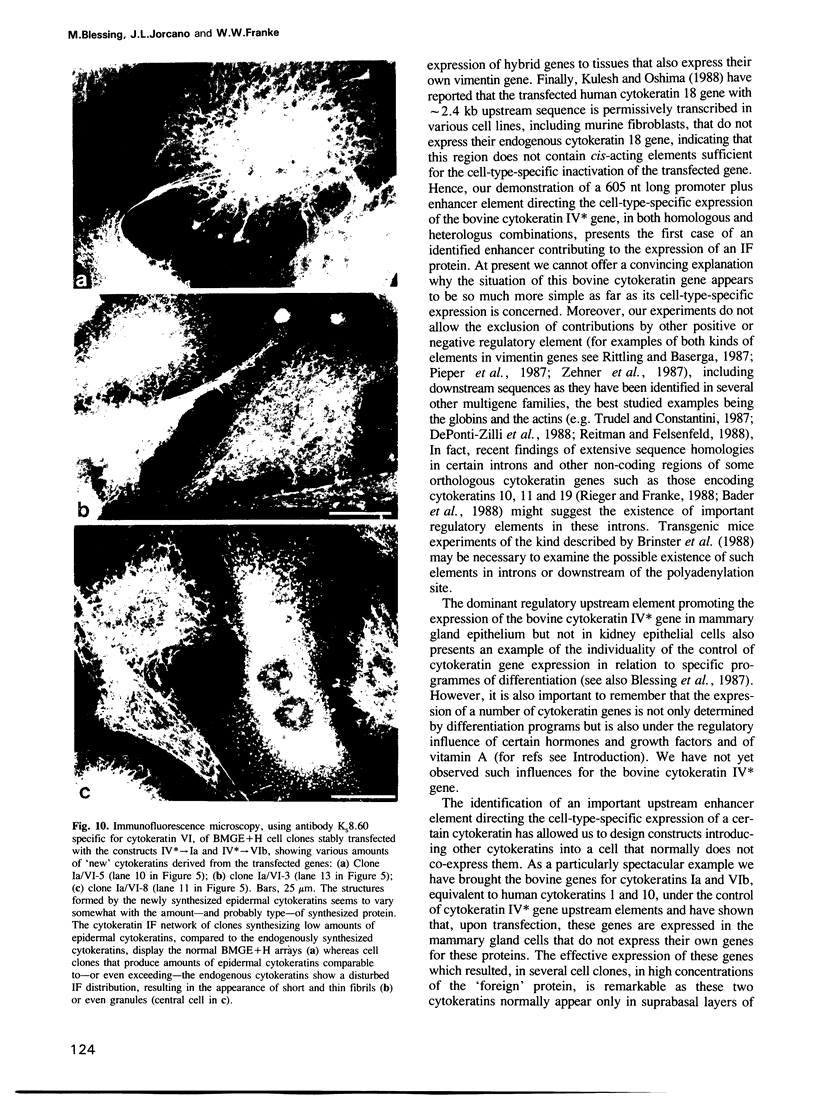
Abstract
The cytokeratins, which form the intermediate filaments (IFs) characteristic of epithelial cells, are encoded by a large family of genes whose members are differentially expressed in patterns different in the various kinds of epithelia. To identify possible cis-regulatory DNA elements involved in the cell-type-specific expression of these genes, we examined, in transfection assays, 5' upstream sequence intercepts of a certain cytokeratin gene, i.e. that for bovine cytokeratin IV* (CKIV*), in combination with the coding portions of either the chloramphenicol acetyltransferase (CAT) gene or other cytokeratin genes. A 5' upstream region located between the cap-site and nucleotide -605 was found to enhance the specific expression of these reporter genes in bovine mammary gland-derived BMGE + H cells, which express the endogenous gene, but not in bovine kidney epithelium-derived MDBK cells which synthesize cytokeratins other than IV*. This epithelium-type-specific expression was also observed in heterologous combinations, e.g. in murine keratinocytes, but not in other murine cell lines such as 3T3 fibroblasts. When a fragment located between -180 and -605 was coupled to the HSV-TK promoter it stimulated the expression of the reporter gene in a cell-type-specific manner. The enhancer character of this 425 nucleotide long region is also demonstrated. Moreover, the CKIV* promoter/enhancer complex was able to direct the expression of epidermal cytokeratins characteristic for suprabasal differentiation, i.e. bovine cytokeratins Ia and VIb, in cells that normally do not express these genes. We show that the newly synthesized cytokeratins integrate into the pre-existing cytokeratin IF system of the transfected cells and that the forced expression of one of these cytokeratins does not induce the endogenous gene encoding its normal pair partner.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtstätter T., Moll R., Moore B., Franke W. W. Cytokeratin polypeptide patterns of different epithelia of the human male urogenital tract: immunofluorescence and gel electrophoretic studies. J Histochem Cytochem. 1985 May;33(5):415–426. doi: 10.1177/33.5.2580881. [DOI] [PubMed] [Google Scholar]
- Albers K., Fuchs E. The expression of mutant epidermal keratin cDNAs transfected in simple epithelial and squamous cell carcinoma lines. J Cell Biol. 1987 Aug;105(2):791–806. doi: 10.1083/jcb.105.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma D. J., Grichnik J. M., Gossett L. M., Schwartz R. J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol Cell Biol. 1986 Jul;6(7):2462–2475. doi: 10.1128/mcb.6.7.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing M., Zentgraf H., Jorcano J. L. Differentially expressed bovine cytokeratin genes. Analysis of gene linkage and evolutionary conservation of 5'-upstream sequences. EMBO J. 1987 Mar;6(3):567–575. doi: 10.1002/j.1460-2075.1987.tb04792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capetanaki Y. G., Ngai J., Lazarides E. Characterization and regulation in the expression of a gene coding for the intermediate filament protein desmin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6909–6913. doi: 10.1073/pnas.81.22.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Cooper D., Sun T. T. Monoclonal antibody analysis of bovine epithelial keratins. Specific pairs as defined by coexpression. J Biol Chem. 1986 Apr 5;261(10):4646–4654. [PubMed] [Google Scholar]
