Abstract
Aim:
To develop a novel vehicle based on cubosomes as an ophthalmic drug delivery system for flurbiprofen (FB) to reduce ocular irritancy and improve bioavailability.
Methods:
FB-loaded cubosomes were prepared using hot and high-pressure homogenization. Cubosomes were then characterized by particle size, zeta potential, encapsulation efficiency, particle morphology, inner cubic structure and in vitro release. Corneal permeation was evaluated using modified Franz-type cells. Ocular irritation was then evaluated using both the Draize method and histological examination. The ocular pharmacokinetics of FB was determined using microdialysis.
Results:
The particle size of each cubosome formulation was about 150 nm. A bicontinuous cubic phase of cubic P-type was determined using cryo-transmission electron microscopy (cryo-TEM) observation and small angle X-ray scattering (SAXS) analysis. In vitro corneal permeation study revealed that FB formulated in cubosomes exhibited 2.5-fold (F1) and 2.0-fold (F2) increase in Papp compared with FB PBS. In the ocular irritation test, irritation scores for each group were less than 2, indicating that all formulations exhibited excellent ocular tolerance. Histological examination revealed that neither the structure nor the integrity of the cornea was visibly affected after incubation with FB cubosomes. The AUC of FB administered as FB cubosome F2 was 486.36±38.93 ng·mL−1·min·μg−1, which was significantly higher than that of FB Na eye drops (P<0.01). Compared with FB Na eye drops, the Tmax of FB cubosome F2 was about 1.6-fold higher and the MRT was also significantly longer (P<0.001).
Conclusion:
This novel low-irritant vehicle based on cubosomes might be a promising system for effective ocular drug delivery.
Keywords: flurbiprofen, cubosomes, ocular drug delivery systems, corneal permeability, ocular irritation
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs), which exert their anti-inflammatory action by inhibiting the enzymes cyclooxygenase (COX1 & COX2), have proven to be an effective alternative to corticosteroids in the topical management of ocular inflammation1. Currently these drugs are used very widely in inhibition of intra-operative miosis, management of post-operative inflammation, treatment of seasonal allergic conjunctivitis, prevention and treatment of cystoid macular edema and control of pain after photo refractive keratectomy2. Because they are acidic, NSAIDs are inherently irritating. Reducing the pH of their formulation further increases their irritation potential2 and decreases their aqueous solubility. Frequently reported adverse reactions of NSAIDs used for ophthalmic therapy include transient burning, stinging, and minor signs of irritation and instillation3. Hypersensitivity reactions with itching, reddening, photosensitivity, and keratitis punctata may occur. Stroobants et al4 investigated the effect of six formulations of NSAID eyedrops on the normal corneal epithelium of rabbits. All six formulations were found to cause changes in the epithelial cells, with severity increasing from the peripheral to the central cornea.
Flurbiprofen (FB), 2-(2-fluorobiphenyl-4-yl)propionic acid, is practically insoluble in water. Aqueous solutions of flurbiprofen sodium are employed to inhibit intraoperative miosis during cataract surgery, control postoperative inflammation, prevent cystoid macular edema and reduce ocular pain5. Nevertheless, transient burning, stinging upon instillation and other minor symptoms of ocular irritation, including fibrosis, miosis and mydriasis, have been reported with the use of flurbiprofen sodium ophthalmic solution6. Flurbiprofen solutions of concentration greater than 0.2% (w/v) are irritating7. Thus, it is of great importance to formulate NSAID formulations that are comfortable when applied topically to the eye.
Great efforts have been made over the past two decades to develop novel ophthalmic drug delivery systems, including liposomes8, microemulsions9 and nanoparticles10. Cubosomes, as dispersed colloidal particles of cubic phase liquid crystals, have stimulated significant research interest as controlled-release drug delivery system. They are commonly prepared from a glycerol monooleate (GMO)-water mixture through high-pressure emulsification, using poloxamer 407 as a stabilizer11. Cubosomes have many advantages for drug delivery, including their ability to incorporate both hydrophilic and hydrophobic drugs12, their characteristics as sustained-release delivery systems13, and their biocompatibility and bioadhesivity properties14, 15.
Research of cubosomes as a drug delivery system has involved oral, intravenous and percutaneous routes of administration16, 17, 18. Transdermal enhancing effect of cubosomes has been reported by some researchers12; this effect might be due to the structural organization of cubosomes, which is similar to that found of biomembranes10, 19.
The aim of this study was to design a novel vehicle based on cubosomes as an ophthalmic delivery system of FB that would reduce ocular irritancy and improve bioavailability. The morphological properties of cubosomes were assessed by atomic force microscopy (AFM) and cryo-TEM. The in vitro release profiles of FB cubosomes were studied using a dynamic dialysis method, and the corneal penetration of the developed vehicle was investigated in vitro. Ocular irritation was assessed by the Draize method, and the possible harmful effect on ocular tissues was evaluated on the basis of histological examination. Finally, the in vivo bioavailability of FB was also investigated.
Materials and methods
Materials
The glyceryl monooleate RYLOTM MG 19 (GMO) was a gift from Danisco Cultor Corp (Grindsted, Denmark). Poloxamer 407 (Lutrol F127) was purchased from BASF (Ludwigshafen, Germany). Flurbiprofen was a gift from Sunve Pharma Co, Ltd (Shanghai, China). Glycerol was purchased from Er-Kang Pharmaceutical Co, Ltd (Hunan, China). All other chemicals and reagents used in the study were of analytical grade.
Male New Zealand albino rabbits weighing 2.0–3.0 kg were provided by the Animal Experimental Center of the Shanghai Institute of Materia Medica. Procedures involving animals were reviewed and approved by the Animal Ethics Committee at the Shanghai Institute of Materia Medica.
