Abstract
Bis(arylmethylidene)acetone derivatives are an important class of curcumin analogues that exhibit various biological and pharmacological activities. We herein report that GO-Y086, a biotinylated bis(arylmethylidene)acetone, inhibits cancer cell growth. We also show that GO-Y086 specifically interacts with the nuclear protein KSRP/FUBP2 by covalent modification. GO-Y086 markedly suppresses the expression of the c-Myc protein, which plays an important role in cellular proliferation and whose expression is regulated by KSRP/FUBP2.
Keywords: KSRP/FUBP2, curcumin analogue, target protein, bis(arylmethylidene)acetone, c-Myc, anticancer
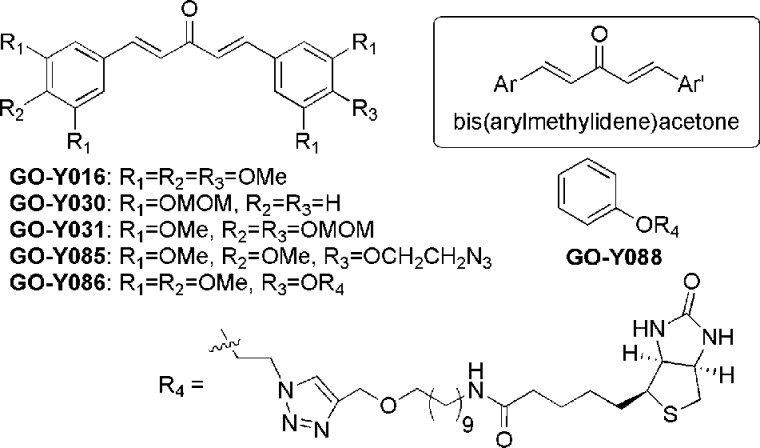
Bis(arylmethylidene)acetone derivatives are an important class of curcumin analogues that exhibit various biological and pharmacological activities, including anti-inflammatory, antioxidant, antibacterial and anticancer activities, without any remarkable toxicity (Figure 1).1−4 Among these attractive properties, we are particularly interested in the potential of bis(arylmethylidene)acetones as anticancer chemotherapeutic agents. Thus, we have synthesized more than 100 bis(arylmethylidene)acetones and tested them for their ability to inhibit cancer cell growth. From these experiments, we found that GO-Y016, GO-Y030, and GO-Y031 exhibited in vitro cancer cell growth inhibition with a GI50 value of 0.3 μM.5 Moreover, GO-Y030 exhibited in vivo chemopreventive activity in the familial adenomatous polyposis (FAP) mouse without apparent toxicity.6 However, its mode of action has remained elusive. Herein, we report that the structurally related biotinylated analogue GO-Y086 inhibits cancer cell growth at a level comparable to that achieved by GO-Y030. We also show that GO-Y086 specifically interacts with the nuclear protein KSRP/FUBP2 by covalent modification. Like GO-Y030, GO-Y086 markedly suppresses the expression of c-Myc protein, which plays an important role in cellular proliferation and whose expression is regulated by KSRP/FUBP2.
Figure 1.
Cytotoxic bis(arylmethylidene)acetones.
Bis(arylmethylidene)acetones have a dienone moiety, which can independently accept a variety of nucleophiles such as thiols and amines via Michael addition.7,8 To determine the molecular target of such protein-reactive small molecules, a pull-down experiment utilizing a biotinylated molecular probe is effective.9,10
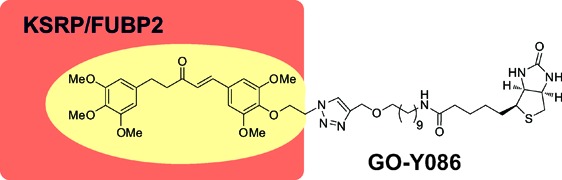
Thus, based on our extensive SAR studies,11 we designed a biotinylated derivative called GO-Y086, which has the GO-Y016-type substitution pattern (3,3′,4,4′,5,5′-hexasubstituted) with a biotin moiety connected at the 4′ position via a linker.
