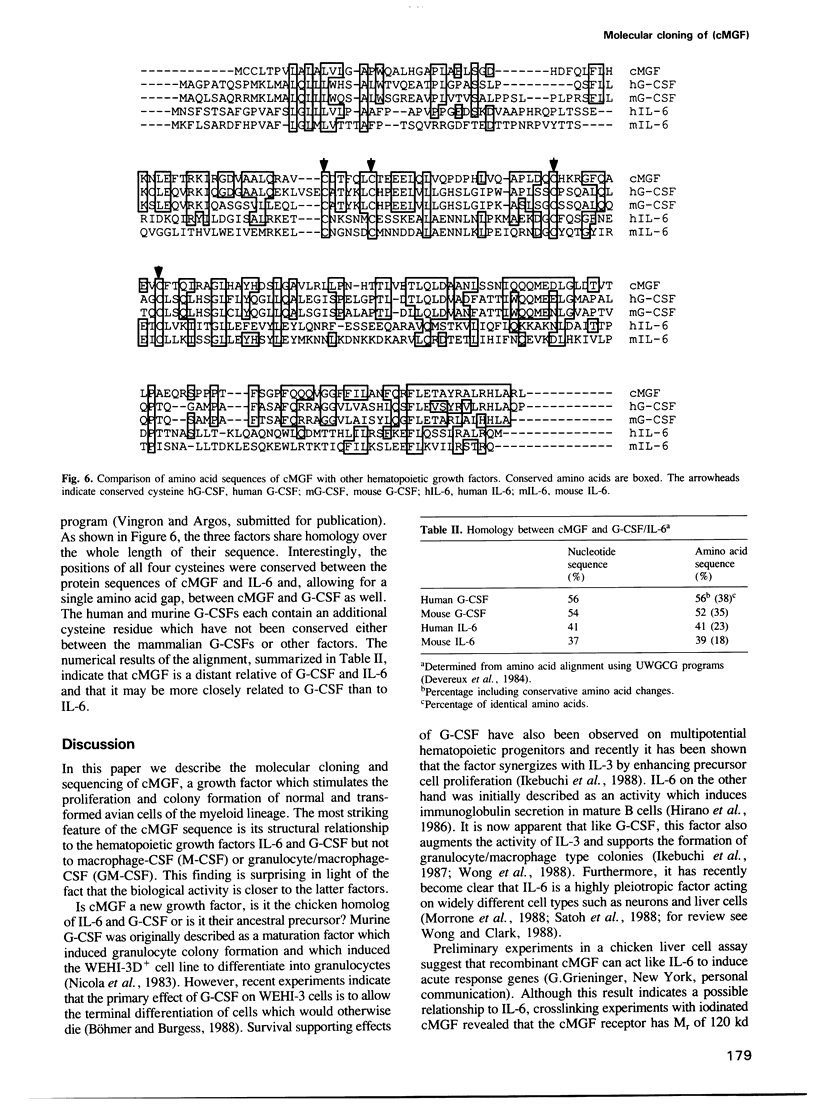
Abstract
Normal as well as retrovirally transformed avian myeloid precursor cells require the colony stimulating factor cMGF for their survival, proliferation and colony formation in vitro. cMGF has been shown to be a glycoprotein which is active in the picomolar concentration range. Co-expression of kinase type oncogenes in v-myb or v-myc transformed myeloid cells induces cMGF expression and confers factor independence via an autocrine mechanism. Here we describe the molecular cloning of cMGF from a myeloblast cDNA library and show that it is a 201 amino acid residue secretory protein which is modified by signal peptide cleavage and glycosylation during translocation into the lumen of membrane vesicles. A bacterially expressed trpE-cMGF fusion protein induces proliferation of E26 transformed myeloblasts in a cMGF bioassay suggesting that glycosylation is not absolutely necessary for biological activity. Sequence comparison reveals that cMGF is distantly related to G-CSF and IL-6.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Anisowicz A., Bardwell L., Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Hayman M. J., Graf T. Myeloblasts transformed by the avian acute leukemia virus E26 are hormone-dependent for growth and for the expression of a putative myb-containing protein, p135 E26. EMBO J. 1982;1(9):1069–1073. doi: 10.1002/j.1460-2075.1982.tb01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhmer R. M., Burgess A. W. Granulocytic colony-stimulating factor (G-CSF) does not induce differentiation of WEHI3B(D+) cells but is required for the survival of the mature progeny. Int J Cancer. 1988 Jul 15;42(1):53–58. doi: 10.1002/ijc.2910420111. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The Drosophila developmental gene, engrailed, encodes a sequence-specific DNA binding activity. Nature. 1985 Dec 19;318(6047):630–635. doi: 10.1038/318630a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue R. E., Wang E. A., Kaufman R. J., Foutch L., Leary A. C., Witek-Giannetti J. S., Metzger M., Hewick R. M., Steinbrink D. R., Shaw G. Effects of N-linked carbohydrate on the in vivo properties of human GM-CSF. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):685–692. doi: 10.1101/sqb.1986.051.01.081. [DOI] [PubMed] [Google Scholar]
- Graf T., von Weizsaecker F., Grieser S., Coll J., Stehelin D., Patschinsky T., Bister K., Bechade C., Calothy G., Leutz A. v-mil induces autocrine growth and enhanced tumorigenicity in v-myc-transformed avian macrophages. Cell. 1986 May 9;45(3):357–364. doi: 10.1016/0092-8674(86)90321-1. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Ikebuchi K., Clark S. C., Ihle J. N., Souza L. M., Ogawa M. Granulocyte colony-stimulating factor enhances interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1988 May;85(10):3445–3449. doi: 10.1073/pnas.85.10.