- DePonti-Zilli L., Seiler-Tuyns A., Paterson B. M. A 40-base-pair sequence in the 3' end of the beta-actin gene regulates beta-actin mRNA transcription during myogenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1389–1393. doi: 10.1073/pnas.85.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner R., Sun T. T., Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J Cell Biol. 1986 May;102(5):1767–1777. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Mayer D., Schmid E., Denk H., Borenfreund E. Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res. 1981 Aug;134(2):345–365. doi: 10.1016/0014-4827(81)90435-3. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Moll R. Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation. 1987;36(2):145–163. doi: 10.1111/j.1432-0436.1987.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W. Nuclear lamins and cytoplasmic intermediate filament proteins: a growing multigene family. Cell. 1987 Jan 16;48(1):3–4. doi: 10.1016/0092-8674(87)90345-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Grund C., Geiger B. Intermediate filament proteins in nonfilamentous structures: transient disintegration and inclusion of subunit proteins in granular aggregates. Cell. 1982 Aug;30(1):103–113. doi: 10.1016/0092-8674(82)90016-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Mittnacht S., Grund C., Jorcano J. L. Integration of different keratins into the same filament system after microinjection of mRNA for epidermal keratins into kidney epithelial cells. Cell. 1984 Apr;36(4):813–825. doi: 10.1016/0092-8674(84)90031-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Schiller D. L., Winter S., Jarasch E. D., Moll R., Denk H., Jackson B. W., Illmensee K. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):431–453. doi: 10.1101/sqb.1982.046.01.041. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Tyner A. L., Giudice G. J., Marchuk D., RayChaudhury A., Rosenberg M. The human keratin genes and their differential expression. Curr Top Dev Biol. 1987;22:5–34. doi: 10.1016/s0070-2153(08)60097-6. [DOI] [PubMed] [Google Scholar]
- Gilfix B. M., Eckert R. L. Coordinate control by vitamin A of keratin gene expression in human keratinocytes. J Biol Chem. 1985 Nov 15;260(26):14026–14029. [PubMed] [Google Scholar]
- Giudice G. J., Fuchs E. The transfection of epidermal keratin genes into fibroblasts and simple epithelial cells: evidence for inducing a type I keratin by a type II gene. Cell. 1987 Feb 13;48(3):453–463. doi: 10.1016/0092-8674(87)90196-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M., Franke W. W. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol. 1985 Nov;101(5 Pt 1):1826–1841. doi: 10.1083/jcb.101.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid H. W., Moll I., Franke W. W. Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. I. Human and bovine hair follicles. Differentiation. 1988;37(2):137–157. doi: 10.1111/j.1432-0436.1988.tb00805.x. [DOI] [PubMed] [Google Scholar]
- Heid H. W., Werner E., Franke W. W. The complement of native alpha-keratin polypeptides of hair-forming cells: a subset of eight polypeptides that differ from epithelial cytokeratins. Differentiation. 1986;32(2):101–119. doi: 10.1111/j.1432-0436.1986.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Huszar M., Gigi-Leitner O., Moll R., Franke W. W., Geiger B. Monoclonal antibodies to various acidic (type I) cytokeratins of stratified epithelia. Selective markers for stratification and squamous cell carcinomas. Differentiation. 1986;31(2):141–153. doi: 10.1111/j.1432-0436.1986.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Jahn L., Fouquet B., Rohe K., Franke W. W. Cytokeratins in certain endothelial and smooth muscle cells of two taxonomically distant vertebrate species, Xenopus laevis and man. Differentiation. 1987;36(3):234–254. doi: 10.1111/j.1432-0436.1987.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Jorcano J. L., Magin T. M., Franke W. W. Cell type-specific expression of bovine keratin genes as demonstrated by the use of complementary DNA clones. J Mol Biol. 1984 Jun 15;176(1):21–37. doi: 10.1016/0022-2836(84)90380-2. [DOI] [PubMed] [Google Scholar]
