Abstract
Objectives. We estimated anogenital wart prevalence from 2003 to 2010 by gender and age group in a large US cohort with private insurance to detect potential decreases among people most likely to be affected by human papillomavirus (HPV) vaccination.
Methods. We restricted health care claims to those from individuals aged 10 to 39 years with continuous insurance within a given year. We derived anogenital wart diagnoses from a diagnosis of condyloma acuminata, or either a less specific viral wart diagnosis or genital wart medication combined with either a benign anogenital neoplasm or destruction or excision of a noncervical anogenital lesion.
Results. Prevalence increased slightly in 2003 to 2006, then significantly declined in 2007 to 2010 among girls aged 15 to 19 years; increased in 2003 to 2007, remained level through 2009, and declined in 2010 among women aged 20 to 24 years; and increased through 2009 but not in 2010 for women aged 25 to 39 years. For males aged 15 to 39 years, prevalence for each 5-year age group increased in 2003 to 2009, but no increases were observed for 2010.
Conclusions. These data indicate reductions in anogenital warts among US females aged 15 to 24 years, the age group most likely to be affected by introduction of the HPV vaccine.
In mid-2006, a quadrivalent human papillomavirus (HPV) vaccine was licensed in the United States for females (Merck & Co., Inc., Whitehouse Station, NJ). This vaccine is specific against HPV types 16 and 18, which cause approximately 70% of cervical cancers worldwide,1,2 as well as types 6 and 11, which are nononcogenic but can cause benign cervical lesions and anogenital warts.1,3,4 A bivalent vaccine (GlaxoSmithKline, Research Triangle Park, NC), specific for only HPV types 16 and 18, was licensed in late 2009. These vaccines are routinely recommended for girls aged 11 to 12 years, with catch-up vaccination through age 26 years.5,6 In late 2011, the quadrivalent vaccine was recommended for boys aged 11 to 12, with catch-up vaccination through age 21 years.7,8 However, HPV vaccine uptake in the United States is relatively low. In 2011, a national survey found that 53% of girls aged 13 to 17 years had received at least 1 dose of the HPV vaccine series, but only 35% had received all 3 doses.9 Vaccine uptake was extremely low among boys.9
Postlicensure monitoring of new vaccines is important to assess the progress of immunization programs, demonstrate population impact, and evaluate policy needs.10–14 Clinical trials have demonstrated the prophylactic efficacy of the quadrivalent HPV vaccine,15,16 and questions of interest about currently available HPV vaccines now center on population effectiveness and cost-effectiveness.17 However, several factors complicate efforts to monitor the population impact of HPV vaccine, including multiple clinical outcomes and variable, often extended, time to outcome development.10,12,14 Cervical cancer is the most important anogenital outcome of HPV infection and may take several decades to develop.18 Cervical intraepithelial neoplasia and adenocarcinoma in situ are the most common cervical cancer precursor lesions, often occurring 1 to 3 years after HPV infection.19–22 In contrast to these outcomes, anogenital warts can develop within months of HPV infection, and therefore monitoring changes in anogenital wart diagnoses can be used to assess the most immediate impact of HPV vaccination.19
The objective of this analysis was to estimate annual prevalence of anogenital wart diagnoses during 2003 to 2010 in a large group of privately insured patients, by gender and age group, to detect potential decreases among people most likely to be affected by quadrivalent HPV vaccination.
METHODS
We obtained data from the Truven Health Analytics MarketScan Commercial Claims and Encounters Database, which contains health care claims data from approximately 100 health insurance plans (Truven Health Analytics, Ann Arbor, MI). The claims records represent the medical experiences of insured employees, early retirees, and their dependents throughout the United States.
Study Population
For this analysis, we used records for inpatient admissions, outpatient (ambulatory) visits, and outpatient pharmaceutical claims from January 2003 through December 2010. We restricted claims records to those from persons aged 10 to 39 years in a given year who were continuously enrolled in participating health insurance plans within that year. Claims records within each year were aggregated so that individual persons were used as the unit of analysis.
Case Definition
We used a number of anogenital wart-related indicators to create the case definition:
International Classification of Disease, 9th Revision, Clinical Modification23 (ICD-9-CM) diagnosis code 078.11, which is specific for condyloma acuminatum (i.e., anogenital warts);
3 less-specific ICD-9-CM codes for viral warts, 078.1 (viral warts; viral warts due to human papillomavirus), 078.10 (viral warts, unspecified; condyloma not otherwise specified; verruca not otherwise specified: vulgaris), and 078.19 (other specified viral warts; genital warts not otherwise specified; verruca plana; verruca plantaris);
National Drug Codes representing formulations of the following anogenital wart treatments24: sinecatechins,25 imiquimod,26 podofilox,27 podophyllum resin,28 and trichloroacetic acid, 2 of which (imiquimod and trichloroacetic acid) are also indicated for conditions other than genital warts;
ICD-9-CM diagnosis codes indicating benign neoplasms of the anogenital region, excluding benign neoplasms of the cervix to avoid including the preponderance of cervical lesions caused by oncogenic HPV types; and
ICD-9-CM; Current Procedural Terminology, 4th Edition29; and Healthcare Financing Association Common Procedural Coding System30 procedure codes indicating destruction or excision of anogenital lesions (excluding the cervix as an anatomic site) or colposcopy of the vulva.
