Abstract
Objectives. We measured symptom and influenza prevalence, and the effectiveness of symptom and temperature screening for identifying influenza, in arriving international airline travelers.
Methods. This cross-sectional study collected data from travelers to Christchurch International Airport, New Zealand, in winter 2008, via a health questionnaire, temperature testing, and respiratory sampling.
Results. Forms were returned by 15 976 (68%) travelers. Of these, 17% reported at least 1 influenza symptom, with runny or blocked nose (10%) and cough (8%) most common. Respiratory specimens were obtained from 3769 travelers. Estimated prevalence of influenza was 1.1% (4% among symptomatic, 0.2% among asymptomatic). The sensitivity of screening criteria ranged from 84% for “any symptom” to 3% for a fever of 37.8 °C or greater. The positive predictive value was low for all criteria.
Conclusions. Border screening using self-reported symptoms and temperature testing has limitations for preventing pandemic influenza from entering a country. Using “any symptom” or cough would lead to many uninfected people being investigated, yet some infected people would remain undetected. If more specific criteria such as fever were used, most infected people would enter the country despite screening.
Border screening to prevent or delay entry of a pandemic influenza virus to a country is intuitively appealing. Identifying infected travelers and turning them back before they commenced travel, or isolating or quarantining them on arrival, would reduce the probability that they would infect people in the destination country.
The evidence base for decisions on border screening has consisted of reviews of previous influenza pandemic experiences and severe acute respiratory syndrome (SARS), and the results of modeling studies. Although entry and exit screening and quarantine measures were successful in delaying entry of the 1918 pandemic virus to some island countries, border screening for SARS was not found to be cost-effective.1 During the pandemic of influenza A (H1N1) 2009, some countries implemented entry screening, although it is unclear how effective it was in delaying entry of the virus2; for example, only 15 of 116 travel-associated cases in Singapore were detected at the airport.3
The results of modeling studies of the effect of border management to prevent the entry of influenza indicate that a very high proportion of infected travelers (> 99%) would need to be prevented from entering the community to have an important impact on the epidemic in a country,4–6 and even then entry screening is unlikely to be effective in preventing importation.7,8 However, all modeling studies must make assumptions about some variables in the models: for example, the proportion of airline travelers who are infected with influenza, symptomatically and asymptomatically.
The World Health Organization’s most recent guidance on pandemic response suggests that countries could consider implementing exit screening (the first few affected countries) or entry screening (as yet unaffected countries) for a limited period at the beginning of a pandemic.9 However, identifying infected arriving travelers is not straightforward. A simple, cheap, quick, and highly accurate test for influenza that could be used on all travelers is not currently available.10 Therefore, signs and symptoms of influenza must be used to screen travelers to identify those most likely to be infected, for further testing. Influenza screening using symptom questionnaires has inherent limitations to its sensitivity and, hence, effectiveness. Travelers who do not identify themselves as symptomatic will not be detected. These will include those who choose not to disclose symptoms or who use medication to suppress them, and those with no or very mild symptoms on arrival in a country. The latter group includes some who will never develop noticeable symptoms11 and those incubating infection acquired before or during the flight.12,13 Conversely, symptom screening will identify a number of travelers with symptoms not caused by influenza; this could overwhelm testing and quarantine or isolation resources.
The aim of this study was to estimate previously unmeasured characteristics of influenza in international travelers, including the prevalence of symptoms and influenza infection (symptomatic and asymptomatic), during a winter influenza season. By assessing the effectiveness of a health questionnaire and temperature testing for entry screening for seasonal influenza, the study aimed to draw conclusions about their likely effectiveness for pandemic influenza.
METHODS
Three airlines agreed to have their staff distribute a questionnaire to travelers (passengers and crew) during flights from Australian airports to Christchurch, New Zealand, arriving during the midafternoon over a 12-week period between June 23 and September 12, 2008. There were 302 flights eligible to take part in the study. Airline staff read out in-flight scripts explaining the study and asking passengers to complete the questionnaire, then distributed the questionnaires to passengers during the flight. The questionnaire, adapted from one we piloted the previous year,14 sought information on symptoms, travel history, and background demographic variables.
