Abstract
An efficient method of constructing recombinant adenoviruses (Ads) has been established. The expression unit to be introduced into recombinant Ad was first inserted into the unique Swa I site of the full-length Ad genome cloned in a cassette cosmid. The cassette bearing the expression unit was then cotransfected into human embryonic kidney 293 cells together with the Ad DNA-terminal protein complex digested at several sites with Eco T22I or Ase I/EcoRI. The use of the parent Ad DNA-terminal protein complex instead of the deproteinized Ad genome DNA allowed very efficient recovery of the desired recombinant Ad, and the above restriction digestion drastically reduced regeneration of the parent virus. Several hundred virus clones were readily obtained in each experiment, and about 70% of the clones were the desired recombinant viruses. Furthermore, because the cassette contained the full-length Ad genome, any position of the genome could be easily modified to develop a new vector design. We established construction systems for two types of Ad vectors, the E1-substitution type and the E4-insertion type. This method may greatly facilitate the application of recombinant Ads and should be useful for further improvement of Ad vectors.
Full text
PDF
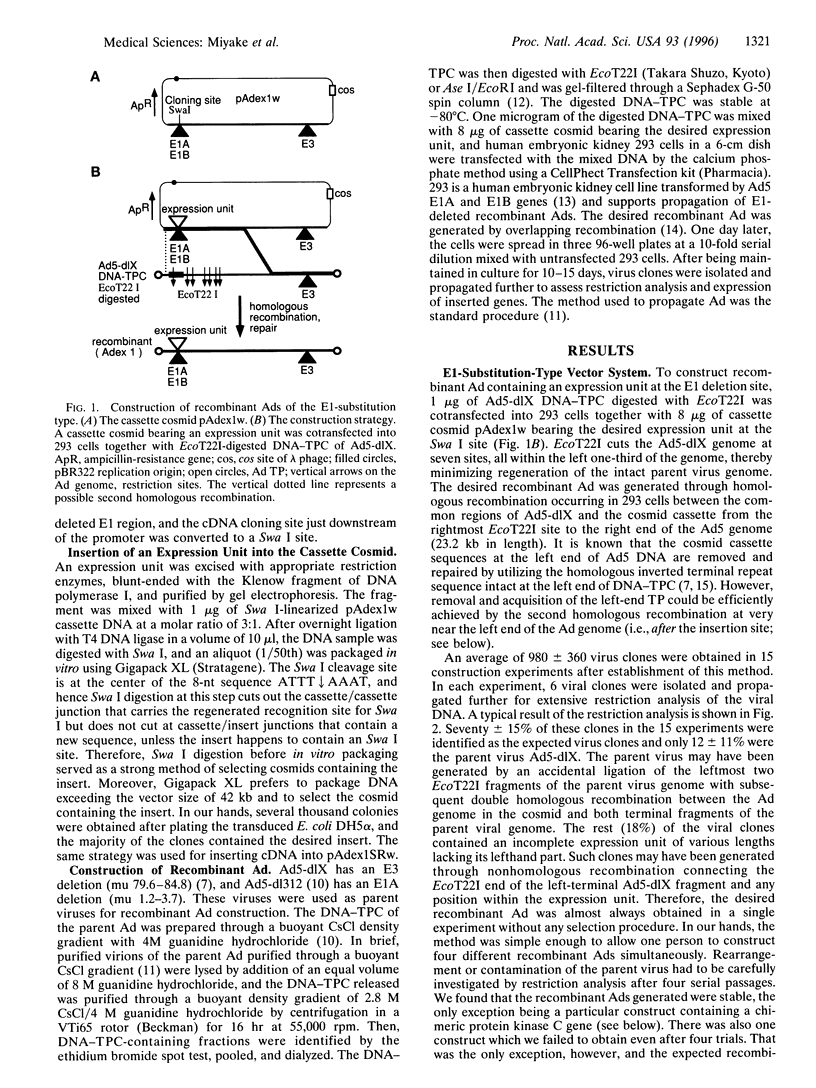
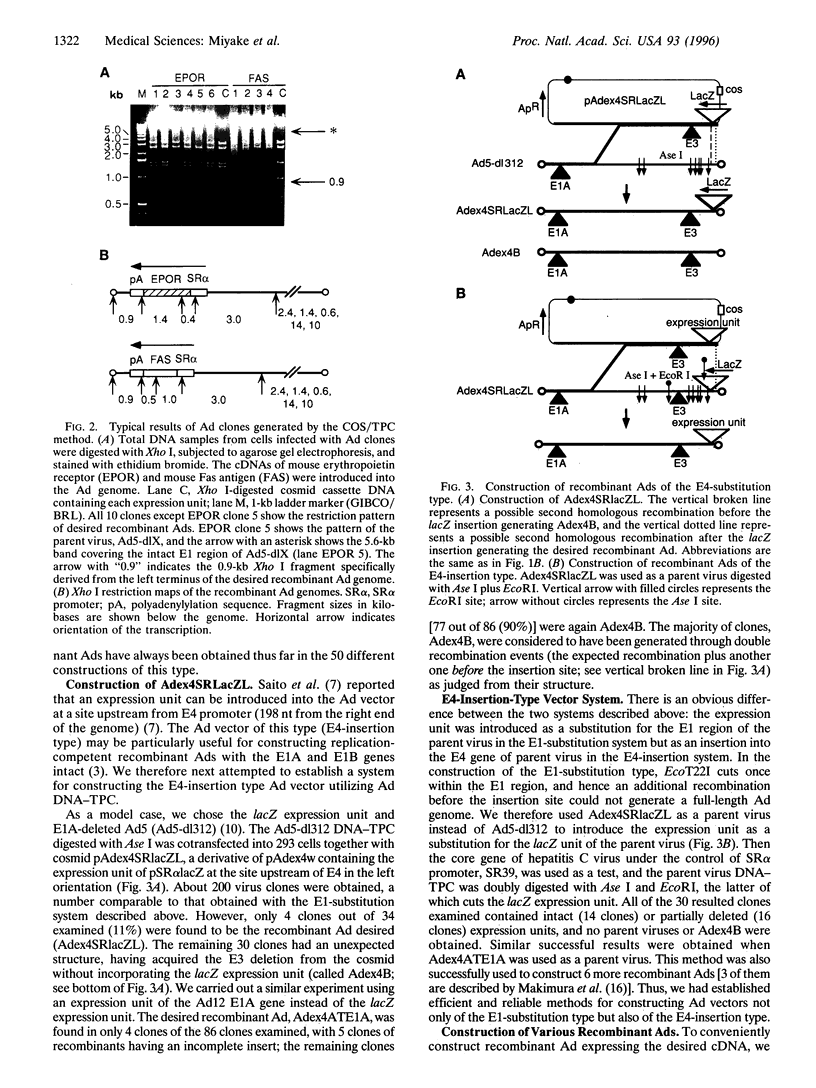


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bett A. J., Haddara W., Prevec L., Graham F. L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda P. K., Natuk R. J., Mason B. B., Bhat B. M., Greenberg L., Dheer S. K., Molnar-Kimber K. L., Mizutani S., Lubeck M. D., Davis A. R. High level expression of the envelope glycoproteins of the human immunodeficiency virus type I in presence of rev gene using helper-independent adenovirus type 7 recombinants. Virology. 1990 Apr;175(2):535–547. doi: 10.1016/0042-6822(90)90438-w. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G., Chinnadurai S., Brusca J. Physical mapping of a large-plaque mutation of adenovirus type 2. J Virol. 1979 Nov;32(2):623–628. doi: 10.1128/jvi.32.2.623-628.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., McElvaney N. G., Rosenfeld M. A., Chu C. S., Mastrangeli A., Hay J. G., Brody S. L., Jaffe H. A., Eissa N. T., Danel C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994 Sep;8(1):42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- Endo F., Katoh H., Yamamoto S., Matsuda I. A murine model for type III tyrosinemia: lack of immunologically detectable 4-hydroxyphenylpyruvic acid dioxygenase enzyme protein in a novel mouse strain with hypertyrosinemia. Am J Hum Genet. 1991 Apr;48(4):704–709. [PMC free article] [PubMed] [Google Scholar]
- Fujita A., Sakagami K., Kanegae Y., Saito I., Kobayashi I. Gene targeting with a replication-defective adenovirus vector. J Virol. 1995 Oct;69(10):6180–6190. doi: 10.1128/jvi.69.10.6180-6190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hata A., Tsuzuki T., Shimada K., Takiguchi M., Mori M., Matsuda I. Structure of the human ornithine transcarbamylase gene. J Biochem. 