Preparation of formulations
The cubosomes were prepared using hot, high-pressure homogenization6. Table 1 lists the major components used in various formulations. The lipid components (GMO), poloxamer 407 and flurbiprofen were dissolved in chloroform followed by evaporation of the solvent. Then, aqueous solution containing 2.6% (w/w) glycerol was added to the mixture. After that, it was roughly dispersed at 80 °C using a high-shear dispersing emulsifier (T25 basic, IKA Guangzhou, China) at 11 000 round per minute for 3 min, after which further size reduction was carried out using a high-pressure homogenizer (Panda 2000, GEA Niro Soavi, Italy) under a pressure of 350 bar for 8 cycles at 60 °C. The nanodispersions were then filtered through a mixed esters cellulose membrane with 0.45 μm pore size.
Table 1. Composition of the investigated cubosome formulations.
| Formulation | GMO | Contents (g/100 g) | Water | ||
|---|---|---|---|---|---|
| Poloxamer 407 | FB | Glycerol | |||
| Blank | 4.5 | 0.5 | - | 2.6 | 92.40 |
| F1 | 4.5 | 0.5 | 0.2 | 2.6 | 92.20 |
| F2 | 4.5 | 0.5 | 0.02 | 2.6 | 92.38 |
Characterization of FB cubosomes
Particle size and zeta potential measurements
The particle size and zeta potential (ξ) of cubosomes were determined using a dynamic light scattering analyzer (Nicomp 380 ZLS, Particle Sizing Systems, Santa Barbara, USA). Each sample was diluted with deionized water and measured at 25 °C. Each experiment was carried out four times.
Drug encapsulation efficiency evaluation
Free FB (non-incorporated in the cubosomes) was separated by ultrafiltration centrifugation (Microcon YM-10, 10 000 MW, Millipore). About 0.5 mL of undiluted sample was placed in Microcon YM-10, and then the unit was centrifuged at 3500 round per minute for 15 min. Free and total FB concentration of cubosomes was measured by HPLC. The encapsulation efficiency (EE) can be calculated by the following equation:
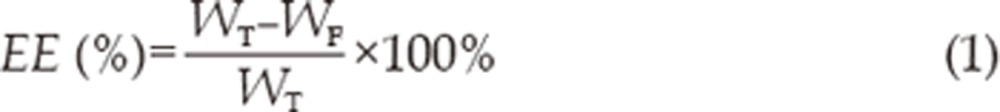 |
where WT is the weight of total drug in cubosomes, and WF is free drug in the ultrafiltrate detected after centrifugation.
The amount of the FB was determined by HPLC (1100 Series, Agilent, USA), which consisted of an autosampler (G1313A ALS), a pump (G1311A Quatpump), a column oven (G1316A Column), a UV detector (G1314A VWD) and data processing software (HP Chemstation Rev.A.10.01). A reversed-phase C18 column (Eclipse XDB, 5 μm, 4.6 mm×150 mm, Agilent, USA) was used. The mobile phase consisted of acetonitrile:water (60:40, v/v) acidified with acetic acid (pH 2.6), with a flow rate of 1.0 mL/min. Detection of FB was performed at 247 nm.
Morphological and structure studies
Morphological investigation was performed with an atomic force microscope (Dimension 3100, Veeco Metrology Group, USA). A few microliters of dispersed cubosomes were placed on a freshly cleaved chip (2.5 cm×2.5 cm) of muscovite mica. The samples were then scanned in a constant force model, and the images were recorded.
The cryo-TEM investigation was performed with a JEOL JEM-2010 TEM (200 kV LaB6 filament). About 4 μL of sample was deposited on a holey carbon grid (R1.2/1.3, Quantifoil Micro Tools GmbH, Jena, Germany). Excess liquid was thereafter removed by means of blotting with filter paper. After blotting, the sample was immediately plunged into pre-cooled liquid ethane for fast freezing. The cryo-grid was then held in a cryo-holder (Gatan 626, Gatan, USA). The sample was observed under low-dose conditions at −172 °C. Digital images were recorded using a Gatan 832 bottom-mounted CCD camera.
The SAXS measurements were performed on a Nanostar U SAXS system (Bruker AXS GmbH, Germany). The instrument source was a copper rotating anode (0.3 mm filament) operating at 40 kV and 40 mA, fitted with cross-coupled Göbel mirrors, resulting in a Cu-Kα radiation wavelength of 1.54 Å. The SAXS camera was fitted with a 2D Hi-STAR detector. Samples were presented in 0.5 mm glass capillaries. The optics and sample chamber were under vacuum to minimize air scatter. Scattering files were background subtracted and normalized to sample transmission, then integrated using SAXS NT. The diffraction patterns obtained were converted to plots of intensity versus s-value, which enabled the identification of Bragg peak reflections; these reflections: were assigned to the different lyotropic liquid crystalline phase using the characteristic spacing ratios: cubic P-type (Im3m): √2: √4: √6: √8 ...; cubic D-type (Pn3m): √2: √3: √4: √6 ...; and cubic G-type (Ia3a): √6: √8: √14: √16 ....
In vitro drug release
In vitro release of FB from cubosomes was evaluated using a dynamic dialysis method20. Briefly, samples of various formulations were placed in dialysis bags (cellulose membrane, MW cut-off at 8000–14000 Da, Sinopharm Chemical Reagent Co, Ltd, China). The dialysis bag was then immersed in 500 mL of simulated tear fluid (STF, NaCl 0.67%, NaHCO3 0.2%, CaCl2·2H2O 0.008%21) at 37±1 °C. The paddle rotation speed was 75 round per minute. At predetermined time intervals, a 5 mL sample was withdrawn and immediately replaced with an equal volume of STF. The concentration of released drug was measured as described in Drug encapsulation efficiency evaluation.
In vitro corneal permeation evaluation
Evaluation procedure
White New Zealand rabbits weighing 2.0–3.0 kg and free of any signs of ocular inflammation or gross abnormalities were obtained from the Animal Experimental Center of the Shanghai Institute of Materia Medica. All studies were approved by the Department of Laboratory Animal Research at the Shanghai Institute of Materia Medica.