Half the questionnaires were marked and placed into the sets of questionnaires in a random order (determined by the RAND function of Microsoft Excel, version 11.6.6, Microsoft Corp, Redmond, WA). Research assistants collected the questionnaires following immigration processing on arrival in Christchurch. All arriving travelers with a marked questionnaire were invited to have throat and nasal swabs (Copan Italia SPA, Brescia, Italy) and their temperature (ThermaScan PRO4000, BRAUN, Kronberg, Germany) taken by trained research nurses. Those travelers with an unmarked questionnaire, but who reported symptoms, were also invited to have swabs and their temperature taken. “Symptomatic” travelers were defined as those who reported 1 or more of: cough, sore throat, sneezing, fever or chills, runny or blocked nose, muscle aches or pains, feeling generally unwell, chest discomfort or breathing difficulties. The nurse also noted on the request form whether the traveler was symptomatic or asymptomatic. Written informed consent was obtained from all participants.
Total plane loading (number on board) was obtained from the New Zealand Customs Service.
Laboratory Analysis
All combined throat and nasal swab samples were analyzed at Canterbury Health Laboratories, Christchurch. A commercial EasyPlex multiplexed tandem polymerase chain reaction system was employed to detect the presence of influenza infection, as described by the manufacturer (EasyPlex Influenza A+B Kit, Cat. No. 3005.01, Ausdiagnostics, Sydney, Australia).
Data Management and Analysis
We double-entered data and transferred them to Stata version 10 (StataCorp LP, College Station, TX) for analysis. We linked data from the health questionnaires to specimen results by using a unique code.
We examined response rates and the prevalence of symptoms and infection in different groups by using the χ2 test and confidence intervals, calculated taking account of the hierarchical nature of the sampling (by flight) using the SVY command in Stata. We estimated the prevalence of influenza infection among all arriving travelers by using the prevalence in holders of marked questionnaires who had provided respiratory samples.
We could not directly calculate the sensitivity and specificity of symptoms for influenza infection from the proportion of cases or noncases with a given symptom because we requested swabs from all symptomatic travelers but only a random sample of asymptomatic travelers. Therefore, we calculated sensitivity and specificity by using the VALIDES command in Stata, which calculates sensitivity and specificity where (as in this study) only a subgroup of those screened are tested with the “gold standard” test. This command cannot account for hierarchical sampling; therefore, the width of the confidence intervals around sensitivity and specificity estimates is underestimated.
We could not directly calculate the positive predictive value (PPV) of symptoms for influenza because symptomatic participants were overrepresented in the group who provided specimens. Therefore, we combined the prevalence of symptoms among all participants with the measured sensitivity and specificity of those symptoms to calculate an estimated PPV.
RESULTS
Of the 302 eligible flights, we received no questionnaires from travelers on 75 flights, mostly because of problems with getting the questionnaires loaded onto the planes. For 40 flights, we received questionnaires from some passengers but the study protocol had not been followed by the crew; for example, questionnaires had been handed out to only some passengers, crew joked about the study, questionnaires were collected by the crew before landing, or questionnaires were handed to passengers on leaving the plane. The response rate for these 40 flights was 38% (2051 of 5460). In 187 flights where, as far as we are aware, the study protocol was followed, the response rate was 67.9% (15 976 of 23 537); range 11% to 91%.
Women were slightly more likely to return questionnaires than were men (69.3% vs 65.9%; P < .001). Response rates increased markedly with age, from 48.7% for children younger than 15 years to 80.0% for people older than 85 years (P < .001 across all age groups). People of New Zealand, Australian, and European nationalities had similar response rates (67.1%, 64.1%, and 66.3%, respectively), whereas persons of other nationalities were less likely to return a questionnaire (57.0%).
Respiratory specimens were obtained from 4252 travelers. Of these, 18 could not be linked to a questionnaire, 8 were incorrectly obtained from asymptomatic travelers holding unmarked questionnaires, and 457 were from travelers on those flights where study protocols had not been followed. A further 43 samples were considered invalid as they contained no human nucleic acid.
