Abstract
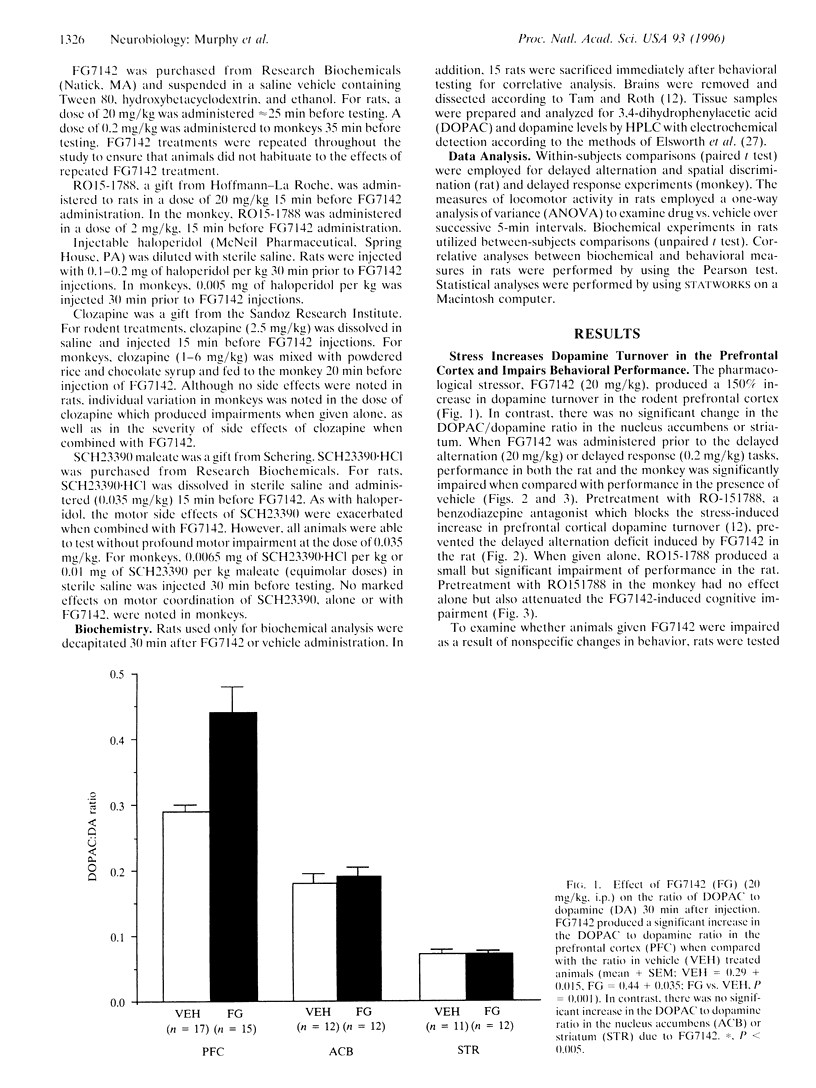
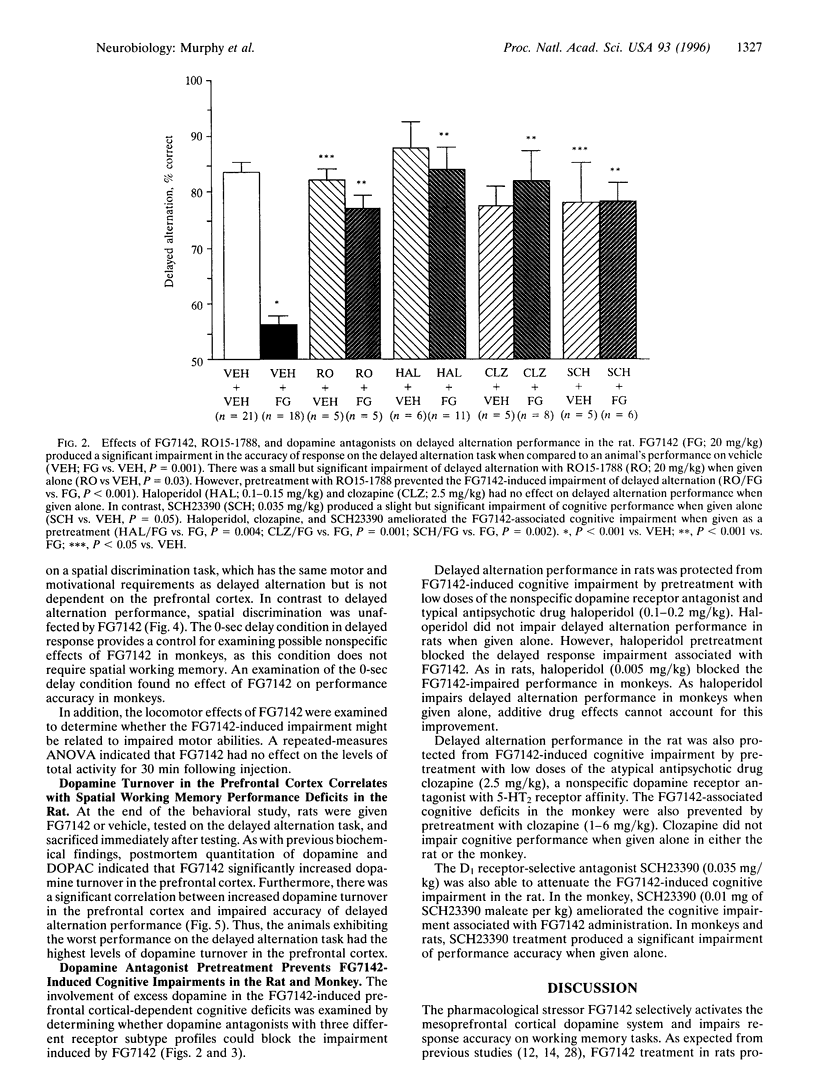
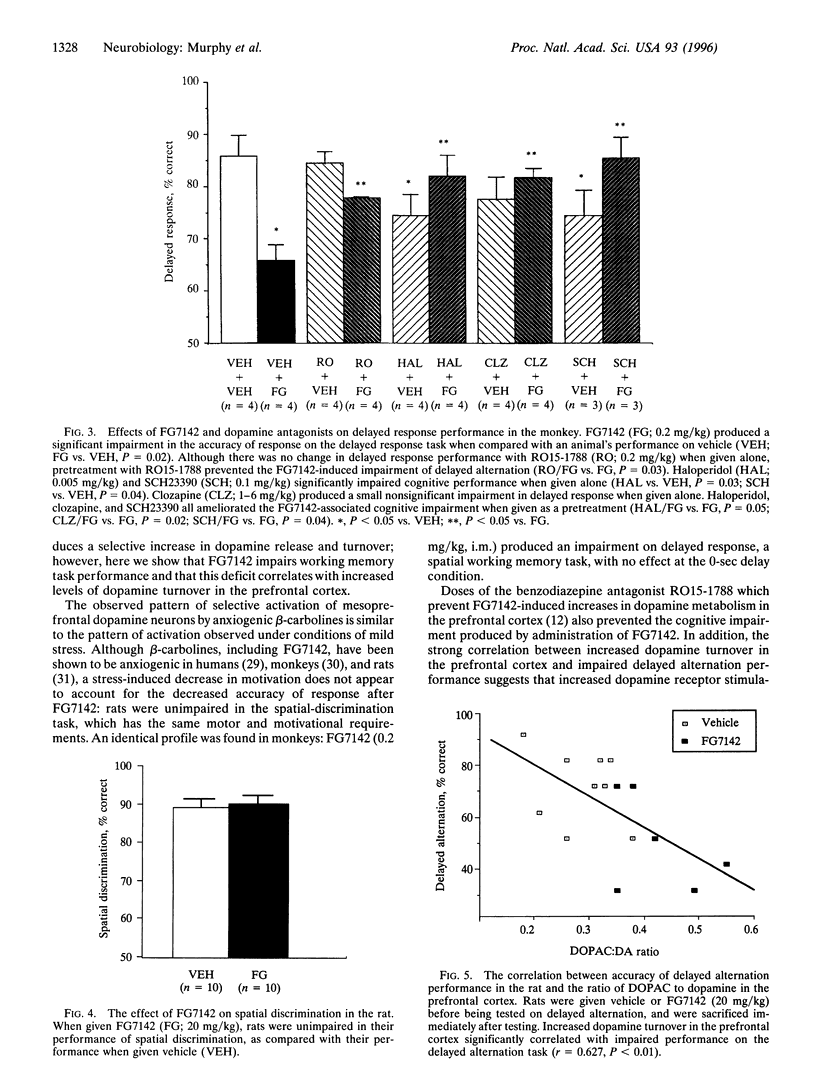
The selective activation of the prefrontal cortical dopamine system by mild stress can be mimicked by anxiogenic beta-carbolines such as FG7142. To investigate the functional relevance of elevated levels of dopamine turnover in the prefrontal cortex, the current study examined the effects of FG7142 on the performance of spatial working memory tasks in the rat and monkey. FG7142 selectively increased prefrontal cortical dopamine turnover in rats and significantly impaired performance on spatial working memory tasks in both rats and monkeys. Spatial discrimination, a task with similar motor and motivational demands (rats), or delayed response performance following zero-second delays (monkeys) was unaffected by FG7142. Further, biochemical analysis in rats revealed a significant positive correlation between dopamine turnover in the prefrontal cortex and cognitive impairment on the delayed alternation task. The cognitive deficits in both rats and monkeys were prevented by pretreatment with the benzodiazepine receptor antagonist, RO15-1788, which blocked the increase in dopamine turnover and by the dopamine receptor antagonists, haloperidol, clozapine, and SCH23390. These findings indicate that excessive dopamine activity in the prefrontal cortex is detrimental to cognitive functions mediated by the prefrontal cortex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnsten A. F., Cai J. X., Murphy B. L., Goldman-Rakic P. S. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994 Oct;116(2):143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Bebbington P., Wilkins S., Jones P., Foerster A., Murray R., Toone B., Lewis S. Life events and psychosis. Initial results from the Camberwell Collaborative Psychosis Study. Br J Psychiatry. 1993 Jan;162:72–79. doi: 10.1192/bjp.162.1.72. [DOI] [PubMed] [Google Scholar]
- Berman K. F., Weinberger D. R. The prefrontal cortex in schizophrenia and other neuropsychiatric diseases: in vivo physiological correlates of cognitive deficits. Prog Brain Res. 1990;85:521–537. doi: 10.1016/s0079-6123(08)62698-9. [DOI] [PubMed] [Google Scholar]