We used these anogenital wart–related indicators to create the case definition as follows:
persons with 1 or more diagnoses of condyloma acuminata (ICD-9-CM code 078.11) occurring within a given year; or
persons with 1 or more less-specific diagnoses of viral warts within a given year, if there was also a procedure for destruction or excision of an anogenital lesion, or a diagnosis of a benign anogenital neoplasm, within 30 days of the diagnosis; or
persons with 1 or more prescriptions for genital wart medications within a given year, if there was also a procedure for destruction or excision of an anogenital lesion, or a diagnosis of a benign anogenital neoplasm, within 30 days of the prescription.
Potential Confounders or Effect Modifiers
We stratified analyses by gender (male or female) and 5-year age group (10–14, 15–19, 20–24, 25–29, 30–34, and 35–39 years). We based receipt of a screening Papanicolaou (Pap) test or routine pelvic examination within a given year on having any 1 of 5 ICD-9-CM diagnosis codes or 2 Healthcare Financing Association Common Procedural Coding System procedure codes indicating a routine gynecologic or pelvic examination or Pap test, or any 1 of 23 Current Procedural Terminology, 4th Edition or 1 of 12 Healthcare Financing Association Common Procedural Coding System codes indicating screening cervical or vaginal cytopathology. Geographic region was the census region in which the insured employee resided (Northeast, North Central, South, and West). We also based residence in a metropolitan statistical area (MSA) or non-MSA on the insured employee’s residence. We categorized insurance plans as noncapitated or capitated (i.e., fee-for-service) as follows: noncapitated plans included basic or major medical, comprehensive, exclusive provider organizations, noncapitated point of service, preferred provider organizations, consumer-driven health plans, and high-deductible health plans; capitated plan types were health maintenance organizations and capitated or partially capitated point-of-service plans.
Statistical Analysis
Annual prevalence of anogenital warts was expressed as number of cases per 1000 person-years. We used the Cochran–Armitage test31,32 to assess statistical significance of anogenital wart prevalence trends for each gender-specific 5-year age group over several time periods, including pre- and postvaccination licensure (2003–2006 and 2007–2010, respectively). We assessed effect modification and confounding (in the absence of effect modification) of anogenital wart rates over time separately within each gender and 5-year age stratum for each potential confounder or effect modifier using Poisson regression analysis33,34 with the log of the stratum-specific denominator as the model offset. We conducted all analyses using SAS/STAT software, version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
More than 64 million person-years of data were represented in this analysis (Table 1). Continuous enrollees aged 10 to 39 years increased from almost 5 million in 2003 to more than 13 million in 2010. Slightly more enrollees were female (51.4% over all 8 years). The highest numbers were in the youngest age groups; more than 38% were aged 10 to 19 years. Most enrollees resided in a MSA (83.54% overall), and more resided in the South (approximately 43%). Sixty-seven percent were in noncapitated health plans in 2003, increasing to 83% in 2010.
TABLE 1—
Demographic Characteristics of Privately Insured Continuous Enrollees Aged 10–39 Years: Truven Health Analytics MarketScan Commercial Claims and Encounters Database, United States, 2003–2010
| Characteristics | 2003 (n = 4 965 887), % | 2004 (n = 5 157 691), % | 2005 (n = 4 985 689), % | 2006 (n = 4 647 945), % | 2007 (n = 8 376 815), % | 2008 (n = 10 179 205), % | 2009 (n = 12 198 813), % | 2010 (n = 13 618 411), % | Total (n = 64 130 456), % |
| Gender | |||||||||
| Male | 48.4 | 48.5 | 48.4 | 48.6 | 48.8 | 48.6 | 48.5 | 48.7 | 48.6 |
| Female | 51.6 | 51.5 | 51.6 | 51.4 | 51.2 | 51.4 | 51.5 | 51.3 | 51.4 |
| Age, y | |||||||||
| 10–14 | 19.6 | 19.8 | 19.7 | 19.9 | 19.4 | 18.9 | 18.6 | 18.7 | 19.1 |
| 15–19 | 18.6 | 18.9 | 18.9 | 19.6 | 19.5 | 19.1 | 18.8 | 19.1 | 19.1 |
| 20–24 | 12.1 | 12.1 | 11.9 | 11.8 | 12.3 | 12.3 | 12.6 | 12.6 | 12.3 |
| 25–29 | 12.6 | 12.6 | 12.8 | 12.6 | 13.1 | 13.7 | 14.1 | 13.6 | 13.3 |
| 30–34 | 17.2 | 17.1 | 17.2 | 16.4 | 16.1 | 16.3 | 16.5 | 16.6 | 16.6 |
| 35–39 | 19.9 | 19.6 | 19.6 | 19.7 | 19.6 | 19.7 | 19.4 | 19.4 | 19.6 |
| Residence | |||||||||
| MSA | 79.8 | 78.9 | 82.9 | 82.3 | 82.5 | 84.3 | 85.2 | 85.7 | 83.5 |
| Non-MSA | 19.4 | 19.3 | 15.8 | 16.5 | 17.1 | 15.3 | 14.7 | 13.9 | 15.9 |
| Missing or unknown | 0.9 | 1.8 | 1.3 | 1.1 | 0.4 | 0.4 | 0.2 | 0.4 | 0.6 |
| Residence region | |||||||||
| Northeast | 9.0 | 8.5 | 7.5 | 9.8 | 8.4 | 9.6 | 12.9 | 14.6 | 10.9 |
| North Central | 21.4 | 22.1 | 22.5 | 26.3 | 28.6 | 25.2 | 27.0 | 24.9 | 25.3 |
| South | 38.5 | 39.1 | 40.7 | 43.1 | 46.5 | 48.2 | 44.4 | 39.2 | 42.9 |
| West | 30.2 | 28.6 | 28.1 | 19.6 | 16.1 | 16.6 | 15.4 | 20.7 | 20.3 |
| Missing or unknown | 0.9 | 1.7 | 1.2 | 1.2 | 0.5 | 0.5 | 0.3 | 0.6 | 0.7 |
| Health plan type | |||||||||
| Capitated | 32.4 | 30.5 | 30.3 | 21.9 | 15.0 | 17.5 | 16.5 | 14.6 | 19.9 |
| Noncapitated | 66.8 | 64.8 | 68.7 | 72.7 | 82.0 | 79.2 | 80.6 | 83.2 | 77.3 |
| Missing or unknown | 0.8 | 4.7 | 1.0 | 5.4 | 3.1 | 3.3 | 2.9 | 2.2 | 2.8 |
Note. MSA = metropolitan statistical area.