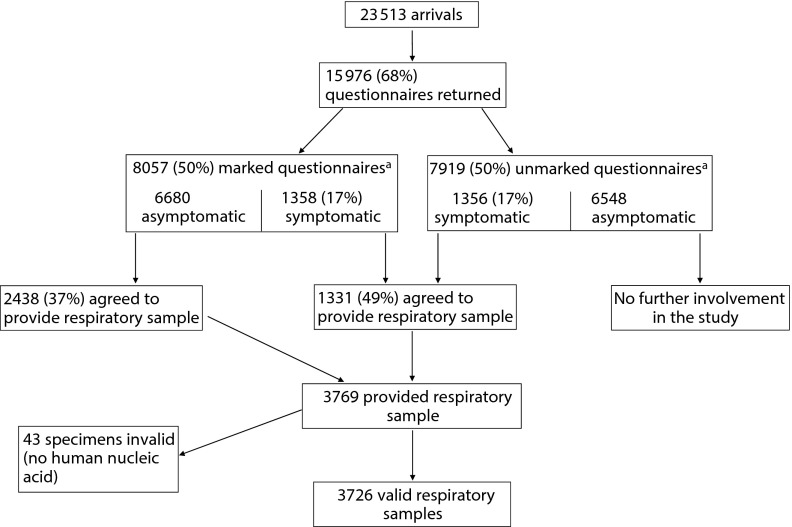
The following analysis includes the 15 976 questionnaires and the 3726 valid specimens correctly obtained (Figure 1). Of asymptomatic travelers who held marked questionnaires, 37% agreed to provide a respiratory sample, as did 49% of symptomatic travelers.
FIGURE 1—
Study flow chart for participants in screening for influenza in arriving airline travelers: Christchurch International Airport, New Zealand, Winter 2008.
a34 participants (19 marked, 15 unmarked) did not give valid answers to the symptom questions
Symptoms
Table 1 shows the symptoms reported by participants. The most common symptoms were runny or blocked nose and cough, and only 0.6% of participants reported fever. Table 2 shows the proportion of participants who were symptomatic, by age, gender, and vaccination, contact, and travel history. There was a significant association between age and symptoms, with a decrease in the prevalence of symptoms with increasing age, except for the small group of those older than 84 years. Influenza vaccination in the past year was not associated with reporting symptoms, but contact with someone who was coughing or sneezing was. Living, traveling, or staying with someone who was coughing or sneezing was the type of contact with the greatest risk of being symptomatic, and having more types of contact increased risk. Having spent time recently in Australia or New Zealand (which were experiencing their annual seasonal influenza epidemic) was associated with a slightly higher prevalence of symptoms than for those who reported travel in northern hemisphere countries during the previous 7 days.
TABLE 1—
Prevalence of Reported Symptoms Among 15 942 Travelers Arriving Into Christchurch International Airport, New Zealand: Winter 2008
| Symptoms | No. (%) | 95% CI |
| Any symptom | 2714 (17.0) | 16.2, 17.8 |
| Cough | 1190 (7.5) | 7.0, 8.0 |
| Blocked or runny nose | 1611 (10.1) | 9.5, 10.7 |
| Sore throat | 654 (4.1) | 3.7, 4.5 |
| Muscle aches or pains | 389 (2.4) | 2.2, 2.7 |
| Sneezing | 582 (3.7) | 3.3, 4.0 |
| Feeling generally unwell | 342 (2.1) | 1.9, 2.4 |
| Fever or chills | 101 (0.6) | 0.5, 0.8 |
| Chest discomfort or breathing difficulties | 171 (1.1) | 0.9, 1.2 |
| ILI (fever and cough or sore throat) | 85 (0.5) | 0.4, 0.7 |
Note. CI = confidence interval; ILI = influenza-like illness. Thirty-four participants did not provide valid information on symptoms.