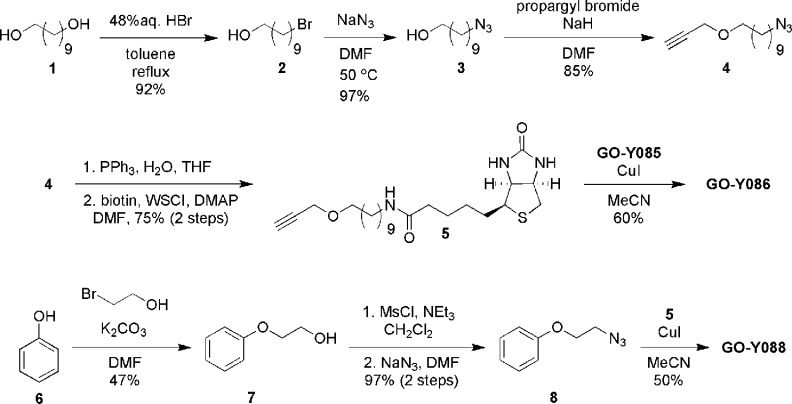
Synthesis of GO-Y086 commenced with monobromination of 1,10-decanediol followed by substitution of the resulting bromide to afford 10-azidodecanol (3) in 89% yield for 2 steps (Figure 2). Introduction of a propargyl group using propargyl bromide and sodium hydride in DMF proceeded to afford propargyl ether 4 in 85% yield. After reduction of the azide moiety with triphenylphosphine and water, the resulting amine was condensed with biotin using water-soluble carbodiimide (WSCI) and (N,N-dimethylamino)pyridine (DMAP) to give biotinylated linker 5 in 75% yield for 2 steps. Finally, the biotinylated linker 5 was connected to GO-Y08511 using a copper-catalyzed Huisgen reaction12 to furnish GO-Y086 in 60% yield. In addition, the biotinylated compound GO-Y088 was also synthesized from phenol (6) in 4 steps. GO-Y086 retained the cell growth inhibitory activity against human colon cancer HCT116 cells as expected (GI50 = 1.7 and 0.3 μM for GO-Y086 and GO-Y030, respectively), whereas GO-Y088 was found to be inactive (GI50 > 50 μM).
Figure 2.
Synthesis of GO-Y086 and GO-Y088.
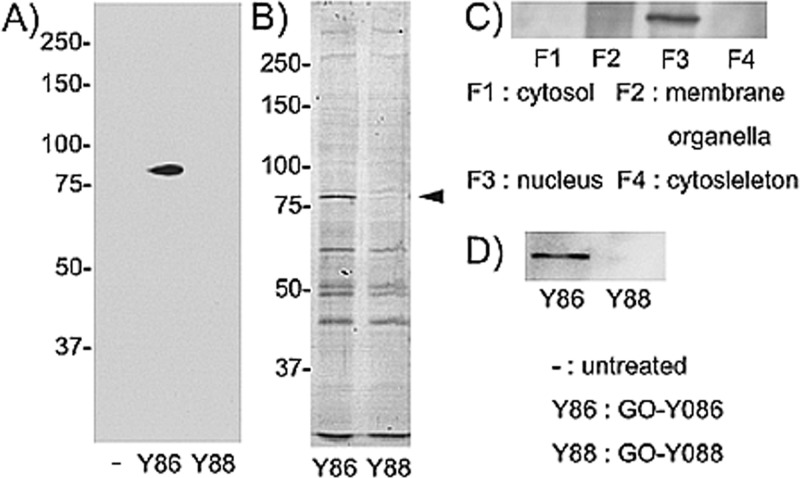
We next carried out pull-down experiments using biotinylated probes and identified the GO-Y086-binding protein (Figure 3). HCT116 cells were incubated with GO-Y086 or GO-Y088 (2.0 μM each) for 9 h. After washing the cells, cell lysates of each sample were prepared, and the biotin-containing complex was isolated using streptavidin-conjugated avidin resin. The biotinylated proteins were analyzed using SDS−polyacrylamide gel.
Figure 3.