Rabbits were sacrificed via a marginal ear vein injection of a lethal dose of urethane. The corneas were immediately excised, weighed and preserved in glutathione bicarbonate ringer (GBR) buffer22. The corneal permeation studies were carried out using modified Franz-type cells. Each sample (200 μL) was added to the donor chamber, and 5.8 mL GBR solution was added to the receptor chamber. Cubosome F1 was diluted to about 0.20 mg/mL with 2.6 % (w/w) glycerol solution. Samples (0.5 mL) from the receptor chamber were withdrawn at the predetermined time intervals and replaced with fresh GBR. All analyses were conducted by HPLC as previously described. Each experiment was repeated three times. The steady-state flux [J, μg/(cm2·s)] and apparent permeability coefficient (Papp, cm/s) were determined using these expressions:
 |
 |
where Q is the total amount of drug permeated at time t, A is the area of exposed corneal surface (0.64 cm2) and C0 is the initial concentration of drug in the donor cell.
Corneal hydration level
At the end of the corneal permeation experiment, each cornea was weighed (Ww). Then it was dried at 70 °C for 12 h and reweighed (Wr). The corneal hydration level (HL%) can be calculated by the following equation:
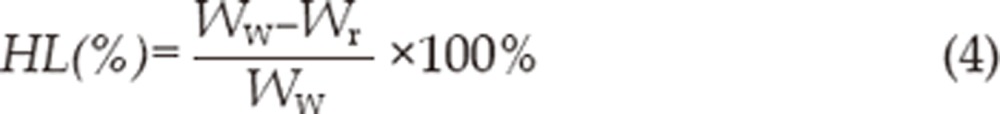 |
Ocular irritation test
Evaluation with the Draize method
Ocular irritation was evaluated according to the Draize method. Rabbits were divided into 2 groups (6 per group) and then treated once every 30 min (1–2 drops in the right eye) for 6 h (12 times) with different formulations. The left eyes served as controls and were treated with saline. The ocular condition was recorded at 1, 2, 4, 24, 48, and 72 h after the last administration. In accordance with the Draize method, ocular irritation scores for every rabbit were calculated by adding the irritation scores for the cornea, iris and conjunctiva. The eye irritation score was obtained by dividing the total score for all rabbits by the number of rabbits. Irritation was classified according to four grades: practically non-irritating, score 0–3; slightly irritating, score 4–8; moderately irritating, score 9–12; and severely irritating (or corrosive), score 13–16.
Histological examination
To examine the effects on corneal structure and integrity, the corneas were removed from the eyes of freshly sacrificed rabbits and incubated at 37 °C for 2 h in the FB cubosome formulations. PBS and sodium dodecylsulfate (SDS) solution in PBS 0.1% (w/w) were used as references.
After incubation, the corneas were washed with PBS and immediately fixed with a formalin solution 8% (w/w). The material was dehydrated with an alcohol gradient, put in melted paraffin and solidified in block form. Cross sections (<1 μm) were cut, stained with haematoxylin and eosin (H&E) and microscopically observed for any pathological modifications23.
In vivo pharmacokinetics
Pupils of the rabbits were dilated with 0.25% (w/v) tropicamide eye drops. Before the probe implantation, the rabbits (n=3) were anesthetized with sodium pentobarbital injected through the marginal ear vein. A CMA20 microdialysis probe (CMA/AB Microdialysis, Sweden) was implanted into the aqueous humor using a 25 G needle. The needle was slowly retracted, leaving the probe with the dialysis membrane in the middle of the anterior chamber. The outlet of the probe was then fixed to prevent any movement during sample collection. The probes were perfused with pH 7.4 isotonic phosphate buffer saline (IPBS) at a flow rate of 2 μL/min using the BAS microdialysis system (Bioanalytical System Inc, USA). After probe implantation, the animals were allowed to stabilize for 2 h. This time period has been shown to be sufficient for the restoration of intraocular pressure and replenishment of the aqueous humor originally lost during probe implantation24.
After that, 100 μL of the formulation (FB, 0.2 mg/mL) was instilled into each eye. The experiment was continued for 6 h after instillation of the formulation. Samples were collected every 20 min and were analyzed using HPLC.
To determine the in vivo relative recovery of drugs through the membrane, the microdialysis probe was perfused at a rate of 2 μL/min with different concentrations of standard flurbiprofen IPBS solution. Dialysates were collected for 20 min after 30 min of perfusion. The dialysate (20 μL) was injected into the HPLC column and drug levels were determined. Relative recovery is expressed as a ratio of drug concentration in dialysate to that in the solution bathing the dialysis membrane. Recovery (R) is the ratio of drug concentration in the dialysate (Cd) to that in the tissue (Cm), calculated according to the following equation:
 |
where Cp is the drug concentration in the perfusate. R is the value of the slope for the plot of Cd–Cp versus Cp.
Statistical analysis
All experiments were performed in replicate for validity of statistical analysis. Results were expressed as mean±SD. ANOVA and Student's t-test were performed on the data sets using Origin for Windows®. Differences were considered significant for P values <0.05.
Results
Characterization of FB cubosomes
Particle size, zeta potential and drug encapsulation efficiency
The physicochemical properties of cubosome formulations are presented in Table 2. The particle size of each formulation ranged from 130 nm to 150 nm, with PI less than 0.132. Only a slight enlargement of particle size could be seen with the increased amount of drug loaded. Zeta potential of the cubosome formulations was about −15 mV. Different cubosome formulations had similarly high drug encapsulation efficiency (>98%) (Table 2), which could be explained by the high solubility of FB in lipid bilayers of cubosomes.