Anogenital Warts Case Definition
Of cases, 87% were diagnosed with condyloma acuminata; 11% had a less specific viral wart diagnosis combined with a procedure for destruction or excision of an anogenital lesion, or a diagnosis of a benign anogenital neoplasm, within 30 days. Only 2% of cases had neither a condyloma acuminata nor a viral warts diagnosis but had 1 or more prescriptions for anogenital wart medications combined with destruction or excision of an anogenital lesion, or a diagnosis of a benign anogenital neoplasm, within 30 days. These percentages did not vary by gender.
Prevalence of Anogenital Warts Over Time
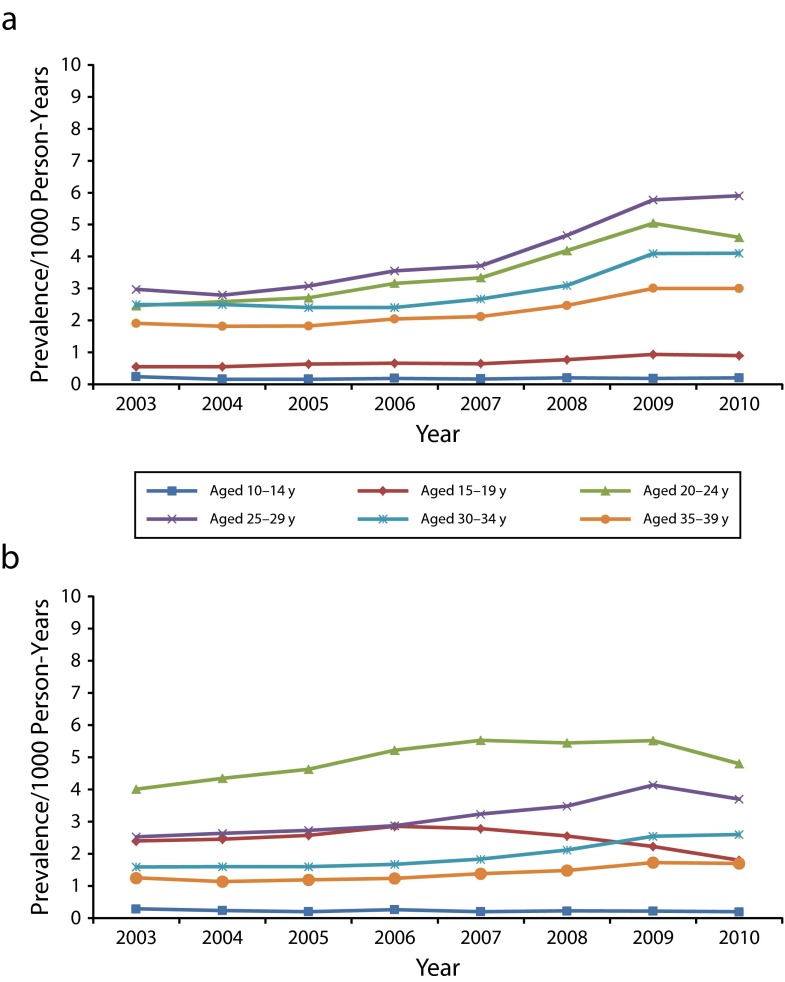
For boys aged 10 to 14 years, prevalence of anogenital warts was very low (0.2 per 1000 person-years) and did not vary over time (Figure 1). Rates were slightly higher in boys aged 15 to 19 years, ranging from 0.5 in 2003 to 0.9 in 2009 and 2010. Rates in men aged 30 to 34 and 35 to 39 years were higher, rising from 2.5 to 4.1 per 1000 person-years for those aged 30 to 34 years and from 1.4 to 2.4 for men aged 35 to 39 years from 2003 to 2009 (P < .001 for both groups). For men in both of these age groups, as for those aged 15 to 19 years, prevalence in 2010 was the same as in 2009.