- Jorcano J. L., Rieger M., Franz J. K., Schiller D. L., Moll R., Franke W. W. Identification of two types of keratin polypeptides within the acidic cytokeratin subfamily I. J Mol Biol. 1984 Oct 25;179(2):257–281. doi: 10.1016/0022-2836(84)90468-6. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Tretjakoff I., Beaudet L., Peterson A. Expression and assembly of a human neurofilament protein in transgenic mice provide a novel neuronal marking system. Genes Dev. 1987 Dec;1(10):1085–1095. doi: 10.1101/gad.1.10.1085. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Stellmach V., Javors J., Fuchs E. Regulation of human mesothelial cell differentiation: opposing roles of retinoids and epidermal growth factor in the expression of intermediate filament proteins. J Cell Biol. 1987 Dec;105(6 Pt 2):3039–3051. doi: 10.1083/jcb.105.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp B., Rentrop M., Schweizer J., Winter H. Nonepidermal members of the keratin multigene family: cDNA sequences and in situ localization of the mRNAs. Nucleic Acids Res. 1986 Jan 24;14(2):751–763. doi: 10.1093/nar/14.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp L. W., Bunn C. L. The experimental manipulation of keratin expression and organization in epithelial cells and somatic cell hybrids. Curr Top Dev Biol. 1987;22:69–96. doi: 10.1016/s0070-2153(08)60099-x. [DOI] [PubMed] [Google Scholar]
- Kopan R., Traska G., Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987 Jul;105(1):427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schmid E., Jorcano J. L., Franke W. W. De novo synthesis and specific assembly of keratin filaments in nonepithelial cells after microinjection of mRNA for epidermal keratin. Cell. 1983 Apr;32(4):1125–1137. doi: 10.1016/0092-8674(83)90296-9. [DOI] [PubMed] [Google Scholar]
- Krimpenfort P. J., Schaart G., Pieper F. R., Ramaekers F. C., Cuypers H. T., van den Heuvel R. M., Vree Egberts W. T., van Eys G. J., Berns A., Bloemendal H. Tissue-specific expression of a vimentin--desmin hybrid gene in transgenic mice. EMBO J. 1988 Apr;7(4):941–947. doi: 10.1002/j.1460-2075.1988.tb02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh D. A., Oshima R. G. Cloning of the human keratin 18 gene and its expression in nonepithelial mouse cells. Mol Cell Biol. 1988 Apr;8(4):1540–1550. doi: 10.1128/mcb.8.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane E. B., Bártek J., Purkis P. E., Leigh I. M. Keratin antigens in differentiating skin. Ann N Y Acad Sci. 1985;455:241–258. doi: 10.1111/j.1749-6632.1985.tb50415.x. [DOI] [PubMed] [Google Scholar]
- Lane E. B., Goodman S. L., Trejdosiewicz L. K. Disruption of the keratin filament network during epithelial cell division. EMBO J. 1982;1(11):1365–1372. doi: 10.1002/j.1460-2075.1982.tb01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. D., Baden H. P. Organisation of the polypeptide chains in mammalian keratin. Nature. 1976 Nov 25;264(5584):377–379. doi: 10.1038/264377a0. [DOI] [PubMed] [Google Scholar]
- Lehnert M. E., Jorcano J. L., Zentgraf H., Blessing M., Franz J. K., Franke W. W. Characterization of bovine keratin genes: similarities of exon patterns in genes coding for different keratins. EMBO J. 1984 Dec 20;3(13):3279–3287. doi: 10.1002/j.1460-2075.1984.tb02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard D. G., Gorham J. D., Cole P., Greene L. A., Ziff E. B. A nerve growth factor-regulated messenger RNA encodes a new intermediate filament protein. J Cell Biol. 1988 Jan;106(1):181–193. doi: 10.1083/jcb.106.