Table 2. Particle size, polydispersity index (PI), zeta potential (ξ) and drug encapsulation efficiency (EE) of formulations (n=4).
| Formulation | FB (mg/mL) | Particle size (nm) | Physicochemical properties | EE (%) | |
|---|---|---|---|---|---|
| PI | ξ (mV) | ||||
| Blank | - | 130.2±1.90 | 0.127±0.007 | −17.92±1.38 | - |
| F1 | 2.0 | 148.6±2.16 | 0.132±0.010 | −13.59±1.37 | 99.38±0.022 |
| F2 | 0.2 | 130.3±1.85 | 0.089±0.012 | −16.46±1.29 | 98.56±0.020 |
Morphological and structural studies
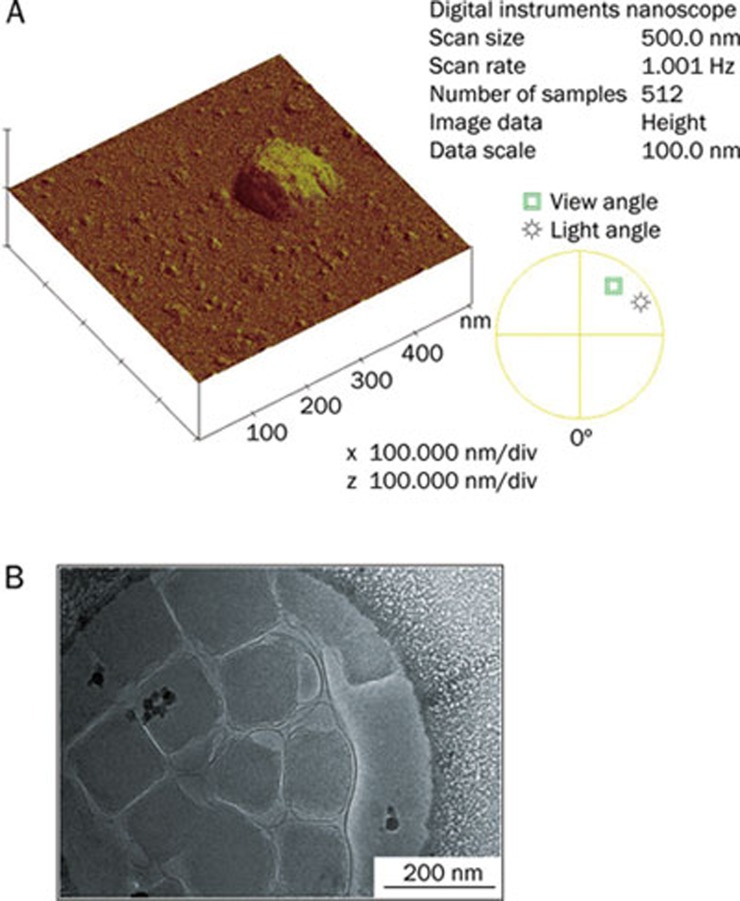
Images of AFM and cryo-TEM observation are shown in Figure 1. The apparent height of cubosomes on the surface of mica was about 29.0 nm, which was in accordance with previous findings for cubosomes observed with AFM25. The particle size of cubosomes observed by AFM and cryo-TEM was about 150 nm, which was similar to the data obtained by dynamic light scattering experiments (148.6±2.16 nm). Cryo-TEM also revealed submicron particles of faceted morphology, showing an inner texture compatible with the expected periodicity from reversed bilayer cubic structure, which was consistent with previously observed cubosome images26.
Figure 1.
(A) AFM image and (B) Cryo-TEM image of FB-cubosomes (F1).
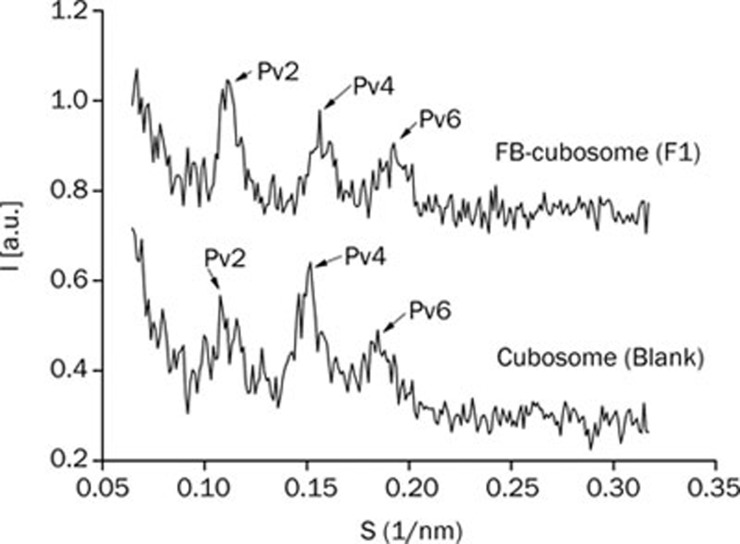
SAXS experiments have been performed to determine the inner structural organization. A series of diffraction profiles, obtained respectively in the absence and the presence of FB (2.0 mg/mL), are shown in Figure 2. Several Bragg peaks could be observed in both samples. The spacing ratio of reflections (√2: √4: √6) was consistent with the formation of a bicontinuous cubic phase of cubic P-type (Im3m). The cubic unit cell dimension was similar in the absence and presence of FB—13.2 nm for blank cubosomes and 12.8 nm for FB-cubosomes (F1)—which indicated that FB did not remarkably alter the cubosome inner structure.
Figure 2.
Small angle X-ray diffraction profiles of blank cubosome and FB-cubosomes (F1).