Identification of a 75 kDa nuclear protein that covalently interacts with GO-Y086. (A) Cell lysates of GO-Y086- or GO-Y088-treated cells were incubated with avidin resin. The resin-bound proteins were analyzed by 8% SDS−PAGE and probed with antibiotin antibody. (B) CBB staining of the membrane used for the Western blot above. (C) Proteins in GO-Y086-treated cells were fractionated in a stepwise manner using ProteoExtract. A GO-Y086-bound protein in each sample was analyzed in the same manner as described in panel A. (D) Western blot analysis of the GO-Y086-bound protein using anti-KSRP antibody.
A specific 75 kD protein was observed by Western blotting in the GO-Y086-treated cells (Figure 3A). A band presumed to be identical was detected after staining the membrane used for the Western blot analysis with Coomassie Brilliant Blue (CBB) (Figure 3B). Fractionation analysis of the GO-Y086-treated cells showed that the GO-Y086-binding protein appeared in the nuclear fraction (Figure 3C). Finally, mass spectrometry-based identification of this protein revealed that the protein was a 75 kDa (711 amino acids) nuclear protein, the KH-type splicing regulatory protein (KSRP), also known as the far-upstream element binding protein 2 (FUBP2) (see Supporting Information). This result was confirmed by Western blotting using anti-KSRP antibody (Figure 3D).
KSRP/FUBP2 is implicated in a variety of cellular processes, including splicing in the nucleus, mRNA decay, maturation of miRNA, and transcriptional control of proto-oncogenes such as c-myc.13−16 The domain structure of KSRP/FUBP2 consists of a PG-rich amino-terminus, four KH-type nucleic acid-binding domains, and a Q-rich carboxy (C)-terminus (Figure 4A).17
Figure 4.
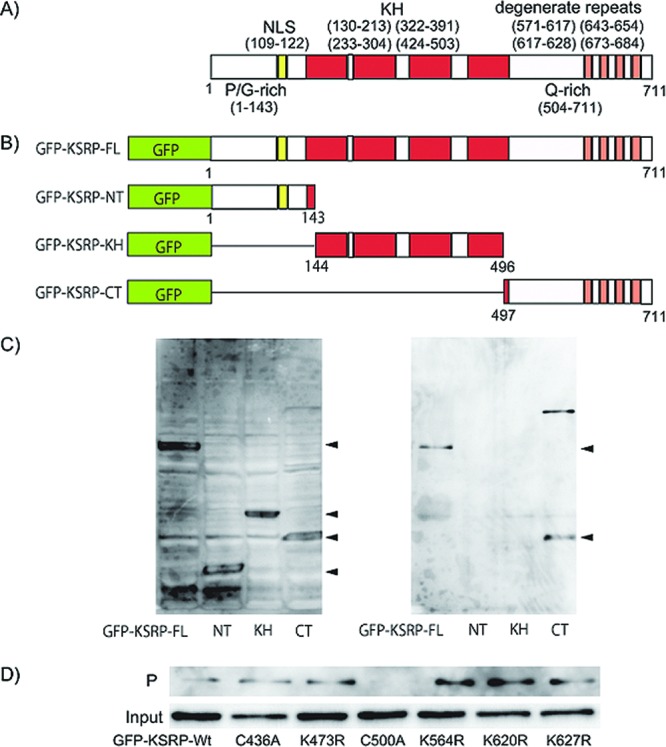
Structure of KSRP/FUBP2 and determination of the GO-Y086-binding site. (A) The domain structure of KSRP/FUBP2. NLS: nuclear localization signal. KH: KH RNA-binding domain. Degenerate repeats: DYTKAWEEYYKK degenerate repeats. P/G-rich: proline/glycine-rich domain. Q-rich: glutamine-rich domain. (B) Structure of the GFP-tagged deletion mutants of KSRP/FUBP2. (C) Western blot analysis of the GO-Y086-bound mutant using anti-GFP antibody. HEK-293T cells were transfected with different GFP-tagged deletion mutants, as shown in panel B. The mutants in the cell lysates were tested for their ability to bind to GO-Y086. Left panel: total cell lysate. Right panel: precipitate. (D) Western blot analysis of GO-Y086-bound point mutants of KSRP/FUBP2 using anti-GFP antibody. Input: total cell lysate. P: precipitate. GFP-KSRP-Wt: GFP-tagged KSRP wild-type.