FIGURE 1—
Annual anogenital wart prevalence per 1000 person-years among private insurance enrollees aged 10–39 years who were (a) male and (b) female: Truven Health Analytics MarketScan Commercial Claims and Encounters Database, United States, 2003–2010.
We observed the highest rates per 1000 person-years among men aged 20 to 24 and 25 to 29 years. In men aged 20 to 24 years, rates increased from 2.5 in 2003 to 5.0 in 2009 (P < .001) and then decreased to 4.6 in 2010 (P = .001). For men aged 25 to 29 years, rates were 3.0 per 1000 person-years in 2003, rising to 5.9 in 2010 (P < .001). Although rates in both of these age groups increased most rapidly between 2007 and 2009, rates in men aged 20 to 24 and 25 to 29 years also increased significantly between 2003 and 2006 (P < .001 for both groups).
Among girls aged 10 to 14 years, as for boys, anogenital wart prevalence was very low and stable (0.2 or 0.3 per 1000 person-years across the 8-year period; Figure 1). Rates in women in the oldest age groups, 30 to 34 and 35 to 39 years, were lower than were those observed for men in the same age groups. For women aged 30 to 34 years, rates rose from 1.6 in 2003 to 2.5 in 2009 (P < .001); for those aged 35 to 39 years, rates were 1.3 in 2003 and 1.7 in 2009 (P < .001). For both of these age groups, prevalence did not significantly increase from 2009 to 2010. We observed higher rates in women aged 25 to 29 years, rising from 2.5 in 2003 to 4.1 in 2009 (P < .001), then declining slightly to 3.7 per 1000 person-years in 2010 (P < .001). In these older age groups, rates in women increased through 2009, although these increases were not as great as those for older men.
However, rates in women aged 20 to 24 years did not show this same increase over time. Although rates in this age group increased through 2006 (P < .001), the first year of HPV vaccine licensure, rates from 2007 through 2009 were essentially unchanged, leveling off at 5.4 to 5.5 per 1000 person-years. In 2010, prevalence in women aged 20 to 24 years decreased to 4.8 (P < .001). In women aged 15 to 19 years, the group most likely to be affected by the introduction of HPV vaccine, the trend was more pronounced. Prevalence per 1000 person-years was 2.9 in 2006, decreasing to 1.8 in 2010. Although this decrease of 1.1 per 1000 is relatively small, it is statistically significant (P < .001) given the large number of observations in our study. The 2010 rate in girls aged 15 to 19 years was derived from 2283 cases, with a denominator of more than 1.2 million person-years.
Within each gender- and age-specific stratum, prevalence of anogenital warts over time was statistically significantly modified by each of the effect modifiers examined: receipt of screening Pap test or routine pelvic examination within the same year (females only), region, residence or nonresidence in an MSA, and health plan capitation or noncapitation. However, graphical examination of the gender- and age-specific rates for each factor level showed similar trends; the only exception were results by routine Pap test or pelvic examination status.
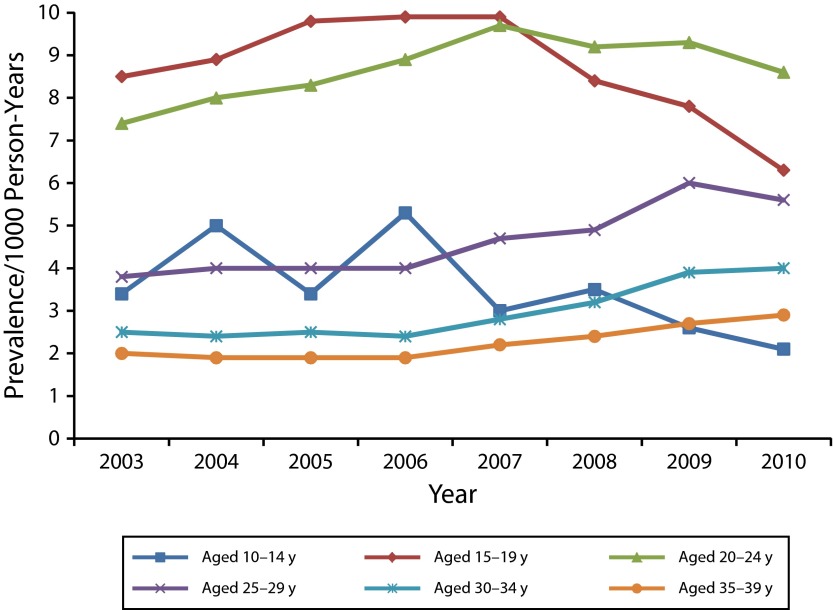
With the exception of girls aged 10 to 14 years, receipt of a routine Pap test or pelvic examination for women in each age group rose slightly in 2003 to 2006, was level in 2006 to 2009, and decreased slightly in 2009 to 2010; in those aged 10 to 14 years, the rate was essentially unchanged over time. The median percentage of females receiving a routine Pap test or pelvic examination across all years was 1% for those aged 10 to 14 years; 21% for those aged 15 to 19 years; 48% for those aged 20 to 24 years; 59% for those aged 25 to 29 years; 55% for those aged 30 to 34 years; and 51% for those aged 35 to 39 years. Among females who did not receive a routine Pap test or pelvic examination within a given year, anogenital wart prevalence was very low for all age groups (< 2.0 per 1000) and did not vary significantly by year (data not shown). By contrast, among those receiving a routine Pap test or pelvic examination, prevalence of anogenital warts by age group decreased significantly during 2007 to 2010 for girls aged 15 to 19 years and women aged 20 to 24 years (Figure 2). In these 2 age groups, anogenital wart prevalence was much higher across all years among those who had a routine Pap test or pelvic examination within the same year, compared with all females in these age groups. Anogenital wart prevalence, although less stable in girls aged 10 to 14 years, also decreased in this age group during 2007 to 2010.