TABLE 2—
Prevalence of Symptoms by Demographic, Vaccination, Contact, and Travel History Among 15 942 Travelers Arriving Into Christchurch International Airport, New Zealand: Winter 2008
| Variable | Total,a No. | Symptomatic,b No. (%) | χ2 (P) or 95% CI | |
| All | 15 942 | 2714 (17.0) | ||
| Gender | ||||
| Male | 8065 | 1299 (16.1) | ||
| Female | 7759 | 1420 (18.0) | 11.0 (.001) | |
| Missing | 103 | 15 | ||
| Age, y | ||||
| 0–14 | 1102 | 266 (24.1) | ||
| 15–24 | 2574 | 615 (23.9) | ||
| 25–44 | 5467 | 948 (17.3) | ||
| 45–64 | 4709 | 609 (12.9) | ||
| 65–84 | 1208 | 147 (12.2) | ||
| ≥ 85 | 23 | 4 (17.4) | 34.0 (< .001) | |
| Missing | 858 | 125 | ||
| Influenza vaccination in past 12 mo | ||||
| Yes | 3375 | 594 (17.6) | ||
| No | 11 967 | 2096 (17.5) | 0.01 (.91) | |
| Missing | 600 | 24 | ||
| Contact with someone who was coughing or sneezing in past 3 d | ||||
| Yes | 3967 | 1330 (33.5) | ||
| No | 8803 | 797 (9.1) | ||
| Do not know | 2603 | 543 (20.9) | 490.5 (< .001) | |
| Invalid or missing | 569 | 44 | ||
| If contact, in contact with | ||||
| Someone lived, traveled, stayed with | 2396 | 884 (36.9) | 34.6, 39.2 | |
| Someone visited or worked with | 1528 | 508 (33.2) | 30.6, 35.9 | |
| A stranger | 585 | 148 (25.3) | 21.9, 28.7 | |
| Missing | 15 | 5 | ||
| 1 type of contactc | 3463 | 1140 (32.9) | ||
| 2 types of contact | 421 | 155 (36.8) | ||
| 3 types of contact | 68 | 30 (44.1) | 2.7 (.073) | |
| Travel historyd—spent any time during the last 7 d in | ||||
| New Zealand | 765 | 149 (19.5) | 16.4, 22.6 | |
| Australia | 14 295 | 2480 (17.3) | 16.5, 18.2 | |
| Asia | 799 | 108 (13.5) | 10.5, 16.5 | |
| Africa, Pacific Islands, or South America | 90 | 13 (14.4) | 6.7, 22.2 | |
| North America or Europe | 535 | 83 (15.5) | 12.1, 18.9 | |
| Invalid or missing | 167 | 30 |
Excludes the 34 participants who did not give valid symptom information.
Symptomatic = having at least 1 of: cough, sore throat, sneezing, fever or chills, runny or blocked nose, muscle aches or pains, feeling generally unwell, chest discomfort, or breathing difficulties.
Number of types of contact = how many of the previous questions a participant said “yes” to (someone lived, traveled, stayed with; someone visited or worked with; a stranger).
Totals do not add to the numbers in row 1 because more than 1 category could be selected.
An aural temperature measurement was obtained from 3589 of the 3726 participants who provided a valid specimen. Among 1276 who were symptomatic, 14 (1%) had a measured temperature of 37.8 °C or greater, and 52 (4%) had a temperature of 37.5 °C or greater. Of the 2313 who reported no symptoms, 6 (0.3%) had a measured temperature of 37.8 °C or greater and 54 (2%) had a temperature of 37.5 °C or greater.
Influenza Infection
Influenza virus was detected in 60 respiratory samples; 51 were type B, 8 type A, and 1 sample had both A and B. The prevalence of influenza infection in holders of marked questionnaires, representing our estimate of the prevalence among all participants, was 35 of 3103 (1.13%; 95% confidence interval [CI] = 0.77, 1.48).
Among participants who provided samples, 56 of 1317 (4.3%) who were symptomatic and 4 of 2409 (0.17%) who were asymptomatic had influenza infection.
The sensitivity, specificity, and estimated PPV for influenza infection of selected symptoms, influenza-like illness, and combinations of “any symptom” and factors associated with being symptomatic are shown in Table 3. “Any symptom” had sensitivity and specificity in the mid-80s, but a low estimated PPV of 5.5%, whereas fever had the highest PPV and very high specificity, but low sensitivity for influenza infection. The combination of symptoms and contact (prevalence 8.6%) had better specificity than “any symptom” alone, and similar PPV, but poor sensitivity. Symptoms or contact (prevalence 34.6%) had slightly better sensitivity, but resulted in a large decrease in specificity and PPV compared with “any symptom.” No other combination of factors was significantly more accurate than those shown in the table (data not shown).
