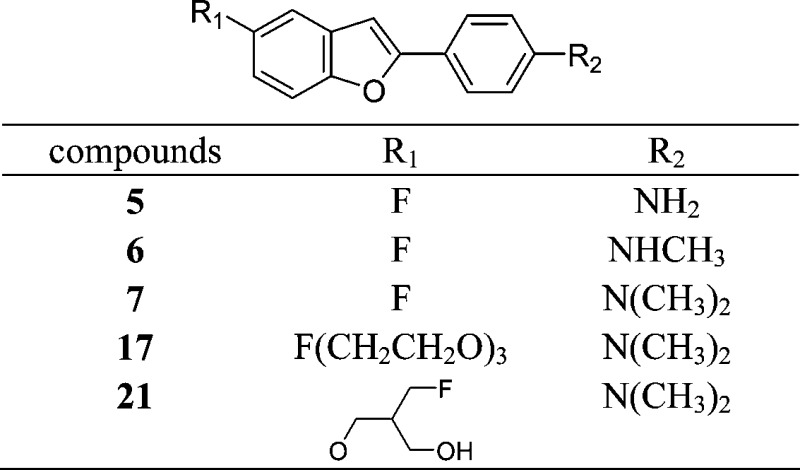
Abstract
A series of fluorinated benzofuran derivatives as potential tracers for positron emission tomography (PET) targeting β-amyloid plaques in the brains of patients with Alzheimer's disease (AD) were synthesized and evaluated. The derivatives were produced using an intramolecular Wittig reaction. In experiments in vitro, all displayed high affinity for Aβ(1−42) aggregates with Ki values in the nanomolar range. Radiofluorinated 17, [18F]17, in particular labeled β-amyloid plaques in sections of Tg2576 mouse brain and displayed high uptake (5.66% ID/g) at 10 min postinjection, sufficient for PET imaging. In addition, in vivo β-amyloid plaque labeling can be clearly demonstrated with [18F]17 in Tg2576 mice. In conclusion, [18F]17 may be useful for detecting β-amyloid plaques in patients with AD.
Keywords: Alzheimer's disease, fluorine-18, benzofuran, positron emission tomography (PET)
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by dementia, cognitive impairment, and memory loss. Autopsied brains of AD patients show neuropathological features such as the presence of senile plaques and neurofibrillary tangles, which contain β-amyloid peptides (Aβ) and highly phosphorylated τ proteins. Aβ aggregates in the brain are a hallmark of AD.1,2 The quantitative evaluation of Aβ aggregates in the brain with noninvasive techniques such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) would allow a presymptomatic diagnosis and the monitoring of putative effects of neuroprotective treatments. Thus, great efforts have been made to develop radiotracers that bind to β-amyloid plaques in vivo.3−5
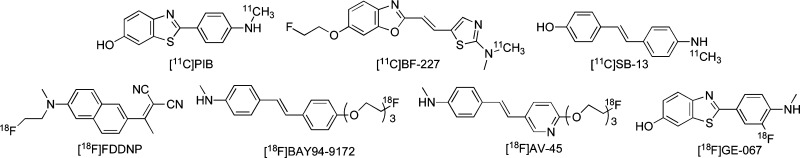
Recent success in developing radiolabeled agents targeting Aβ aggregates has provided a window of opportunity to improve the diagnosis of AD. Preliminary reports of PET imaging suggested that [11C]4-N-methylamino-4′-hydroxystilbene (SB-13),6 [11C]2-(4′-(methylaminophenyl)-6-hydroxybenzothiazole (PIB),7,8 and [11C]2-(2-[2-dimethylaminothiazol-5-yl]ethenyl)-6-(2-[fluoro]ethoxy)benzoxazole (BF-227)9 showed differential uptake and retention in the brain of AD patients as compared to controls. However, 11C is a positron-emitting isotope with a short t1/2 (20 min), which limits its clinical application. Recent efforts have focused on the development of comparable agents labeled with a longer half-life isotope, 18F (t1/2, 110 min). Preliminary studies with [18F]-2-(1-(2-(N-(2-fluoroethyl)-N-methylamino)naphthalene-6-yl)ethylidene)malononitrile ([18F]FDDNP)10,11 showed differential uptake and retention in the brain of AD patients for the first time. More recently, a stilbene derivative, [18F]BAY94-9172,12,13 and a styrylpyridine derivative, [18F]AV-45,14,15 and a fluorinated PIB analogue, [18F]GE-067,16 have proven useful in the imaging of β-amyloid plaques in living human brain tissue in clinical trials (Figure 1).