1988 Feb;103(2):302–308. doi: 10.1093/oxfordjournals.jbchem.a122265. [DOI] [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Hino M., Tojo A., Misawa Y., Morii H., Takaku F., Shibuya M. Unregulated expression of the erythropoietin receptor gene caused by insertion of spleen focus-forming virus long terminal repeat in a murine erythroleukemia cell line. Mol Cell Biol. 1991 Nov;11(11):5527–5533. doi: 10.1128/mcb.11.11.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978 Jan;13(1):181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Takemura R., Oshima T., Mori H., Ihara Y., Yanagisawa M., Masaki T., Hirokawa N. Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol. 1989 Sep;109(3):1173–1184. doi: 10.1083/jcb.109.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegae Y., Lee G., Sato Y., Tanaka M., Nakai M., Sakaki T., Sugano S., Saito I. Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res. 1995 Oct 11;23(19):3816–3821. doi: 10.1093/nar/23.19.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Spencer F., Tugendreich S., Connelly C., Hieter P. Efficient manipulation of the human adenovirus genome as an infectious yeast artificial chromosome clone. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6186–6190. doi: 10.1073/pnas.91.13.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. W., Uetsuki T., Kaziro Y., Yamaguchi N., Sugano S. Use of the human elongation factor 1 alpha promoter as a versatile and efficient expression system. Gene. 1990 Jul 16;91(2):217–223. doi: 10.1016/0378-1119(90)90091-5. [DOI] [PubMed] [Google Scholar]
- Kobune F., Funatu M., Takahashi H., Fukushima M., Kawamoto A., Iizuka S., Sakata H., Yamazaki S., Arita M., Xu W. Characterization of measles viruses isolated after measles vaccination. Vaccine. 1995 Mar;13(4):370–372. doi: 10.1016/0264-410x(95)98259-d. [DOI] [PubMed] [Google Scholar]
- Komase K., Haga T., Yoshikawa Y., Sato T. A., Yamanouchi K. Molecular analysis of structural protein genes of the Yamagata-1 strain of defective subacute sclerosing panencephalitis virus. III. Nucleotide sequence of the hemagglutinin gene. Virus Genes. 1990 Jul;4(2):163–172. doi: 10.1007/BF00678407. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G., Robert J. J., Berrard S., Ridoux V., Stratford-Perricaudet L. D., Perricaudet M., Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993 Feb 12;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Lomax K. J., Volpp B. D., Nunoi H., Sechler J. M., Nauseef W. M., Clark R. A., Gallin J. I., Malech H. L. Cloning of a 67-kD neutrophil oxidase factor with similarity to a noncatalytic region of p60c-src. Science. 1990 May 11;248(4956):727–730. doi: 10.1126/science.1692159. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Jinno A., Takagi N., Shibuya M. A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol Cell Biol. 1990 May;10(5):2261–2268. doi: 10.1128/mcb.10.5.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y., Harada S., Suzuki R., Watanabe Y., Inoue Y., Saito I., Miyamura T. Expression of processed envelope protein of hepatitis C virus in mammalian and insect cells. J Virol. 