- Bradberry C. W., Lory J. D., Roth R. H. The anxiogenic beta-carboline FG 7142 selectively increases dopamine release in rat prefrontal cortex as measured by microdialysis. J Neurochem. 1991 Mar;56(3):748–752. doi: 10.1111/j.1471-4159.1991.tb01987.x. [DOI] [PubMed] [Google Scholar]
- Brozoski T. J., Brown R. M., Rosvold H. E., Goldman P. S. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979 Aug 31;205(4409):929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Bubser M., Schmidt W. J. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav Brain Res. 1990 Mar 5;37(2):157–168. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- Davis K. L., Kahn R. S., Ko G., Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991 Nov;148(11):1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Deutch A. Y. Prefrontal cortical dopamine systems and the elaboration of functional corticostriatal circuits: implications for schizophrenia and Parkinson's disease. J Neural Transm Gen Sect. 1993;91(2-3):197–221. doi: 10.1007/BF01245232. [DOI] [PubMed] [Google Scholar]
- Deutch A. Y., Roth R. H. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- Dorow R., Horowski R., Paschelke G., Amin M. Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet. 1983 Jul 9;2(8341):98–99. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Elsworth J. D., Deutch A. Y., Redmond D. E., Jr, Taylor J. R., Sladek J. R., Jr, Roth R. H. Symptomatic and asymptomatic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates: biochemical changes in striatal regions. Neuroscience. 1989;33(2):323–331. doi: 10.1016/0306-4522(89)90212-1. [DOI] [PubMed] [Google Scholar]
- Gedye A. Tourette syndrome attributed to frontal lobe dysfunction: numerous etiologies involved. J Clin Psychol. 1991 Mar;47(2):233–252. doi: 10.1002/1097-4679(199103)47:2<233::aid-jclp2270470209>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Goldstein M., Deutch A. Y. Dopaminergic mechanisms in the pathogenesis of schizophrenia. FASEB J. 1992 Apr;6(7):2413–2421. [PubMed] [Google Scholar]