To identify the GO-Y086-binding site on KSRP/FUBP2, pull down assays were carried out using different GFP-tagged deletion mutants of KSRP/FUBP2 (Figure 4B). The mutants were expressed in HEK-293T cells and tested for their ability to bind to GO-Y086 (Figure 4C). The results showed that only the proteins having a C-terminal region (497−711) retained the ability to bind to GO-Y086, suggesting that the GO-Y086 binding site is located in this region.
Because GO-Y086 would act as a Michael acceptor, a nucleophilic cysteine or lysine residue in the C-terminal region is predicted to be the binding site. Among the nucleophilic cysteine and lysine residues, C500, K564, K620, and K627 are thought to be candidates because the other residues either were found in the peptide fragments identified in the PMF analysis (K646, K653, K654), were necessary for the digested peptide to be observed (K628), or were not found in the C-terminal region above (K494) (see Supporting Information). Thus, six GFP-tagged KSRP/FUBP2 mutants, including C500A, K564R, K620R, and K627R, each harboring one point mutation, were prepared and examined for their binding ability (Figure 4D). Among the mutants tested, only the C500A mutant lost its ability to bind to GO-Y086. These results strongly suggested that GO-Y086 binds to the Cys-500 residue of the KSRP/FUBP2 protein.
Next, we examined whether the binding of GO-Y086 to the KSRP/FUBP2 protein is important for its anticancer activity. The binding of FUBPs to the FUSE (far-upstream element) sequence of the human c-myc proto-oncogene is believed to be vital to the regulation of c-Myc.18−21 Indeed, knockdown of KSRP/FUBP2 reduced the expression of c-Myc and inhibited cell growth (see Supporting Information). We therefore investigated the effect of GO-Y030 and GO-Y086 on the level of c-Myc expression.
HCT116 cells were treated with different concentrations of GO-Y030, GO-Y086 or GO-Y088 for 24 h, and then the c-Myc protein expression level was analyzed by Western blot analysis. Treatment with GO-Y030 or GO-Y086 markedly reduced the amount of c-Myc protein, whereas treatment with GO-Y088 did not (Figure 5A). In addition, the downregulation was also observed at the mRNA level (Figure 5B). These results suggest that the binding of the bis(arylmethylidene)acetones to the Cys-500 residue of KSRP/FUBP2 downregulates the expression of c-Myc, and thereby inhibits cell proliferation (Figure 6).
Figure 5.
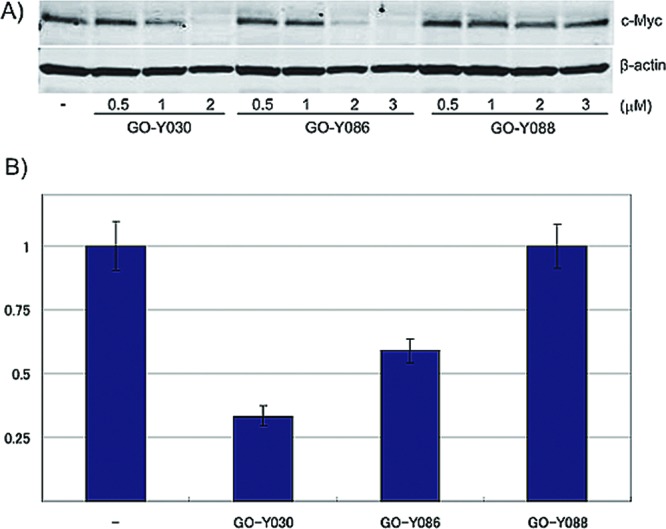
Downregulation of c-Myc in HCT116 cells treated with GO-Y086 and GO-Y030. (A) HCT116 cells were treated with the compounds for 24 h and the cell lysates were analyzed by Western blotting. (B) After the HCT116 cells were treated with 3 μM of the compounds for 24 h, the relative c-myc mRNA abundance was determined by quantitative RT-PCR analysis.
Figure 6.
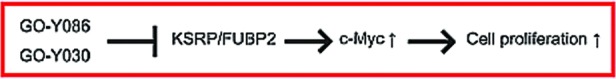
Possible mechanism of action of anticancer bis(arylmethylidene)acetones.