TABLE 3—
Sensitivity, Specificity, and Positive Predictive Value of Symptoms and Other Screening Criteria for Identifying Influenza in Arriving International Airline Travelers, Christchurch International Airport, New Zealand, Winter 2008
| Symptom | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Estimated PPV, % |
| Any symptom | 84.0 (70.3, 97.6) | 83.6 (83.0, 84.2) | 5.5 |
| Cough | 59.6 (44.9, 74.3) | 93.1 (92.6, 93.5) | 9.0 |
| Sore throat | 25.0 (15.5, 34.4) | 96.2 (95.9, 96.5) | 7.0 |
| Fever, self-report | 11.3 (5.4, 17.1) | 99.5 (99.4, 99.6) | 20.5 |
| ILI, self-report | 11.5 (5.6, 17.3) | 99.6 (99.5, 99.7) | 24.7 |
| Measured temperature ≥ 37.8 °C | 2.6 (0.5, 4.8) | 99.9 (99.9, 100.0) | …a |
| ILI, using measured temperature | 2.5 (0.4, 4.6) | 100 | …a |
| History of contact with someone who was coughing or sneezing | 43.2 (30.8, 55.5) | 75.5 (74.9, 76.2) | 2.0 |
| Any symptom or contact | 86.4 (74.4, 98.3) | 67.2 (66.4, 67.9) | 2.9 |
| Any symptom and contact | 33.5 (22.2, 44.7) | 92.0 (91.6, 92.5) | 4.6 |
| Any symptom and contact with someone visited, worked, lived, traveled, or stayed with | 29.8 (19.3, 40.3) | 92.4 (92.0, 92.8) | 4.3 |
| Any symptom and age < 25 y | 14.6 (7.2, 22.0) | 94.6 (94.3, 95.0) | 3.0 |
Note. CI = confidence interval; ILI = influenza-like illness (fever and cough or sore throat); PPV = positive predictive value.
At near 100% specificity, the PPV cannot be reliably estimated.
DISCUSSION
In this study carried out during the winter influenza season of 2008, 68% of airline travelers arriving into Christchurch, New Zealand, on flights from Australia, returned our study questionnaire. Of these, 17% reported at least 1 symptom that could be caused by influenza, with younger participants and those who had been in contact with someone who was coughing or sneezing in the previous 3 days having a higher prevalence of symptoms than others. The most commonly reported symptoms were blocked or runny nose (10.1%) and cough (7.5%). In some travelers, the dry, cold air in the plane cabin may have caused these symptoms, which would limit their specificity for influenza.
The prevalence of influenza infection among study participants was estimated to be slightly more than 1%. Only the broad screening definition of “any symptom” was able to identify more than two thirds of such cases (84%; higher than suggested by volunteer infection studies11), with more specific individual symptoms such as cough and sore throat having much lower sensitivity. Sensitivity was particularly low for fever-based criteria; reported fever (11.3%), measured fever of 37.8 °C or greater (2.6%), and “influenza-like illness” (fever and cough or sore throat; 11.5%). Because of the low prevalence of influenza infection, the PPV was low for all criteria, even though some had high specificity. For example, the PPV of “any symptom” was 5.5%, and that of self-reported fever was only 20.5% despite its higher-than-99% specificity.
Strengths and Limitations
An important strength of this study is that it is the only one we are aware of that has screened large numbers of arriving airline travelers with a health questionnaire and then tested respiratory samples of both symptomatic and asymptomatic travelers for influenza infection. This approach, which required considerable effort to gain airline cooperation,14 is necessary to estimate the sensitivity and specificity of different screening criteria and the prevalence of infection among travelers.
Study limitations include the short-haul nature of the flights included, possible bias from variations in response rate by age, low response rates to requests for respiratory specimens, and the relatively homogeneous travel histories of participants.
We were able to gain airline approval only to include flights arriving from Australia (flight time 3–4 hours) in the study. It is possible that over longer flights some more infected passengers may have become symptomatic, in which case we have underestimated the sensitivity of symptoms for influenza infection after long-haul flights. However, our other main findings of the high prevalence of symptoms among passengers and the low PPV of screening criteria would be unaffected.
Lower response rates to our questionnaire at younger ages may have led to some bias in our estimates of the prevalence of symptoms and of influenza. Younger respondents had a higher prevalence of symptoms than did older respondents, so if those who did respond were representative of all younger travelers, our estimate of symptom (and possibly influenza) prevalence will be an underestimate. On the other hand, if questionnaires were returned preferentially for symptomatic young people, our estimates will be overestimates of the true population prevalence. We are unable to assess this, but the generally higher incidence of influenza among children15 suggests that the latter is not the case, and our estimates of 17% symptom prevalence and 1% influenza prevalence can be considered minimum estimates.
The proportion of travelers who were willing to provide respiratory specimens for influenza testing was 49% for symptomatic and 37% for asymptomatic travelers. Although these low response rates affect the precision of our estimates, we believe that they are unlikely to be biased as it is unlikely that influenza-infected travelers would be more or less likely to agree to provide specimens than those not infected.
Because most of the travelers in this study were currently living in or visiting geographic regions with high seasonal influenza activity, we were unable to assess the use of travel history as a screening criterion.