Figure 1.
Chemical structures of PET imaging agents targeting β-amyloid plaques in AD patients.
To search for more candidates for 18F-labeled tracers for PET, we planned to evaluate a new series of benzofuran derivatives previously reported as useful radioiodinated or 11C-labeled probes for imaging β-amyloid plaques.17,18 The derivatives showed good affinity for Aβ aggregates in vitro in binding experiments using synthetic Aβ aggregates and neuropathological staining of AD brain sections. We report here the in vitro and in vivo evaluation of a series of fluorinated benzofuran derivatives as probes for imaging β-amyloid plaques by PET.
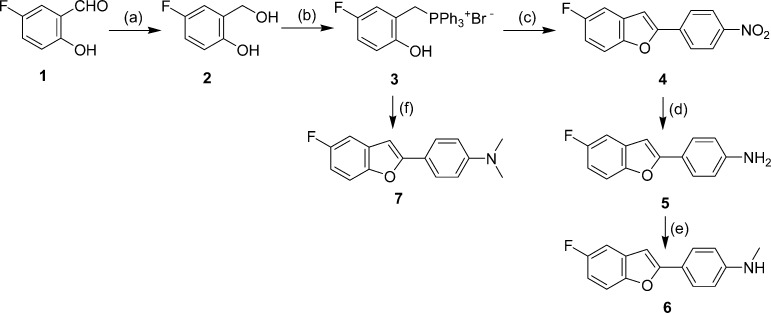
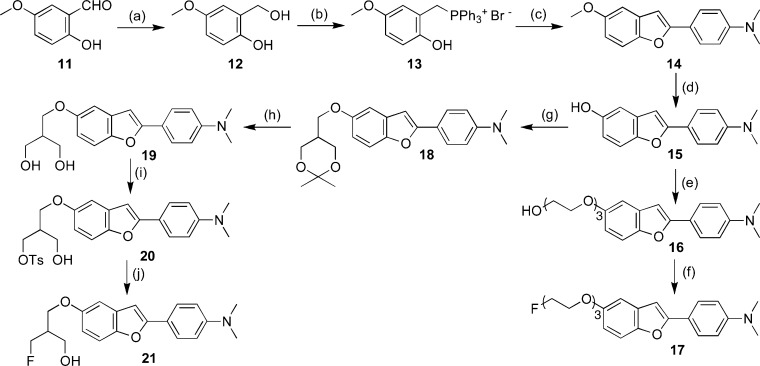
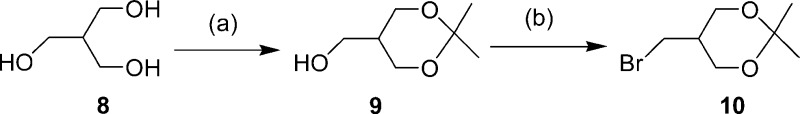
The synthesis of the fluorinated benzofuran derivatives is outlined in Schemes 1−3. The key step in the formation of the benzofuran backbone is accomplished by an intramolecular Wittig reaction between triphenyl phosphonium salt and 4-nitrobenzoyl chloride or 4-dimethylaminobenzoyl chloride.17 The desired Wittig reagent, 3, was readily prepared from 5-fluoro-2-hydroxybenzyl alcohol and triphenylphosphine hydrobromide (yield 71%). Another Wittig reagent, 13, was readily prepared from 2-hydroxy-5-methoxybenzyl alcohol and triphenylphosphine hydrobromide (yield 84%). Wittig reactions afforded the desired benzofurans (4, 7, and 14) in yields of 31, 55, and 27%, respectively. To prepare the amine compound 5, the nitro group was reduced with SnCl2 in ethanol (yield 93%). Conversion of 5 to the corresponding monomethylamino derivative 6 was achieved by monomethylation with paraformaldehyde and NaOMe (yield 30%). The synthesis outlined in Schemes 2 and 3 was achieved using methods reported previously.18−22
Scheme 1.
Scheme 3.
Scheme 2.