- Gotham A. M., Brown R. G., Marsden C. D. 'Frontal' cognitive function in patients with Parkinson's disease 'on' and 'off' levodopa. Brain. 1988 Apr;111(Pt 2):299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Jonides J., Smith E. E., Koeppe R. A., Awh E., Minoshima S., Mintun M. A. Spatial working memory in humans as revealed by PET. Nature. 1993 Jun 17;363(6430):623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984 Nov;320(1):65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- McCarthy G., Blamire A. M., Puce A., Nobre A. C., Bloch G., Hyder F., Goldman-Rakic P., Shulman R. G. Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane H., Shimizu N., Hori T. Stress-induced norepinephrine release in the rat prefrontal cortex measured by microdialysis. Am J Physiol. 1994 Dec;267(6 Pt 2):R1559–R1566. doi: 10.1152/ajpregu.1994.267.6.R1559. [DOI] [PubMed] [Google Scholar]
- Ninan P. T., Insel T. M., Cohen R. M., Cook J. M., Skolnick P., Paul S. M. Benzodiazepine receptor-mediated experimental "anxiety" in primates. Science. 1982 Dec 24;218(4579):1332–1334. doi: 10.1126/science.6293059. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Clinical and experimental approaches to varieties of memory. Int J Neurosci. 1991 Jun;58(3-4):135–150. doi: 10.3109/00207459108985429. [DOI] [PubMed] [Google Scholar]
- Owen A. M., James M., Leigh P. N., Summers B. A., Marsden C. D., Quinn N. P., Lange K. W., Robbins T. W. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992 Dec;115(Pt 6):1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Pellow S., File S. E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986 Mar;24(3):525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Robbins T. W. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16(3):391–402. doi: 10.1093/schbul/16.3.391. [DOI] [PubMed] [Google Scholar]
- Roth R. H., Tam S. Y., Ida Y., Yang J. X., Deutch A. Y. Stress and the mesocorticolimbic dopamine systems. Ann N Y Acad Sci. 1988;537:138–147. doi: 10.1111/j.1749-6632.1988.tb42102.x. [DOI] [PubMed] [Google Scholar]
- Růzicka E., Roth J., Spacková N., Mecír P., Jech R. Apomorphine induced cognitive changes in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1994 Aug;57(8):998–1001. doi: 10.1136/jnnp.57.8.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shue K. L., Douglas V. I. Attention deficit hyperactivity disorder and the frontal lobe syndrome. Brain Cogn. 1992 Sep;20(1):104–124. doi: 10.1016/0278-2626(92)90064-s. [DOI] [PubMed] [Google Scholar]