There is no good evidence available on influenza virus transmissibility during the various phases of infection (including asymptomatic or afebrile infection); however, detection of viral RNA on a respiratory sample does not necessarily imply infectiousness. Specimens taken in this study were not able to be cultured for influenza virus, so it is possible that some of the positive RNA results represented shedding of nonviable influenza virus. If so, our data would overestimate the proportion of infectious travelers that would enter the community despite screening.
Generalizability of These Findings
The premise of this study is that lessons learned from seasonal influenza can be applied to pandemic influenza. Although any new influenza strain will have some different characteristics from previous ones, we believe that the results of this study are sufficiently generalizable to be useful for pandemic planning.
This study was carried out during a “normal” influenza season, when the predominant strain was influenza B.16 There were no travel restrictions in operation during the study, so our results are likely to be broadly generalizable to other seasonal influenza epidemics. If, as suggested by volunteer studies,11 influenza B causes fewer symptoms than influenza A, our study will underestimate symptom prevalence and sensitivity for influenza A epidemics. However, the performance of symptom-based screening criteria depends importantly on influenza prevalence.
During influenza pandemics the prevalence of influenza among travelers might be higher than in this study, given that the prevalence in the population is likely to be higher than during seasonal influenza (although in 2 planes that departed from high-prevalence areas early in the 2009 H1N1 pandemic the prevalence of infection was only 2%13,17). However, people with severe symptoms would be unlikely to commence travel, so if a pandemic strain caused mainly severe disease then the prevalence of pandemic influenza among travelers could be very low. Travel restrictions and advisories during a pandemic may also reduce the prevalence among arriving travelers. In addition, if quarantine or isolation are likely, some travelers will not admit to symptoms or contact with symptomatic others, which would reduce the sensitivity of a questionnaire for influenza infection. On balance, it seems unlikely that the screening criteria we have evaluated would perform better during a pandemic than during this study.
Implications for Entry Screening Policy
The purpose of entry screening at the border as part of a pandemic response strategy is to identify “high-risk” travelers for further testing to establish whether they are infected with influenza. To prevent transmission to the community this group should be quarantined (at home or in an institution) and possibly treated with antivirals pending the test results, so for this to be feasible the group must be not too large. On the other hand, the number of infected people who are missed by the screening and enter the community should be very small. Criteria that ensure that no infected people “slip through” will inevitably identify larger numbers of uninfected people (who will be tested unnecessarily) than more restrictive criteria. In low-prevalence situations, the number of uninfected people who are sent for further action can be large compared with the number of infected people who are detected.
Symptom-based screening for influenza has inherent limitations to its sensitivity, as a proportion of those infected with influenza will be asymptomatic on arrival and some people with symptoms will not report them. Although sensitivity and PPV can be traded off to some extent, in this study the use of any screening criterion with reasonable sensitivity (e.g., > 80%) would mean that more than 15% of travelers would require further testing. Until high-sensitivity rapid tests are available, these travelers would all require isolation pending influenza test results, although fewer than 10% of those thus isolated are likely to be infected. In addition, some arriving passengers who are infected with influenza will not be detected at the border. This includes those whose infection was not detectable on arrival and those missed by screening—the latter alone would be 0.2% of travelers in this study (using criteria with 80% sensitivity, 20% of the 1.13% infected would not be detected). Thus, in countries with large numbers of arriving airline travelers, many infected people would enter the community undetected by screening.
Other Approaches to Border Screening
There is an argument for shifting the focus of border screening to exit screening, to reduce the number of infected people boarding flights. However, the same issues of sensitivity and specificity of symptoms would apply. For example, using the criterion of cough (which had the highest sensitivity of the individual symptoms) to exclude passengers would have resulted in refusing to board 7.5% of the passengers in this study, of whom 90% were uninfected. If a very virulent influenza virus caused a pandemic, the balance of cost and benefit for border screening may be different from that for a virus more like a seasonal influenza virus.
An evaluation of infrared thermal image scanning undertaken during a part of this study found that it was not effective for influenza screening at borders, mainly because fever was not a particularly common symptom among infected travelers.18
In the very early phase of a pandemic with a limited geographic focus, screening arriving travelers from the relevant country and testing and quarantining all those who are symptomatic may be logistically feasible. Prevalence may be higher in this situation, which would improve PPVs but also mean that more asymptomatic infected travelers would enter the community (because of the poor sensitivity of screening criteria). And if the relevant country is a common source of travelers (e.g., the United States, as in the H1N1 2009 pandemic), the logistic feasibility and cost of entry screening may outweigh the likely benefit.