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984 Oct;38(3):731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leutz A., Beug H., Graf T. Purification and characterization of cMGF, a novel chicken myelomonocytic growth factor. EMBO J. 1984 Dec 20;3(13):3191–3197. doi: 10.1002/j.1460-2075.1984.tb02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutz A., Beug H., Walter C., Graf T. Hematopoietic growth factor glycosylation. Multiple forms of chicken myelomonocytic growth factor. J Biol Chem. 1988 Mar 15;263(8):3905–3911. [PubMed] [Google Scholar]
- Linzer D. I., Lee S. J., Ogren L., Talamantes F., Nathans D. Identification of proliferin mRNA and protein in mouse placenta. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4356–4359. doi: 10.1073/pnas.82.13.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. H., Castro M. M., Aboul-ela F., Tinoco I., Jr Base pairing involving deoxyinosine: implications for probe design. Nucleic Acids Res. 1985 Dec 20;13(24):8927–8938. doi: 10.1093/nar/13.24.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone G., Ciliberto G., Oliviero S., Arcone R., Dente L., Content J., Cortese R. Recombinant interleukin 6 regulates the transcriptional activation of a set of human acute phase genes. J Biol Chem. 1988 Sep 5;263(25):12554–12558. [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Nicola N. A., Peterson L. Identification of distinct receptors for two hemopoietic growth factors (granulocyte colony-stimulating factor and multipotential colony-stimulating factor) by chemical cross-linking. J Biol Chem. 1986 Sep 15;261(26):12384–12389. [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakamura S., Taga T., Matsuda T., Hirano T., Kishimoto T., Kaziro Y. Induction of neuronal differentiation in PC12 cells by B-cell stimulatory factor 2/interleukin 6. Mol Cell Biol. 1988 Aug;8(8):3546–3549. doi: 10.1128/mcb.8.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Ziltener H. J., Leslie K. B. Structural homologies among the hemopoietins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2458–2462. doi: 10.1073/pnas.83.8.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek-Giannotti J. S., Temple P. A., Kriz R., Ferenz C., Hewick R. M., Clark S. C., Ikebuchi K., Ogawa M. Stimulation of murine hemopoietic colony formation by human IL-6. J Immunol. 1988 May 1;140(9):3040–3044. [PubMed] [Google Scholar]
- Yamasaki K., Taga T., Hirata Y., Yawata H., Kawanishi Y., Seed B., Taniguchi T., Hirano T., Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988 Aug 12;241(4867):825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Weizsäcker F., Beug H., Graf T. Temperature-sensitive mutants of MH2 avian leukemia virus that map in the v-mil and the v-myc oncogene respectively. EMBO J. 1986 Jul;5(7):1521–1527. doi: 10.1002/j.1460-2075.1986.tb04392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]