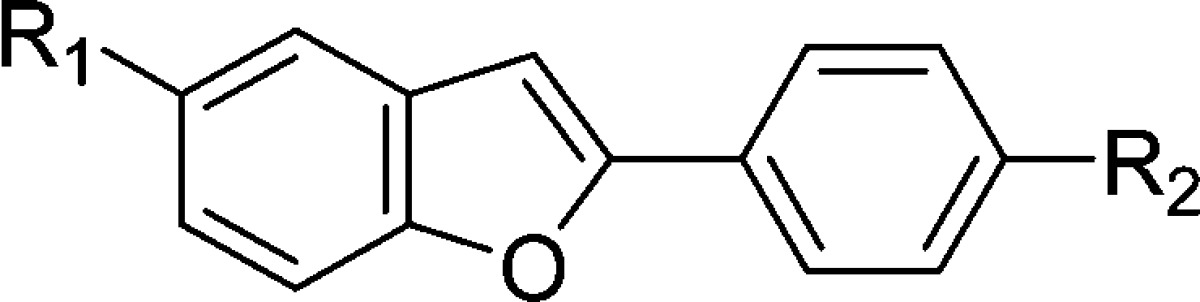
The binding experiments were carried out as described previously.17,23 Assays using Aβ(1−42) aggregates demonstrated that these fluorinated benzofuran derivatives competed with [125I]IMPY to bind β-amyloid plaques with excellent affinity (Table 1).24,25 Compound 7 with a dimethylaminophenyl moiety in the phenylbenzofuran molecule displayed slightly lower values (higher affinity) than 5 with an aminophenyl moiety or 6 with a monomethylaminophenyl moiety. However, all of the derivatives maintained good binding affinity with Ki values in the nanomolar range. The results strongly support our previous finding that benzofuran derivatives have considerable tolerance for structural modification.17,18 Among the derivatives with high affinity for Aβ aggregates, 17 was tested further because of the ease with which it could be labeled with 18F.
Table 1. Chemical Structures and Inhibition Constants of Fluorinated Benzofuran Derivatives.
 |
Inhibition constants (Ki, nM) of compounds for the binding of [125I]IMPY to Aβ(1−42) aggregates. Values are the means ± standard errors of the mean for 3−6 independent experiments.
The 18F-labeled 17 ([18F]17) was prepared from a tosyl precursor (22) via a nucleophilic displacement reaction with the fluoride anion (Scheme 4).23 A solution of 22 (1.0 mg) in acetonitrile (200 μL) was added to a reaction vessel containing 18F. The mixture was heated at 120 °C for 10 min. Radiolabeling of the precursor generated [18F]17 with an average radiochemical yield of 10.0% and radiochemical purity of >99%. The specific activity of [18F]17 was 242 GBq/μmol. The identity of [18F]17 was verified by a comparison of the retention time with the nonradioactive compound. Initially, 21 was expected to show similar radiolabeling to 17. However, the radiolabeling of 21 under the various reaction conditions normally used for 18F radiolabeling gave a radiochemical yield (<0.1%) too low to conduct a subsequent distribution experiment.
Scheme 4.

To evaluate the uptake of [18F]17 in the brain, a biodistribution experiment was performed in normal mice (Table 2). [18F]17 displayed high uptake (5.66% ID/g) at 10 min postinjection, sufficient for PET imaging, and the radioactivity in the brain cleared with time. At 60 min postinjection, the uptake was 2.80% ID/g, indicating a relatively slow washout from the brain. One way to select a ligand with appropriate kinetics in vivo is to use the brain2 min/brain60 min ratio as an index to compare the washout rate. Although the brain2 min/brain60 min ratio of [18F]17 (1.0) was still lower than that of [11C]PIB (12.0)7,8 or [18F]AV-45 (3.80),14,15 it was much improved as compared to the values for iodinated benzofuran derivatives (0.47−0.48) reported previously.17 Furthermore, among these benzofuran derivatives, the washout rate improved as the lipophilicity decreased (log P values of [18F]17 and the iodinated benzofuran derivatives were 1.20 and 2.12−2.35, respectively).17 Thus, lipophilicity is important to improving washout from the brain. To further enhance the washout rate, the synthesis of less lipophilic benzofuran derivatives, for example, by replacing the dimethylamino group with another hydrophilic group, is now under investigation. Uptake in the bone at 60 min was measurable (2.74% ID/g), suggesting little defluorination in vivo.13,19,26 However, the free fluoride was not taken up by brain tissue, and so, interference with the imaging is expected to be relatively minor.
Table 2. Biodistribution of Radioactivity after Injection of [18F]17 in Normal Micea.
| organ | 2 min | 10 min | 30 min | 60 min |
|---|---|---|---|---|
| blood | 1.64 ± 0.07 | 2.48 ± 0.18 | 2.68 ± 0.26 | 3.41 ± 0.56 |
| brain | 2.88 ± 0.46 | 5.66 ± 0.31 | 3.14 ± 0.26 | 2.80 ± 0.06 |
| bone | 1.19 ± 0.18 | 1.76 ± 0.13 | 3.63 ± 1.11 | 2.74 ± 0.59 |
Expressed as % of injected dose per gram. Each value represents the mean ± SD for five mice.