FIGURE 2—
Annual anogenital wart prevalence per 1000 person-years among female private insurance enrollees aged 10–39 years who received a screening Papanicolaou test: Truven Health Analytics MarketScan Commercial Claims and Encounters Database, United States, 2003–2010.
Within each gender, the trends and rates for each age group were comparable for capitated and noncapitated health plans. Although the gender- and age-specific trends were also similar for those residing in MSAs and non-MSAs, rates were generally higher in men aged 20 to 39 years who resided in MSAs. Gender- and age-specific rates also varied somewhat across census regions, but trends appeared fairly similar across regions (Figure A; available as a supplement to the online version of this article at http://www.ajph.org); the decline in rates among girls aged 15 to 19 years was consistent across regions, whereas rates in other age groups generally increased. We observed the highest prevalence rates for men aged 25 to 29 years in the Northeast, ranging from 4.8 in 2005 to 9.2 per 1000 person-years in 2010. Among women, prevalence spiked slightly among those aged 20 to 24 years in the Northeast in 2007 (7.8 per 1000 person-years), then decreased to 5.7 in 2010.
DISCUSSION
Prevalence of anogenital warts decreased significantly among females in the age groups most likely to have been affected by the introduction of the quadrivalent HPV vaccine in mid-2006. For those aged 15 to 19 years, the decline in anogenital warts began in 2007 and continued through 2010. Among women aged 20 to 24 years, anogenital wart prevalence, which had been increasing from 2003 through 2007, was stable from 2007 to 2009 and then decreased in 2010. Although prevalence in women aged 25 to 29 years increased through 2009, we also observed a decrease for this group in 2010. These declines are what we would expect to see several years after initiating routine HPV vaccination for girls aged 11 to 12 years, with catch-up vaccination through age 26 years.
Although anogenital wart prevalence in women aged 30 to 34 and 35 to 39 years did not increase between 2009 and 2010, more years of data are needed to interpret these observations. Women aged 30 to 39 years in 2010 would have been 26 to 35 years old in 2006, the year the quadrivalent HPV vaccine was first available in the United States. Catch-up vaccination has only been recommended through age 26 years, making most of these women ineligible for vaccination; however, some of these women were likely vaccinated despite their age. Overall, between 0.8% and 2% of all US women older than 26 years are estimated to have initiated HPV vaccination35–37; although HPV vaccine may possibly be more readily available to privately insured, older women than those with public or no insurance, no estimates of HPV vaccine initiation by insurance status for women aged 26 years or older are currently available. However, several studies have shown that among women aged 18 or 19 through 26 years, initiation was 3- to 10-fold higher among those with (predominately) private insurance compared with those with no insurance.35,36
That we observed a decline in anogenital wart prevalence in 2010 for men aged 20 to 24 years is intriguing. Men in this age group are possibly the most likely sexual partners of women aged 15 to 19 years,38–40 and if so, these men would be the first to be affected by decreased prevalence among their cohort of sexual partners. These declines are consistent with herd immunity against HPV types 6 and 11, which has been demonstrated among young women attending primary care clinics in Cincinnati, Ohio, and which we presume extended to the women’s male sexual partners,41 although additional years of data are needed to confirm whether rates among men aged 20 to 24 years are on the decline. Prevalence of anogenital warts among men in all other age groups increased through 2009, then stabilized from 2009 to 2010; again, more years of data are needed to discern whether anogenital wart rates have stabilized in these groups or are in fact declining.
In 2007, Australia implemented a national HPV vaccination program for girls and young women, achieving 65% to 80% coverage with 1 or more doses. Several studies have subsequently observed reductions of 73% to 90% in genital wart presentations among age-eligible resident women.42,43 Reductions in genital warts among heterosexual men have also been found.43,44 In Sweden, decreases in incidence of anogenital warts were observed during 2006 to 2010 only for women aged 15 to 19 and 20 to 24 years, whereas rates for women in other age groups, and men in all age groups, were relatively stable.45 Bauer et al.46 examined data from the California Family Planning Access Care and Treatment program, which serves low-income clients. Between 2007 and 2010, prevalence among women younger than 21 years decreased by almost 35% (9.4 per 1000 in 2007 to 6.1 in 2010); smaller reductions were observed among women aged 21 to 25 years and men younger than 21 and aged 21 to 25 years. The smaller declines observed by these investigators, and in our study relative to those reported from Australia, are consistent with lower HPV vaccination coverage in the United States.