1992 Mar;66(3):1425–1431. doi: 10.1128/jvi.66.3.1425-1431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Otsuka T., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for granulocyte-macrophage colony stimulating factor: comparison of the mouse and human genes. EMBO J. 1985 Oct;4(10):2561–2568. doi: 10.1002/j.1460-2075.1985.tb03971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Kaibuchi K., Arai K. A protein kinase C cDNA without the regulatory domain is active after transfection in vivo in the absence of phorbol ester. Mol Cell Biol. 1989 Feb;9(2):831–836. doi: 10.1128/mcb.9.2.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991 Dec 15;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Ragot T., Vincent N., Chafey P., Vigne E., Gilgenkrantz H., Couton D., Cartaud J., Briand P., Kaplan J. C., Perricaudet M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993 Feb 18;361(6413):647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Saito I., Oya Y., Yamamoto K., Yuasa T., Shimojo H. Construction of nondefective adenovirus type 5 bearing a 2.8-kilobase hepatitis B virus DNA near the right end of its genome. J Virol. 1985 Jun;54(3):711–719. doi: 10.1128/jvi.54.3.711-719.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Stark G. R. Charomids: cosmid vectors for efficient cloning and mapping of large or small restriction fragments. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8664–8668. doi: 10.1073/pnas.83.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Ochiya T., Sato Y., Tsukamoto M., Konishi H., Saito I., Sugimura T., Terada M. Adenovirus-mediated transfer of the HST-1 (FGF4) gene induces increased levels of platelet count in vivo. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12368–12372. doi: 10.1073/pnas.91.26.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Stein C., Gieselmann V., Kreysing J., Schmidt B., Pohlmann R., Waheed A., Meyer H. E., O'Brien J. S., von Figura K. Cloning and expression of human arylsulfatase A. J Biol Chem. 1989 Jan 15;264(2):1252–1259. [PubMed] [Google Scholar]
- Sternberg N., Sauer B., Hoess R., Abremski K. Bacteriophage P1 cre gene and its regulatory region. Evidence for multiple promoters and for regulation by DNA methylation. J Mol Biol. 1986 Jan 20;187(2):197–212. doi: 10.1016/0022-2836(86)90228-7. [DOI] [PubMed] [Google Scholar]
- Stow N. D. The infectivity of adenovirus genomes lacking DNA sequences from their left-hand termini. Nucleic Acids Res. 1982 Sep 11;10(17):5105–5119. doi: 10.1093/nar/10.17.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., North D. L., Lakich M. M., Russell S. D., Whalen R. G. A possible regulatory role for conserved promoter motifs in an adult-specific muscle myosin gene from mouse. J Biol Chem. 1992 Aug 25;267(24):16957–16967. [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Itoh N., Yonehara S., Copeland N. G., Jenkins N. A., Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992 Feb 15;148(4):1274–1279. [PubMed] [Google Scholar]
- Yamasaki K., Taga T., Hirata Y., Yawata H., Kawanishi Y., Seed B., Taniguchi T., Hirano T., Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988 Aug 12;241(4867):825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- Zabner J., Couture L. A., Gregory R. J., Graham S. M., Smith A. E., Welsh M. J. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993 Oct 22;75(2):207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]