In vitro drug release
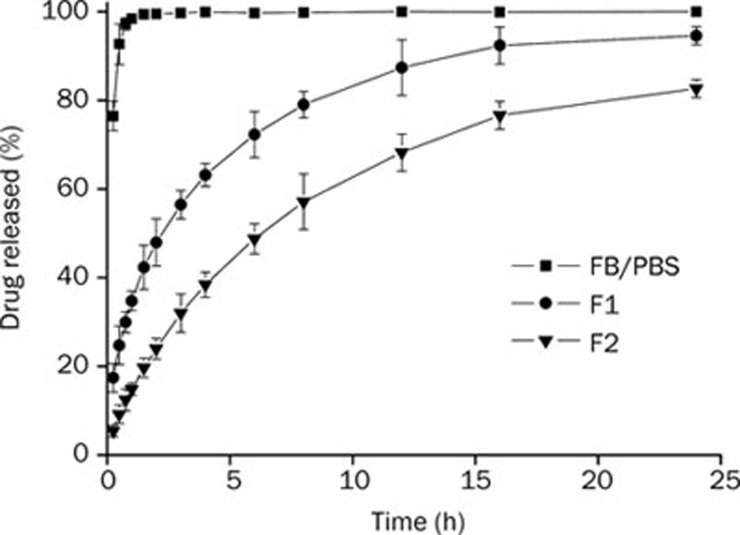
In vitro release profile of FB from cubosomes was performed in pH 7.4 STF using the dynamic dialysis bag method (Figure 3). The diffusion of FB from PBS solution was very fast; it was completed within 2 h. However, the release of FB from cubosomes exhibited much slower profiles. For cubosome F1, about 90% of the drug was released in 24 h. For cubosome F2, about 40% of the drug had been released at the first 4 h and about 80% was released at 24 h. The release rate constant (K), correlation coefficient (R) and simulated profiles are summarized in Table 3. The correlation coefficients (R) calculated for each formulation were above 0.99, which indicated that in vitro drug release profiles of FB-cubosomes fit well with the Higuchi model. Other researchers27 have also found that in vitro drug release profiles of cubosomes followed the Higuchi square root model. As shown in Figure 3, the drug release rate could be raised with an increase of drug loading.
Figure 3.
In vitro release profiles of FB from FB in PBS, FB-cubosomes F1, and FB-cubosomes F2 in STF (n=3).
Table 3. Release rate constants and correlation coefficients calculated after fitting the release profiles obtained using Higuchi model.
| Formulation | K | R | Equation |
|---|---|---|---|
| F1 | 30.64 | 0.9975 | R=30.64 t1/2+3.41 |
| F2 | 22.53 | 0.9989 | R=22.53 t1/2−7.12 |
In vitro corneal permeation evaluation
Results of coneal permeation profiles
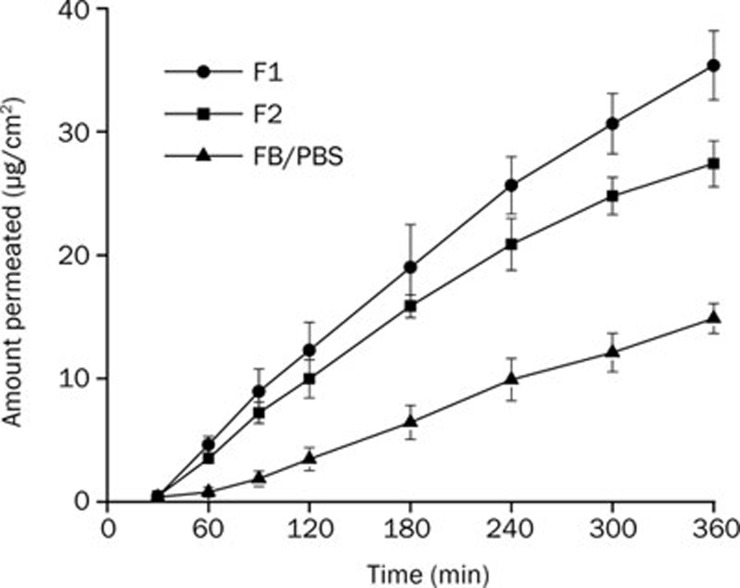
The transcorneal permeation profiles of FB are shown in Figure 4. After a time lag of about 30 min, a nearly linear relationship between accumulative permeated FB and time could be observed. As listed in Table 4, the Papp of FB in F1, F2, and PBS solution were 8.74±0.20×10−6, 6.85±0.66×10−6 and 3.47±0.51×10−6, respectively. Compared with PBS solution, FB formulated in cubosomes exhibited a 2.5-fold (F1) and 2.0-fold (F2) increase in Papp, which indicated the penetration enhancement effect of cubosomes.
Figure 4.
In vitro permeation profiles of various FB-formulations (n=3).
Table 4. Steady state flux (J), apparent permeability coefficient (Papp) and corneal hydration levels (HL) of various formulations (n=3). cP<0.01 vs FB/PBS.
| Formulation | J×103 [μg/(cm2·s)] | Papp×106 (cm/s) | HL (%) |
|---|---|---|---|
| FB/PBS | 0.85±0.13 | 3.47±0.51 | 79.43±0.32 |
| F1 | 1.74±0.041 | 8.74±0.20c | 80.89±0.11 |
| F2 | 1.53±0.15 | 6.85±0.66c | 80.76±0.34 |
On the other hand, a significant difference (P<0.01) in Papp values between F1 and F2 could be seen in this study. This might be due to the higher release rate of FB from its carrier in the dispersion medium, as manifested in the in vitro release experiment.
Corneal hydration levels
Hydration levels in the cornea are frequently used to evaluate in vitro corneal damage. The normal cornea has a hydration level of 76%–80%28. When a hydration level higher than 83% is detected, the cornea has experienced damage. The hydration levels of the excised cornea exposed to the test formulations are presented in Table 4. Each was less than 83%, indicating that no damage to the epithelium or endothelium had occurred during experiments.
Ocular irritation test
Evaluation with the Draize method
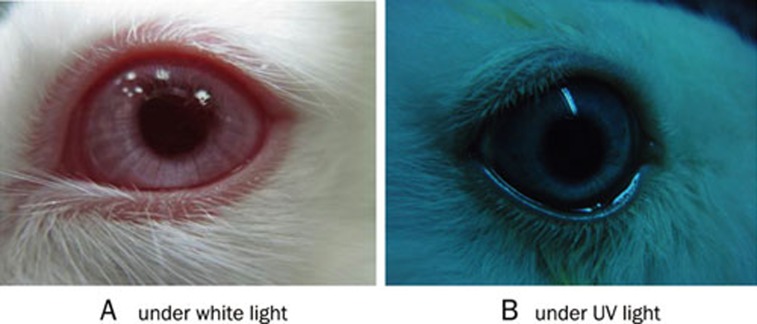
Albino rabbits (n=12) were treated with the formulations listed above to investigate ocular irritation. The results of multiple administrations are summarized in Table 5. The irritation scores for each group were less than 2, indicating that all formulations exhibited excellent ocular tolerance. No ocular damage or clinically abnormal signs were observed in the cornea, conjunctiva or iris, as illustrated in photographs of rabbit eyes in ocular irritation tests (Figure 5).