In the past decade, several studies have estimated genital wart prevalence in various populations, using different methodologies and case definitions. Our prevaccination era findings are reasonably similar to those previously reported, after these differences are taken into consideration. Genital wart prevalence estimates using the MarketScan Commercial Claims and Encounters Database year 2000 data47 were slightly higher than those reported here; however, the case definition used in that study included imputed cases with nonspecific viral wart diagnoses, but without any genital-specific procedure. An analysis of health claims data from the Integrated Health Care Information Services National Benchmark Database during 1998 to 2001 found the highest rates among men and women aged 20 to 29 years (approximately 2.7 and 3.2 per 1000 person-years, respectively).48 An analysis of claims from 5 geographically dispersed Blue Cross/Blue Shield health plans, gender- and age-adjusted to the mid-2004 US civilian population, also found the highest rates among men and women aged 20 to 29 years (for men, 2.4 per 1000 for ages 20–24 years and 2.7 for ages 25–29 years; for women, 4.6 for ages 20–24 years and 2.7 for ages 25–29 years)49; these rates are almost identical to the 2004 rates reported here.
The increases in anogenital wart prevalence found in this study in the years before introduction of HPV vaccine have been observed in other data sources. Between 1998 and 2001, anogenital wart incidence per 1000 increased from 1.2 to 2.1 in the Integrated Health Care Information Services National Benchmark Database.48 Data from the National Disease and Therapeutic Index also suggested that initial anogenital wart presentations to physicians’ offices increased during 1998 to 2005.50 It is not possible to discern from these data whether anogenital wart incidence in the United States is truly increasing or whether these observations are the result of greater clinician or patient awareness of anogenital warts.
This study has a number of limitations. Most important, it is a purely ecological analysis and as such we cannot definitely conclude that HPV vaccination is the cause of the changes in anogenital wart prevalence observed in young women. HPV vaccination status is incomplete for the MarketScan study population used in this analysis because it can be determined with reasonable certainty only for female patients continuously enrolled in participating health plans since late 2006, when the HPV vaccine was first licensed for use in the United States; quadrivalent HPV vaccination for males was not recommended until 2011. We instead focused on the prevalence of anogenital warts in the overall population of enrollees in private health plans participating in MarketScan because our interest is in the impact of HPV vaccination at the population level. Clinical trials have already demonstrated the prophylactic efficacy of quadrivalent HPV vaccine,15,16 and as has been noted in the scientific literature, questions of interest about currently available HPV vaccines now center on population effectiveness and cost-effectiveness.17
Unmeasured confounding may possibly be responsible for the observed declines in anogenital warts, such as changes in plans participating in MarketScan resulting in lower risk enrollees, or less detection or treatment of anogenital warts. Although the number of MarketScan enrollees increased substantially after 2006, their distribution by gender and age was remarkably stable over the 8-year study period; the increase in enrollment seems unlikely to be associated with HPV vaccine licensure but is simply the result of increased participation in MarketScan by employer health plans. Unmeasured confounding also seems unlikely to differentially affect female enrollees in the younger age groups across the pre- and post-HPV vaccine licensure time periods, but we cannot rule out this possibility. Although distinguishing incident from recurrent infections was not possible in this analysis, each person meeting our case definition was only counted once per calendar year, and it is unlikely that recurrences would differ by age group and time period in ways that would result in the observed declines in prevalence.
HPV vaccination coverage was generally higher in the Northeast and lower in the South.9,51–54 Although state-specific data on HPV vaccine coverage are not available for 2007,55 in 2008 the median rate of HPV vaccine uptake among adolescents aged 13 to 17 years for states in the Northeast was 50.2%, compared with 30.9% for North Central states, 33.6% for Southern states, and 36.2% for Western states.51 These differences in coverage were somewhat attenuated by 2010 (median 2010 rates were 54.6% for the Northeast, 46.1% for North Central, 43.5% for the South, and 52.5% for the West),9 and this pattern may account for the somewhat delayed decrease in anogenital wart prevalence observed for Southern girls aged 15 to 19 years. Higher vaccine uptake in the Northeast in the years immediately after vaccine introduction may be correlated with increased patient or provider awareness of genital warts, resulting in more presentations or detection. The differential participation in MarketScan across regions precludes the ability to infer that the results from this study apply to a broader, more well-defined population such as all privately insured people aged 10 to 39 years in the United States. In addition, 20% of enrollees over the entire 8-year period participated in capitated health plans, to which claims for all genital wart diagnoses or treatments may not be submitted; the result may have been a slight underestimation of anogenital wart prevalence in this study, although capitated plan enrollment decreased over the course of the study period.
This study has several strengths. Data were available for large numbers of people, ranging from 4.6 to 13.6 million 10- to 39-year-old enrollees per year. Because 87% of cases had a diagnosis of condyloma acuminata, the case definition used for this study is likely to be reasonably specific. Because administrative claims data are used for billing purposes, diagnosis codes derived from claims may include conditions that are considered but ultimately ruled out; however, the potential for prevalence overestimation resulting from this factor is unlikely for such a straightforward diagnosis as anogenital warts.
We were able to address the possibility that declines in anogenital wart prevalence among young women may be the result of decreased routine Pap test or pelvic examination. Among women continuously enrolled in a health plan participating in MarketScan, Pap tests and pelvic examinations did not significantly decline between 2003 and 2010. Most anogenital wart diagnoses did occur in women who had a routine Pap test or pelvic examination within the same calendar year; however, restricting the analyses to only these women did not change the observed trends in anogenital wart prevalence.