In conclusion, we designed and synthesized GO-Y086 and found that this curcumin analogue binds covalently to KSRP/FUBP2 in cells. This is the first example of a bis(arylmethylidene)acetone that forms a covalent linkage with its binding protein under physiological conditions. Moreover, GO-Y086 was found to downregulate the expression of c-Myc. These results raise the possibility that KSRP/FUBP2 is an important target of the anticancer bis(arylmethylidene)acetones.22 Since KSRP/FUBP2 is known to regulate miRNAs and other mRNAs, additional studies would unravel the pathways activated by anticancer bis(arylmethylidene)acetones. These efforts are now underway.
Acknowledgments
We gratefully acknowledge Professor Douglas L. Black (University of California, Los Angeles) for his provision of the GFP-tagged full-length and deletion mutants of KSRP, and Ms. Hisae Kondo for her help with the PMF analysis.
Supporting Information Available
Experimental procedures for biological analysis and chemical synthesis, and characterization of compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
NOTE ADDED AFTER ASAP PUBLICATION.
This paper was published on the Web on June 4, 2010, with an error in the structure of the abstract graphic. The corrected version was reposted on June 9, 2010.
This work was partly supported by a Grant-in-Aid for the Research Fellowship for Young Scientists (H.Y.) and by a Grant-in-Aid from the Tohoku University Global COE Project, “International Center of Research & Education for Molecular Complex Chemistry”, of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Supplementary Material
References
- Masuda T.; Jitoe A.; Isobe J.; Nakatani N.; Yonemori S. Antioxidative and Antiinflammatory Curcumin-Related Phenolics from Rhizomes of Curcuma-Domestica. Phytochemistry 1993, 32, 1557–1560. [Google Scholar]
- Schraufstätter E.; Bernt H. Antibacterial Action of Curcumin and Related Compounds. Nature 1949, 164, 456–457. [DOI] [PubMed] [Google Scholar]
- Adams B. K.; Ferstl E. M.; Davis M. C.; Herold M.; Kurtkaya S.; Camalier R. F.; Hollingshead M. G.; Kaur G.; Sausville E. A.; Rickles F. R.; Snyder J. P.; Liotta D. C.; Shoji M. Synthesis and Biological Evaluation of Novel Curcumin Analogs as Anti-cancer and Anti-angiogenesis Agents. Bioorg. Med. Chem. 2004, 12, 3871–3883. [DOI] [PubMed] [Google Scholar]
- Anand P.; Thomas S. G.; Kunnumakkara A. B.; Sundaram C.; Harikumar K. B.; Sung B.; Tharakan S. T.; Misra K.; Priyadorsini I. K.; Rajasekharan K. N.; Aggarwal B. B. Biological Activities of Curcumin and Its Analogues (Congeners) Made by Man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [DOI] [PubMed] [Google Scholar]
- Ohori H.; Yamakoshi H.; Tomizawa M.; Shibuya M.; Kakudo Y.; Takahashi A.; Takahashi S.; Kato S.; Suzuki T.; Ishioka C.; Iwabuchi Y.; Shibata H. Synthesis and Biological Analysis of New Curcumin Analogues Bearing an Enhanced Potential for the Medicinal Treatment of Cancer. Mol. Cancer Ther. 2006, 5, 2563–2571. [DOI] [PubMed] [Google Scholar]
- Shibata H.; Yamakoshi H.; Sato A.; Ohori H.; Kakudo Y.; Kudo C.; Takahashi Y.; Watanabe M.; Takano H.; Ishioka C.; Noda T.; Iwabuchi Y. Newly Synthesized Curcumin Analog Has Improved Potential to Prevent Colorectal Carcinogenesis in vivo. Cancer Sci. 2009, 100, 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. M.; Lu Y. J.; Hu H. P.; Shoji M.; Liotta D. C.; Snyder J. P. Curcumin Analog Cytotoxicity Against Breast Cancer Cells: Exploitation of a Redox-Dependent Mechanism. Bioorg. Med. Chem. Lett. 2009, 19, 6627–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amolins M. W.; Peterson L. B.; Blagg B. S. J. Synthesis and Evaluation of Electron-Rich Curcumin Analogues. Bioorg. Med. Chem. 2009, 17, 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T.; Watanabe H.; Nakayama H.; Tada Y.; Kanoh N.; Kondoh M.; Asao T.; Takio K.; Watanabe H.; Nishikawa K.; Kitahara T.; Osada H. The Anticancer Natural Product Pironetin Selectively Targets Lys352 of Alpha-Tubulin. Chem. Biol. 2004, 11, 799–806. [DOI] [PubMed] [Google Scholar]