Table 5. Results of ocular irritation test of FB-cubosomes after multiple administrations (n=6).
| Group | Parameters | Eye irritation scores | |||||
|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 4 h | 24 h | 48 h | 72 h | ||
| FB cubosome F1 | Corneal opacity | 0 | 0 | 0 | 0 | 0 | 0 |
| Iris | 0 | 0 | 0 | 0 | 0 | 0 | |
| Conjunctivae | 9 | 7 | 3 | 0 | 0 | 0 | |
| Total score | 9 | 7 | 3 | 0 | 0 | 0 | |
| Average | 1.5 | 1.17 | 0.5 | 0 | 0 | 0 | |
| Result | Nonirritant | ||||||
| FB cubosome F2 | Corneal opacity | 0 | 0 | 0 | 0 | 0 | 0 |
| Iris | 0 | 0 | 0 | 0 | 0 | 0 | |
| Conjunctivae | 6 | 4 | 1 | 0 | 0 | 0 | |
| Total score | 6 | 4 | 1 | 0 | 0 | 0 | |
| Average | 1 | 0.67 | 0.17 | 0 | 0 | 0 | |
| Results | Nonirritant | ||||||
Figure 5.
Photographs of rabbit eyes in ocular irritation tests.
Histological examination
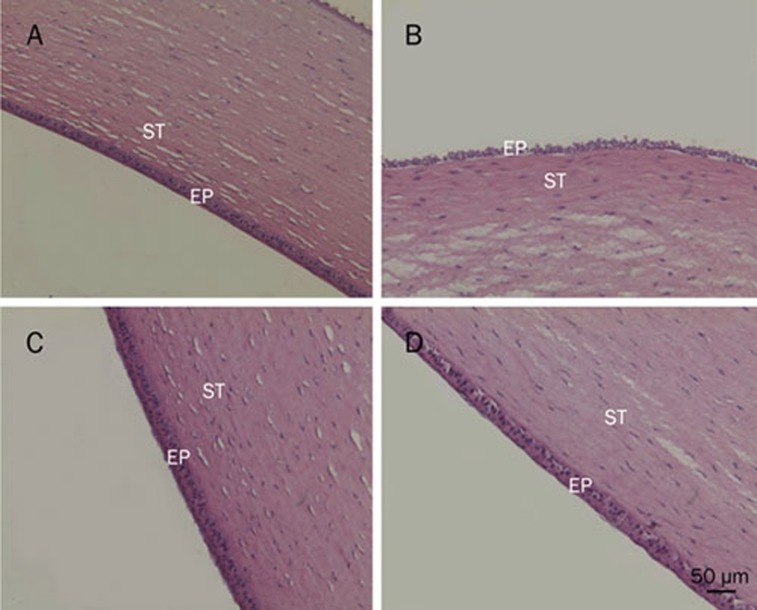
Figure 6 presented corneal cross sections after incubation of freshly excised rabbit corneas with various formulations. As shown in Figure 6A, the structure of the epithelium (EP) and stroma (ST) were maintained after incubation in physiological IPBS. When corneal epithelium was exposed to 0.1% SDS (Figure 6B), the structure of EP was destroyed as superficial epithelial cells detached from tissue assembly. Neither the structure nor the integrity of the corneas was visibly affected after incubation with FB cubosome F1 and F2, which indicated that, even when FB concentration was 0.2%, the cubosome formulations were not irritating to the cornea.
Figure 6.
Histological cross sections of excised rabbit cornea showing epithelium (EP) and stroma (ST), stained with hematoxylin-eosin (scale bar 50 μm) after incubation at 37 °C for 0.5 h. (A) Isotonic phosphate buffer (PBS) pH 7.4; (B) Sodium dodecylsulfate (SDS) solution 0.1% (w/w); (C) FB cubosome F1 (0.2%); (D) FB cubosome F2 (0.02%).
In vivo pharmacokinetics
The in vivo probe recovery (R) was 38.21% with linear regression of Cd–Cp=−0.3821Cp–7.217. In vivo probe recoveries were usually determined before the experiments to ensure the proper functioning of the probe; recoveries ranged from 10% to 40%29, 30. In this study, it remained constant throughout the whole experiment.
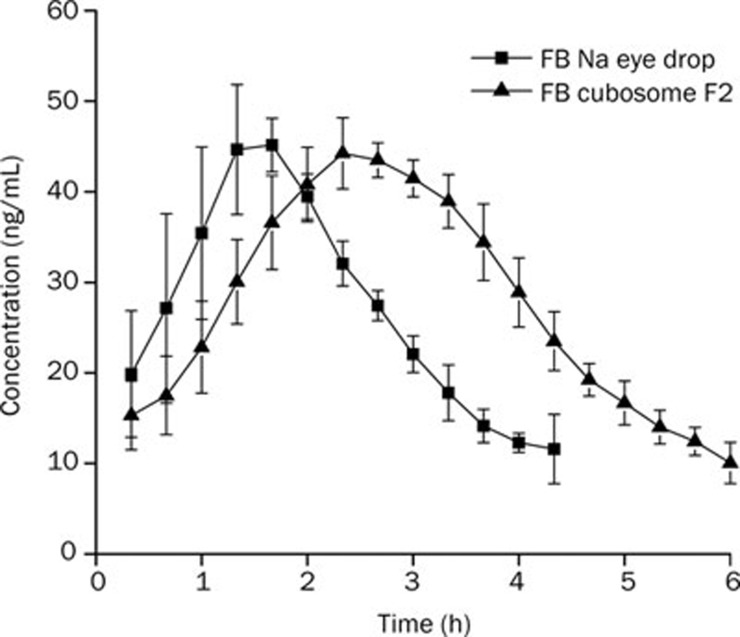
Figure 7 shows the profiles of flurbiprofen concentration in aqueous humor over time. Pharmacokinetic parameters are presented in Table 6. The AUC of FB administered as FB cubosome F2 was 486.36±38.93 ng·mL−1·min·μg−1, which was significantly higher than that for FB Na eye drops (P<0.01). The relative bioavailability of FB cubosome F2 was 172.8%, which was calculated according to the following equation: Bioavailability(%)=AUCcubosome/AUCeyedrop×100%. Compared with FB Na eye drops, the Tmax of FB cubosome F2 was about 1.6-fold higher and the MRT was also significantly longer (P<0.001).