Our observations indicate that reductions in prevalence of anogenital warts among young women may be occurring in the United States, despite fairly low rates of HPV vaccine uptake. Although the introduction of bivalent HPV vaccine for females in 2009 may complicate these efforts, continued surveillance of anogenital wart prevalence using available health claims databases will be a cost-effective method of monitoring the population impact of HPV vaccination.
Human Participant Protection
Institutional review board approval was not needed because only secondary, deidentified data were used for this analysis.
References
- 1.Garland SM, Steben M, Sings HL et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 2.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiley DJ, Douglas J, Beutner K et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis. 2002;35(suppl 2):S210–S224. doi: 10.1086/342109. [DOI] [PubMed] [Google Scholar]
- 4.Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnurch HG, zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):626–629. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–1708. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):630–632. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13–17 years—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(34):671–677. [PubMed] [Google Scholar]
- 10.Brotherton JML, Kaldor JM, Garland SM. Monitoring the control of human papillomavirus (HPV) infection and related diseases in Australia: towards a national HPV surveillance strategy. Sex Health. 2010;7(3):310–319. doi: 10.1071/SH09137. [DOI] [PubMed] [Google Scholar]
- 11.Fairley CK, Donovan B. What can surveillance of genital warts tell us? Sex Health. 2010;7(3):325–327. doi: 10.1071/SH09145. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz LE, Hariri S, Unger ER, Saraiya M, Datta SD, Dunne EF. Post-licensure monitoring of HPV vaccine in the United States. Vaccine. 2010;28(30):4731–4737. doi: 10.1016/j.vaccine.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Schuchat A, Bell BP. Monitoring the impact of vaccines postlicensure: new challenges, new opportunities. Expert Rev Vaccines. 2008;7(4):437–456. doi: 10.1586/14760584.7.4.437. [DOI] [PubMed] [Google Scholar]
- 14.Wong CA, Saraiya M, Hariri S et al. Approaches to monitoring biological outcomes for HPV vaccination: challenges of early adopter countries. Vaccine. 2011;29(5):878–885. doi: 10.1016/j.vaccine.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Dillner J, Kjaer SK, Wheeler CM et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;341 doi: 10.1136/bmj.c3493. c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garland SM, Hernandez-Avila M, Wheeler CM et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 17.Castle PE, Zhao F-H. Population effectiveness, not efficacy, should decide who gets vaccinated against human papillomavirus via publicly funded programs. J Infect Dis. 2011;204(3):335–337. doi: 10.1093/infdis/jir287. [DOI] [PubMed] [Google Scholar]
- 18.Egelkrout EM, Galloway DA. The biology of genital human papillomaviruses. In: Holmes KK, Sparling PF, Stamm WE, editors. Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw Hill Medical; 2008. pp. 463–487. [Google Scholar]
- 19.Winer RL, Kiviat NB, Hughes JP et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191(5):731–738. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 20.Moscicki AB, Hills N, Shiboski S et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285(23):2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 21.Woodman CBJ, Collins S, Winter H et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357(9271):1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 22.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32(suppl 1):S16–S24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 23.International Classification of Disease, 9th Revision, Clinical Modification. Hyattsville, MD: National Center for Health Statistics; 2011. DHHS Publication PHS 11-1260. [Google Scholar]
- 24.Workowski KA, Berman S, Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2010 [published correction appears in MMWR Recomm Rep. 2011;60(1):18. (Note: Dosage error in article text)] MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 25.McEvoy GK, editor. Sinecatechins (topical) AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc.; 2012. STAT!Ref Online Electronic Medical Library. Available at: http://online.statref.com/Document.aspx?docAddress=pDsr3ldZEAdtFTdz9Db3Dw%3d%3d&SessionId=1A73E85IJTYCPFIL. Accessed May 10, 2012. [Google Scholar]
- 26.McEvoy GK, editor. Imiquimod (topical) AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc.; 2012. STAT!Ref Online Electronic Medical Library. Available at: http://online.statref.com/Document.aspx?docAddress=pDsr3ldZEAdtFTdz9Db3Dw%3d%3d&SessionId=1A73E85IJTYCPFIL. Accessed May 10, 2012. [Google Scholar]
- 27.McEvoy GK, editor. Podofilox (topical) AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc.; 2012. STAT!Ref Online Electronic Medical Library. Available at: http://online.statref.com/Document.aspx?docAddress=pDsr3ldZEAdtFTdz9Db3Dw%3d%3d&SessionId=1A73E85IJTYCPFIL. Accessed May 10, 2012. [Google Scholar]
- 28.McEvoy GK, editor. Podophyllum resin (topical) AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc.; 2012. STAT!Ref Online Electronic Medical Library. Available at: http://online.statref.com/Document.aspx?docAddress=pDsr3ldZEAdtFTdz9Db3Dw%3d%3d&SessionId=1A73E85IJTYCPFIL. Accessed May 10, 2012. [Google Scholar]