Conclusions
This study has provided estimates of the prevalence of respiratory symptoms and influenza infection among travelers arriving in New Zealand from Australia during the Southern Hemisphere influenza season, which can be used to support future assessments of border screening strategies.
Among this population, entry screening for influenza infection would be either very resource intensive (testing and potentially quarantining 17% of arriving travelers using “any symptom”) or ineffective (allowing 97% of infected arrivals to enter the community if measured fever was used). Public health resources, particularly trained staff, are finite, and the considerable effort required for border screening reduces staff available for other aspects of pandemic management. Although the severity of a future pandemic threat may mean that border screening is attempted, it is likely that for most situations the poor effectiveness and high opportunity costs of current methods for border screening will limit its usefulness as a pandemic response strategy.
Acknowledgments
This study was funded by the US Centers for Disease Control and Prevention (grant 1 U01 CI000445-01).
We are grateful to Christchurch International Airport Limited, New Zealand Customs Service, and the participating airlines for their cooperation and assistance; Andrew Strathdee and Patalee Mahagamasekera for the laboratory testing; and Claire Cameron for data cleaning and preparation for analysis.
Note. The study funder had no role in study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the article for publication.
Human Participant Protection
This study was approved by the New Zealand Health and Disability Multiregion Ethics Committee (MEC/06/12/172).
References
- 1.Bell DM, World Health Organization Writing Group Non-pharmaceutical interventions for pandemic influenza, international measures. Emerg Infect Dis. 2006;12(1):81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowling BJ, Lau LL, Wu Pet al. Entry screening to delay local transmission of 2009 pandemic influenza A (H1N1). BMC Infect Dis. 2010;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherjee P, Lim PL, Chow Aet al. Epidemiology of travel-associated pandemic (H1N1) 2009 infection in 116 patients, Singapore. Emerg Infect Dis. 2010;16(1):21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper BS, Pitman RJ, Edmunds WJ, Gay NJ. Delaying the international spread of pandemic influenza. PLoS Med. 2006;3(6):e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442(7101):448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishiura H, Wilson N, Baker MG. Quarantine for pandemic influenza control at the borders of small island nations. BMC Infect Dis. 2009;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitman RJ, Cooper BS, Trotter CL, Gay NJ, Edmunds WJ. Entry screening for severe acute respiratory syndrome (SARS) or influenza: policy evaluation. BMJ. 2005;331(7527):1242–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caley P, Becker NG, Philp DJ. The waiting time for inter-country spread of pandemic influenza. PLoS ONE. 2007;2(1):e143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandemic Influenza Preparedness and Response: A WHO Guidance Document. Geneva, Switzerland: World Health Organization; 2010 [PubMed] [Google Scholar]
- 10.Uyeki TM, Prasad R, Vukotich Cet al. Low sensitivity of rapid diagnostic test for influenza. Clin Infect Dis. 2009;48(9):e89–e92 [DOI] [PubMed] [Google Scholar]
- 11.Carrat F, Vergu E, Ferguson NMet al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775–785 [DOI] [PubMed] [Google Scholar]
- 12.Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9(5):291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker MG, Thornley CN, Mills Cet al. Transmission of pandemic A/H1N1 2009 influenza on passenger aircraft: retrospective cohort study. BMJ. 2010;340:c2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan AR, Priest PC, Jennings LC, Brunton CR, Baker MG. Screening for influenza infection in international airline travelers. Am J Public Health. 2009;99(Suppl 2):S360–S362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monto AS. Epidemiology of influenza. Vaccine. 2008;26(Suppl 4):D45–D48 [DOI] [PubMed] [Google Scholar]
- 16.Lopez L, Huang QS. Influenza in New Zealand 2008. Wellington, New Zealand: Institute of Environmental Science and Research; 2009 [Google Scholar]
- 17.Foxwell AR, Roberts L, Lokuge K, Kelly PM. Transmission of influenza on international flights, May 2009. Emerg Infect Dis. 2011;17(7):1188–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priest PC, Duncan AR, Jennings LC, Baker MG. Thermal image scanning for influenza border screening: results of an airport screening study. PLoS ONE. 2011;6(1):e14490. [DOI] [PMC free article] [PubMed] [Google Scholar]