Figure 7.
Aqueous humor flurbiprofen concerntration-time profiles following a topical dose in each eye of rabbits. Each point represents the mean±SD (n=3).
Table 6. Pharmacokinetic parameters of flurbiprofen in aqueous humor after topical administration in each eye of rabbit (n=3). cP<0.01 vs the data of FB Na eye drops.
| Samples | AUC/dose (ng·mL−1·min·μg−1) | Cmax/dose (ng·mL−1·μg−1) | tmax (min) | MRT (min) |
|---|---|---|---|---|
| FB Na eye drops | 281.38±35.80 | 1.92±0.22 | 93.32±11.56 | 118.11±5.20 |
| FB cubosome F2 | 486.36±38.93c | 2.25±0.12 | 153.3±11.55c | 171.7±7.71c |
Note: The AUC and Cmax were dose-normalized.
Discussion
In the present study, GMO cubosomes loaded with FB were prepared using hot, high-pressure homogenization. The results of zeta potential showed that both blank and drug-loaded cubosomes carried a negative charge, which might be due to the trace amount of free fatty acid in GMO. The purity of commercially available GMO (RYLOTM MG 19) used in this study was 92.5%, and it contained a small amount of free oleic acid. Because it is lipophilic, free oleic acid should be absorbed onto the surface of the cubosomes, some of which might ionize to carry a negative charge. Therefore, the negative charge on the surface of cubosomes was due mainly to the ionization of the carboxylic end group of oleic acid. Although the zeta potential in this system was not high enough (>30 mV31) to provide effective electric repulsion to avoid the aggregation of particles, poloxamer 407, acting as a steric stabilizer, would not only stabilize the cubic phase dispersions efficiently but also preserve the inner cubic structure of the particles.
In vitro corneal permeation results showed that FB formulated in cubosomes exhibited a 2.5-fold (F1) and 2.0-fold (F2) increase in Papp. One main mechanism for this effect might be the absorption and/or surface lipid exchange between the cubosome particles and corneal epithelial cells. The close adhesion of the small lipid colloidal carriers with a large surface area to the lipophilic epithelium surface could promote the permeation of the lipophilic drug (unionized form) through corneal epithelium and supply more drug to the anterior eye. Moreover, GMO, as the major structure lipid of the cubosomes, has been reported to be an effective transdermal enhancer32, which also seems to enhance the transcorneal permeability of FB.
In this study, both the Draize method and histological examination were used to evaluate the ocular irritation of FB cubosomes. No ocular damage or clinically abnormal signs were observed, which indicated good biocompatibility of FB cubosome formulations. This may be the result of the carboxy-group shielding effect by the carrier. Encapsulation efficiencies of cubosome formulations were above 98% (Table 2), which meant that most of the drug was encapsulated in the cubosomes. Thus, few drugs partitioned in the aqueous phase, leaving few carboxy groups to exert irritation.
In the in vivo pharmacokinetic experiments, the AUC of FB administered as FB cubosome F2 was about 1.7-fold higher than that of FB Na eye drops (P<0.01), the MRT and Tmax were also significantly greater. As shown in the in vitro penetration experiments, the transcorneal penetration of FB might be enhanced by the interaction between nano-sized cubosome particles and corneal epithelium and the existence of GMO. Furthermore, with the close adhesion of cubosome particles to corneal epithelium and the subsequent absorption and/or surface lipid exchange effect, drug depot might form at the corneal epithelium, which would supply more drugs to the anterior tissue of the eye. This might contribute to the prolonged MRT and Tmax of FB in pharmacokinetic experiments.
Conclusion
In this study, a novel vehicle based on cubosomes was designed as an ocular drug delivery system. The cubosomes exhibited low ocular irritating properties as evaluated by both the Draize method and histological examination. In vitro corneal penetration assessment revealed that cubosomes could enhance the transcorneal permeation of FB. In vivo, the AUC and MRT of FB administered as FB cubosome F2 was significantly higher than those of FB Na eye drops. In conclusion, cubosomes are a promising low-irritant vehicle for the effective ocular drug delivery of FB.
Acknowledgments
The authors are grateful to Danisco Cultor, Denmark, for providing samples of GMO. We thank National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” (No 2009ZX09301-001) for the financial support. This work was also supported in part by the National Basic Research Program of China (No 2009CB930300).