- 29.Ingenix® CPT with RVUs Data File. Eden Prairie, MN: Ingenix®/St. Anthony Publishing/Medicode; 2010. STAT!Ref Online Electronic Medical Library. Available at: http://online.statref.com/Document.aspx?docAddress=OWcsDkcRlp3abrwTKPObeQ%3d%3d&SessionId=1A73E85IJTYCPFIL. Accessed September 27, 2010. [Google Scholar]
- 30.Healthcare Common Procedure Coding System (HCPCS) Baltimore, MD: Centers for Medicare and Medicaid Services; 2010. [Google Scholar]
- 31.SAS Institute, Inc. Base SAS 9.3 Procedures Guide. Cary, NC: SAS Institute, Inc.; 2011. The FREQ procedure; pp. 72–238. [Google Scholar]
- 32.Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11(3):375–386. [Google Scholar]
- 33.SAS Institute Inc. SAS/STAT 9.3 User’s Guide. Cary, NC: SAS Institute, Inc.; 2011. The GENMOD procedure; p. 5. [Google Scholar]
- 34.Berk R, MacDonald J. Overdispersion and Poisson regression. J Quant Criminol. 2008;24(3):269–284. [Google Scholar]
- 35.Taylor LD, Hariri S, Sternberg M, Dunne EF, Markowitz LE. Human papillomavirus vaccine coverage in the United States, National Health and Nutrition Examination Survey, 2007-2008. Prev Med. 2011;52(5):398–400. doi: 10.1016/j.ypmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Jain N, Euler GL, Shefer A, Lu P, Yankey D, Markowitz L. Human papillomavirus (HPV) awareness and vaccination initiation among women in the United States, National Immunization Survey—Adult 2007. Prev Med. 2009;48(5):426–431. doi: 10.1016/j.ypmed.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Schiller JS, Euler GL. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Health Statistics; 2009. Vaccination coverage estimates from the National Health Interview Survey: United States, 2008. [Google Scholar]
- 38.Ford K, Sohn W, Lepkowsk J. American adolescents: sexual mixing patterns, bridge partners, and concurrency. Sex Transm Dis. 2002;29(1):13–19. doi: 10.1097/00007435-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Stein CR, Kaufman JS, Ford CA, Feldblum PJ, Leone PA, Miller WC. Partner age difference and prevalence of chlamydial infection among young adult women. Sex Transm Dis. 2008;35(5):447–452. doi: 10.1097/OLQ.0b013e3181659236. [DOI] [PubMed] [Google Scholar]
- 40.Aral SO, Hughes JP, Stoner B et al. Sexual mixing patterns in the spread of gonococcal and chlamydial infections. Am J Public Health. 1999;89(6):825–833. doi: 10.2105/ajph.89.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahn JA, Brown DR, Ding L et al. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;130(2):e249–e256. doi: 10.1542/peds.2011-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donovan B, Guy R, Ali H et al. O5-S2.01 a national program with a national impact: quadrivalent HPV vaccination and genital warts in Australia, 2004-2010. Sex Transm Infect. 2011;87(suppl 1) A91. [Google Scholar]
- 43.Read TRH, Hocking JS, Chen MY, Donovan B, Bradshaw CS, Fairley CK. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect. 2011;87(7):544–547. doi: 10.1136/sextrans-2011-050234. [DOI] [PubMed] [Google Scholar]
- 44.Fairley CK, Hocking JS, Gurrin LC, Chen MY, Donovan B, Bradshaw CS. Rapid decline in presentations of genital warts after the implementation of a national quadrivalent human papillomavirus vaccination programme for young women. Sex Transm Infect. 2009;85(7):499–502. doi: 10.1136/sti.2009.037788. [DOI] [PubMed] [Google Scholar]
- 45.Leval A, Herweijer E, Arnheim-Dahlstrom L et al. Incidence of genital warts in Sweden before and after quadrivalent human papillomavirus vaccine availability. J Infect Dis. 2012;206(6):860–866. doi: 10.1093/infdis/jis405. [DOI] [PubMed] [Google Scholar]
- 46.Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data. Am J Public Health. 2012;102(5):833–835. doi: 10.2105/AJPH.2011.300465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis. 2003;36(11):1397–1403. doi: 10.1086/375074. [DOI] [PubMed] [Google Scholar]
- 48.Koshiol JE, Laurent SA, Pimenta JM. Rates and predictors of new genital warts claims and genital warts-related healthcare utilization among privately insured patients in the United States. Sex Transm Dis. 2004;31(12):748–752. doi: 10.1097/01.olq.0000145851.76025.ad. [DOI] [PubMed] [Google Scholar]
- 49.Hoy T, Singhal PK, Willey VJ, Insinga RP. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin. 2009;25(10):2343–2351. doi: 10.1185/03007990903136378. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2010. Atlanta, GA: Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of STD Prevention; 2011. [Google Scholar]
- 51.Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(36):997–1001. [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(32):1018–1023. [PubMed] [Google Scholar]
- 53.Bach PB. Gardasil: from bench, to bedside, to blunder. Lancet. 2010;375(9719):963–964. doi: 10.1016/S0140-6736(09)62029-8. [DOI] [PubMed] [Google Scholar]
- 54.Dickerson JB, Smith ML, Ory MG. Increasing uptake of Gardasil among American adolescents. Comparisons with the history of hepatitis B vaccination. Hum Vaccin. 2011;7(2):211–219. doi: 10.4161/hv.7.2.13633. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. Vaccination coverage among adolescents aged 13-17 years—United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(40):1100–1103. [PubMed] [Google Scholar]