- Teruya T.; Simizu S.; Kanoh N.; Osada H. Phoslactomycin Targets Cysteine-269 of the Protein Phosphatase 2A Catalytic Subunit in Cells. FEBS Lett. 2005, 579, 2463–2468. [DOI] [PubMed] [Google Scholar]
- Yamakoshi H.; Ohori H.; Kudo C.; Sato A.; Kanoh N.; Ishioka C.; Shibata H.; Iwabuchi Y. Structure-Activity Relationship of C-5-Curcuminoids and Synthesis of Their Molecular Probes Thereof. Bioorg. Med. Chem. 2010, 18, 1083–1092. [DOI] [PubMed] [Google Scholar]
- Kolb H. C.; Finn M. G.; Sharpless K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. [DOI] [PubMed] [Google Scholar]
- Min H. S.; Turck C. W.; Nikolic J. M.; Black D. L. A New Regulatory Protein, KSRP, Mediates Exon Inclusion Through an Intronic Splicing Enhancer. Genes Dev. 1997, 11, 1023–1036. [DOI] [PubMed] [Google Scholar]
- He L. S.; Weber A.; Levens D. Nuclear Targeting Determinants of the Far Upstream Element Binding Protein, a c-Myc Transcription Factor. Nucleic Acids Res. 2000, 28, 4558–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabucchi M.; Briata P.; Garcia-Mayorai M.; Haase A. D.; Filipowicz W.; Ramos A.; Gherzi R.; Rosenfeld M. G. The RNA-Binding Protein KSRP Promotes the Biogenesis of a Subset of MicroRNAs. Nature 2009, 459, 1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherzi R.; Lee K.-Y.; Briata P.; Wegmüller D.; Moroni C.; Karin M.; Chen C.-Y. A KH Domain RNA Binding Protein, KSRP, Promotes ARE-Directed mRNA Turnover by Recruiting the Degradation Machinery. Mol. Cell 2004, 14, 571–583. [DOI] [PubMed] [Google Scholar]
- Hall M. P.; Huang S.; Black D. L. Differentiation-induced Colocalization of the KH-Type Splicing Regulatory Protein with Polypyrimidine Tract Binding Protein and the c-Src Pre-mRNA. Mol. Biol. Cell 2004, 15, 774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.-J.; Liu J.; Dundr M.; Nie Z.; Sanford S.; Levens D. FBPs Are Calibrated Molecular Tools to Adjust Gene Expression. Mol. Cell. Biol. 2006, 26, 6584–6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubaidah R. M.; Tan G. S.; Tan S. B. E.; Lim S. G.; Lin Q.; Chung M. C. M. 2-D DIGE Profiling of Hepatocellular Carcinoma Tissues Identified Isoforms of Far Upstream Binding Protein (FUBP) as Novel Candidates in Liver Carcinogenesis. Proteomics 2008, 8, 5086–5096. [DOI] [PubMed] [Google Scholar]
- Liu J.; Kouzine F.; Nie Z.; Chung H.-J.; Elisha-Feil Z.; Weber A.; Zhao K.; Levens D. The FUSE/FBP/FIR/TFIIH System Is a Molecular Machine Programming a Pulse of c-Myc Expression. EMBO J. 2006, 25, 2119–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.; Liu J.; Collins I.; Sanford S.; O’Connell B.; Benham C. J.; Levens D. Loss of FBP Function Arrests Cellular Proliferation and Extinguishes c-Myc Expression. EMBO J. 2000, 19, 1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcumin and its analogues including bis(arylmethylidene)acetones are considered multitarget compounds that exhibit various biological activities. Accordingly, KSRP/FUBP2 is assumed to be one of the targets of cytotoxic bis(arylmethylidene)acetones. Thus, we also intend to explore other targets of bis(arylmethylidene)acetones by other approaches. These efforts are now underway.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.