- Simon H. Neurones dopaminergiques A10 et système frontal. J Physiol (Paris) 1981 Apr;77(1):81–95. [PubMed] [Google Scholar]
- Stam C. J., Visser S. L., Op de Coul A. A., De Sonneville L. M., Schellens R. L., Brunia C. H., de Smet J. S., Gielen G. Disturbed frontal regulation of attention in Parkinson's disease. Brain. 1993 Oct;116(Pt 5):1139–1158. doi: 10.1093/brain/116.5.1139. [DOI] [PubMed] [Google Scholar]
- Stam C. J., de Bruin J. P., van Haelst A. M., van der Gugten J., Kalsbeek A. Influence of the mesocortical dopaminergic system on activity, food hoarding, social-agonistic behavior, and spatial delayed alternation in male rats. Behav Neurosci. 1989 Feb;103(1):24–35. doi: 10.1037//0735-7044.103.1.24. [DOI] [PubMed] [Google Scholar]
- Tam S. Y., Roth R. H. Modulation of mesoprefrontal dopamine neurons by central benzodiazepine receptors. I. Pharmacological characterization. J Pharmacol Exp Ther. 1990 Mar;252(3):989–996. [PubMed] [Google Scholar]
- Tam S. Y., Roth R. H. Selective increase in dopamine metabolism in the prefrontal cortex by the anxiogenic beta-carboline FG 7142. Biochem Pharmacol. 1985 May 1;34(9):1595–1598. doi: 10.1016/0006-2952(85)90708-7. [DOI] [PubMed] [Google Scholar]
- Thierry A. M., Tassin J. P., Blanc G., Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976 Sep 16;263(5574):242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Weinberger D. R., Berman K. F., Illowsky B. P. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. III. A new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry. 1988 Jul;45(7):609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- Williams G. V., Goldman-Rakic P. S. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995 Aug 17;376(6541):572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Wörtwein G., Mogensen J., Divac I. Retention and relearning of spatial delayed alternation in rats after combined or sequential lesions of the prefrontal and parietal cortex. Acta Neurobiol Exp (Wars) 1993;53(2):357–366. [PubMed] [Google Scholar]
- de Brabander J. M., de Bruin J. P., van Eden C. G. Comparison of the effects of neonatal and adult medial prefrontal cortex lesions on food hoarding and spatial delayed alternation. Behav Brain Res. 1991 Jan 31;42(1):67–75. doi: 10.1016/s0166-4328(05)80041-5. [DOI] [PubMed] [Google Scholar]