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienbaum A., Legagneux V., Portier M. M., Dellagi K., Paulin D. Vimentin gene: expression in human lymphocytes and in Burkitt's lymphoma cells. EMBO J. 1986 Nov;5(11):2809–2814. doi: 10.1002/j.1460-2075.1986.tb04572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchuk D., McCrohon S., Fuchs E. Remarkable conservation of structure among intermediate filament genes. Cell. 1984 Dec;39(3 Pt 2):491–498. doi: 10.1016/0092-8674(84)90456-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Heber A., Schmid W., Danesch U., Posseckert G., Beato M., Schütz G. Glucocorticoid responsiveness of the transcriptional enhancer of Moloney murine sarcoma virus. Cell. 1986 Jul 18;46(2):283–290. doi: 10.1016/0092-8674(86)90745-2. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Levy R., Czernobilsky B., Hohlweg-Majert P., Dallenbach-Hellweg G., Franke W. W. Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Lab Invest. 1983 Nov;49(5):599–610. [PubMed] [Google Scholar]
- Ngai J., Bond V. C., Wold B. J., Lazarides E. Expression of transfected vimentin genes in differentiating murine erythroleukemia cells reveals divergent cis-acting regulation of avian and mammalian vimentin sequences. Mol Cell Biol. 1987 Nov;7(11):3955–3970. doi: 10.1128/mcb.7.11.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Ouhayoun J. P., Gosselin F., Forest N., Winter S., Franke W. W. Cytokeratin patterns of human oral epithelia: differences in cytokeratin synthesis in gingival epithelium and the adjacent alveolar mucosa. Differentiation. 1985;30(2):123–129. doi: 10.1111/j.1432-0436.1985.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Pieper F. R., Slobbe R. L., Ramaekers F. C., Cuypers H. T., Bloemendal H. Upstream regions of the hamster desmin and vimentin genes regulate expression during in vitro myogenesis. EMBO J. 1987 Dec 1;6(12):3611–3618. doi: 10.1002/j.1460-2075.1987.tb02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portier M. M., de Néchaud B., Gros F. Peripherin, a new member of the intermediate filament protein family. Dev Neurosci. 1983;6(6):335–344. doi: 10.1159/000112360. [DOI] [PubMed] [Google Scholar]
- Quax W., van den Broek L., Egberts W. V., Ramaekers F., Bloemendal H. Characterization of the hamster desmin gene: expression and formation of desmin filaments in nonmuscle cells after gene transfer. Cell. 1985 Nov;43(1):327–338. doi: 10.1016/0092-8674(85)90038-8. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Cohlberg J. A., Schiller D. L., Hatzfeld M., Franke W. W. Heterotypic tetramer (A2D2) complexes of non-epidermal keratins isolated from cytoskeletons of rat hepatocytes and hepatoma cells. J Mol Biol. 1984 Sep 15;178(2):365–388. doi: 10.1016/0022-2836(84)90149-9. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Schiller D. L., Hatzfeld M., Achtstätter T., Moll R., Jorcano J. L., Magin T. M., Franke W. W. Patterns of expression and organization of cytokeratin intermediate filaments. Ann N Y Acad Sci. 1985;455:282–306. doi: 10.1111/j.1749-6632.1985.tb50418.x. [DOI] [PubMed] [Google Scholar]
- Reitman M., Felsenfeld G. Mutational analysis of the chicken beta-globin enhancer reveals two positive-acting domains. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6267–6271. doi: 10.1073/pnas.85.17.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger M., Jorcano J. L., Franke W. W. Complete sequence of a bovine type I cytokeratin gene: conserved and variable intron positions in genes of polypeptides of the same cytokeratin subfamily. EMBO J. 1985 Sep;4(9):2261–2267. doi: 10.1002/j.1460-2075.1985.tb03924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling S. R., Baserga R. Functional analysis and growth factor regulation of the human vimentin promoter. Mol Cell Biol. 1987 Nov;7(11):3908–3915. doi: 10.1128/mcb.7.11.