References
- Waterbury L, Kunysz EA, Bewerman R. Effect of steroidal and non steroidal anti-inflammatory agents on corneal wound healing. J Ocul Pharmacol. 1987;3:43–54. doi: 10.1089/jop.1987.3.43. [DOI] [PubMed] [Google Scholar]
- Schalnus R. Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica. 2003;217:89–98. doi: 10.1159/000068563. [DOI] [PubMed] [Google Scholar]
- Joseph TM. A critical look at ocular allergy drugs. Am Fam Physician. 1996;53:2637–42. [PubMed] [Google Scholar]
- Stroobants A, Fabre K, Maudgal PC. Effect of non-steroidal antiinflammatory drugs (NSAID) on the rabbit corneal epithelium studied by scanning electron microscopy. Bull Soc Belge Ophtalmol. 2000;276:73–81. [PubMed] [Google Scholar]
- Araújo J, Vega E, Lopes C, Egea MA, Garcia ML, Souto EB. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloid Surfaces B. 2009;72:48–56. doi: 10.1016/j.colsurfb.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Label information of OcufenLabel approved on 08/25/2003. Available from: : http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/19404scp020_ocufen_lbl.pdf
- Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008;10:229–41. doi: 10.1208/s12248-008-9024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhuang CY, Wang M, Sun XY, Nie SF, Pan WS. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int J Pharm. 2009;379:131–8. doi: 10.1016/j.ijpharm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Gan L, Gan Y, Zhu CL, Zhang XX, Zhu JB. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: in vitro and in vivo results. Int J Pharm. 2009;365:143–9. doi: 10.1016/j.ijpharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Ibrahim HK, El-Leithy IS, Makky AA. Mucoadhesive nanoparticles as carrier systems for prolonged ocular delivery of gatifloxacin/prednisolone bitherapy. Mol Pharm. 2010;7:576–85. doi: 10.1021/mp900279c. [DOI] [PubMed] [Google Scholar]
- Nakano M, Sugita A, Matsuoka H, Handa T. Small-angle X-ray scattering and 13C-NMR investigation on the internal structure of “cubosomes”. Langmuir. 2001;17:3917–22. [Google Scholar]
- Malmsten M. Soft drug delivery systems. Soft Matter. 2006;2:760–9. doi: 10.1039/b608348j. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Zhang J, Zheng LQ, Li DH. Studies of cubosomes as a sustained drug delivery system. J Disp Sci Tech. 2004;25:795–9. [Google Scholar]
- Larsson K. Aqueous dispersion of cubic lipid water phases. Curr Opin Colloid Interface Sci. 2000;5:64–9. [Google Scholar]
- Garg G, Saraf S, Saraf S. Cubosomes: an overview. Biol Pharm Bull. 2007;30:350–3. doi: 10.1248/bpb.30.350. [DOI] [PubMed] [Google Scholar]
- Johnsson M, Barauskas J, Norlin A, Tiberg F. Physicochemical and drug delivery aspects of lipid-based liquid crystalline nanoparticles: a case study of intravenously administered propofol. J Nanosci Nanotechnol. 2006;6:3017–24. doi: 10.1166/jnn.2006.402. [DOI] [PubMed] [Google Scholar]
- Chung H, Kim J, Um JY, Kwon IC, Jeong SY. Self-assembled “nanocubicle” as a carrier for peroral insulin delivery. Diabetologia. 2002;45:448–51. doi: 10.1007/s00125-001-0751-z. [DOI] [PubMed] [Google Scholar]
- Esposito E, Cortesi R, Drechsler M, Paccamiccio L, Mariani P, Contado C, et al. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res. 2005;22:2163–73. doi: 10.1007/s11095-005-8176-x. [DOI] [PubMed] [Google Scholar]
- Larsson K. Cubic lipid-water phases: Structures and biomembrane aspects. J Phys Chem. 1989;93:7304–14. [Google Scholar]
- Gupta PK, Hung CT, Perrier DG. Quantitation of the release of doxorubicin from colloidal dosage forms using dynamic dialysis. J Pharm Sci. 1987;76:141–5. doi: 10.1002/jps.2600760211. [DOI] [PubMed] [Google Scholar]
- Rozier A, Manuel C, Groove J, Plazonet B. Gelrite: a novel ion activated, in situ gelling polymer for ophthalmic vehicles. Effect on bioavailability of timolol. Int J Pharm. 1989;57:163–8. [Google Scholar]
- O'Brien WJ, Edelhauser HF. The corneal penetration of trifluorothymidine, adenine -arabinoside, and idoxuidine: a comparative study. Invest Ophthalmol Vis Sci. 1977;16:1093–103. [PubMed] [Google Scholar]
- Baydoun L, Furrer P, Gurny R, Muller-Goymann CC. New surface-active polymers for ophthalmic formulations: evaluation of ocular tolerance. Eur J Pharm Biopharm. 2004;58:169–75. doi: 10.1016/j.ejpb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Tak RV, Pal D, Gao H, Dey S, Mitra AK. Transport of acyclovir ester prodrugs through rabbit cornea and SIRC-rabbit corneal epithelial cell line. J Pharm Sci. 2001;90:1505–15. doi: 10.1002/jps.1101. [DOI] [PubMed] [Google Scholar]
- Neto C, Aloisi G, Baglioni P, Larsson K. Imaging soft-matter with the atomic force microscopy: cubosomes and hexosomes. J Phys Chem B. 1999;103:3896–9. [Google Scholar]
- Spicer PT. Progress in liquid crystalline dispersions: cubosomes. Curr Opin Colloid Interf Sci. 2005;10:274–9. [Google Scholar]
- Boyd BJ. Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int J Pharm. 2003;260:239–47. doi: 10.1016/s0378-5173(03)00262-x. [DOI] [PubMed] [Google Scholar]
- Saettone MF, Chetoni P, Cerbai R, Mazzanti G, Braghiroli L. Evaluation of ocular permeation enhancers: In vitro effects on corneal transport of four [beta]-blockers and in vitro/in vivo toxic activity. Int J Pharm. 1996;142:103–13. [Google Scholar]
- Rittenhouse KD, Peiffer RL, Pollack GM. Evaluation of microdialysis sampling of aqueous humor for in vivo models of ocular absorption and disposition. J Pharmaceut Biomed. 1998;16:951–9. doi: 10.1016/s0731-7085(97)00060-5. [DOI] [PubMed] [Google Scholar]
- Katragadda S, Gunda S, Hariharan S, Mitra AK. Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: Evaluation of their utility in treating ocular HSV infections. Int J Pharm. 2008;359:15–24. doi: 10.1016/j.ijpharm.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MY, Schutze W, Fuhrer C, Benita S. Characterization of diazepam submicron emulsion interface: role of oleic acid. J Microencapsul. 1994;11:79–92. doi: 10.3109/02652049409040440. [DOI] [PubMed] [Google Scholar]
- Lopes LB, Speretta FFF, Bentley MVLB. Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. Eur J Pharmaceut Sci. 2007;32:209–15. doi: 10.1016/j.ejps.2007.07.006. [DOI] [PubMed] [Google Scholar]