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop D. R., Huitfeldt H., Kilkenny A., Yuspa S. H. Regulated expression of differentiation-associated keratins in cultured epidermal cells detected by monospecific antibodies to unique peptides of mouse epidermal keratins. Differentiation. 1987;35(2):143–150. doi: 10.1111/j.1432-0436.1987.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Roop D. R. Regulation of keratin gene expression during differentiation of epidermal and vaginal epithelial cells. Curr Top Dev Biol. 1987;22:195–207. doi: 10.1016/s0070-2153(08)60104-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., RayChaudhury A., Shows T. B., Le Beau M. M., Fuchs E. A group of type I keratin genes on human chromosome 17: characterization and expression. Mol Cell Biol. 1988 Feb;8(2):722–736. doi: 10.1128/mcb.8.2.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D. L., Franke W. W., Geiger B. A subfamily of relatively large and basic cytokeratin polypeptides as defined by peptide mapping is represented by one or several polypeptides in epithelial cells. EMBO J. 1982;1(6):761–769. doi: 10.1002/j.1460-2075.1982.tb01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E., Franke W. W., Grund C., Schiller D. L., Kolb H., Paweletz N. An epithelial cell line with elongated myoid morphology derived from bovine mammary gland. Expression of cytokeratins and desmosomal plaque proteins in unusual arrays. Exp Cell Res. 1983 Jul;146(2):309–328. doi: 10.1016/0014-4827(83)90133-7. [DOI] [PubMed] [Google Scholar]
- Schmid E., Schiller D. L., Grund C., Stadler J., Franke W. W. Tissue type-specific expression of intermediate filament proteins in a cultured epithelial cell line from bovine mammary gland. J Cell Biol. 1983 Jan;96(1):37–50. doi: 10.1083/jcb.96.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Steven A. C., Roop D. R. The molecular biology of intermediate filaments. Cell. 1985 Sep;42(2):411–420. doi: 10.1016/0092-8674(85)90098-4. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Tseng S. C., Huang A. J., Cooper D., Schermer A., Lynch M. H., Weiss R., Eichner R. Monoclonal antibody studies of mammalian epithelial keratins: a review. Ann N Y Acad Sci. 1985;455:307–329. doi: 10.1111/j.1749-6632.1985.tb50419.x. [DOI] [PubMed] [Google Scholar]
- Trudel M., Costantini F. A 3' enhancer contributes to the stage-specific expression of the human beta-globin gene. Genes Dev. 1987 Nov;1(9):954–961. doi: 10.1101/gad.1.9.954. [DOI] [PubMed] [Google Scholar]
- Tyner A. L., Fuchs E. Evidence for posttranscriptional regulation of the keratins expressed during hyperproliferation and malignant transformation in human epidermis. J Cell Biol. 1986 Nov;103(5):1945–1955. doi: 10.1083/jcb.103.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tölle H. G., Weber K., Osborn M. Keratin filament disruption in interphase and mitotic cells--how is it induced? Eur J Cell Biol. 1987 Feb;43(1):35–47. [PubMed] [Google Scholar]
- Walsh K., Schimmel P. DNA-binding site for two skeletal actin promoter factors is important for expression in muscle cells. Mol Cell Biol. 1988 Apr;8(4):1800–1802. doi: 10.1128/mcb.8.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Zehner Z. E., Li Y., Roe B. A., Paterson B. M., Sax C. M. The chicken vimentin gene. Nucleotide sequence, regulatory elements, and comparison to the hamster gene. J Biol Chem. 1987 Jun 15;262(17):8112–8120. [PubMed] [Google Scholar]
- van den Heuvel R. M., van Eys G. J., Ramaekers F. C., Quax W. J., Vree Egberts W. T., Schaart G., Cuypers H. T., Bloemendal H. Intermediate filament formation after transfection with modified hamster vimentin and desmin genes. J Cell Sci. 1987 Nov;88(Pt 4):475–482. doi: 10.1242/jcs.88.4.475. [DOI] [PubMed] [Google Scholar]