Next, to confirm the specific binding of radiofluorinated ligands to β-amyloid plaques, we performed autoradiographic imaging of [18F]17 with sections (10 μm) of Tg2576 mouse brain. Tg2576 transgenic mice express human APP695 with the K670N, M671L Swedish double mutation.27 They show marked Aβ deposition in the cingulated cortex, entorhinal cortex, dentate gyrus, and CA1 hippocampal subfield by 11−13 months of age and have been frequently used to evaluate the specific binding of β-amyloid plaques in experiments in vitro and in vivo.15,18,19,23 Autoradiographic images of [18F]17 showed high levels of radioactivity in the brain sections (Figure 2A). Furthermore, the hot spots of [18F]17 corresponded with those of thioflavin-S, a pathological dye commonly used to stain β-amyloid plaques (Figure 2B). The results suggest that [18F]17 shows affinity for β-amyloid plaques in the mouse brain in addition to binding synthetic Aβ aggregates.
Figure 2.
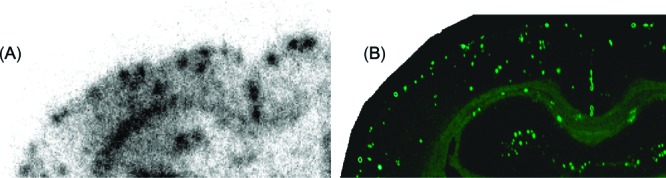
Autoradiography of a section (10 μm) of Tg2576 mouse brain with [18F]17. [18F]17 showed excellent binding to β-amyloid plaques (A). β-Amyloid plaques were confirmed present by staining of the section with thioflavin-S (B).
To further characterize the potential of [18F]17 as an agent for imaging β-amyloid plaques in living brain tissue, we carried out autoradiography ex vivo in a Tg2576 mouse (36 months, male). The autoradiography showed distinctive labeling of β-amyloid plaques in the brain (Figure 3A), which was confirmed by costaining of the sections with thioflavin-S (Figure 3B). Wild-type mouse brain showed no β-amyloid plaques (Figure 3C). This is consistent with results in vitro showing [18F]17 to be highly selective in binding to β-amyloid plaques in the brain.
Figure 3.
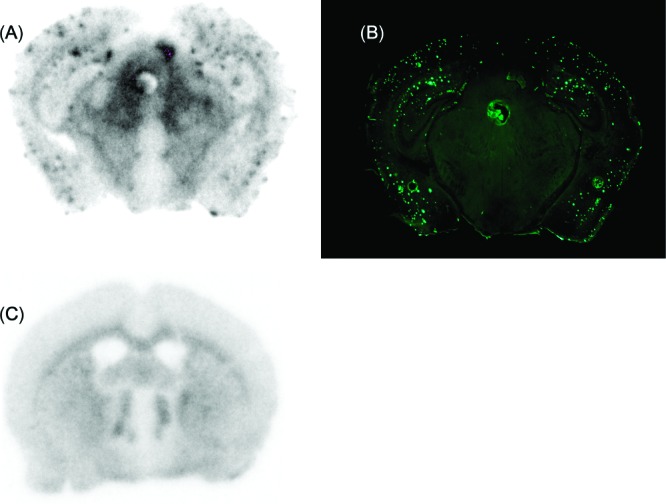
Labeling of β-amyloid plaques in vivo was visualized by autoradiography ex vivo with [18F]17 in sections of Tg2576 mouse brain (A). The same section was also stained with thioflavin-S (B). Wild-type mouse brain showed no β-amyloid plaques (C).
In summary, we produced a series of fluorinated benzofuran derivatives that bind well to Aβ(1−42) aggregates and clearly stain β-amyloid plaques. In experiments in vitro and ex vivo using an animal model of AD, [18F]17 intensely labeled β-amyloid plaques. These newly synthesized derivatives may become PET radiotracers for imaging β-amyloid plaques in the brain and deserve further investigation by optimizing the substituted groups into the benzofuran backbone.
Supporting Information Available
Procedures for the preparation of new fluorinated benzofuran derivatives, in vitro binding assay, in vitro autoradiography using Tg2576 mouse brain sections, and ex vivo autoradiography using Tg2576 mice. This material is available free of charge via the Internet at http://pubs.acs.org.
This study was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO), a Health Labour Sciences Research Grant, and a Grant-in-Aid for Young Scientists (A) and Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Supplementary Material
References
- Selkoe D. J. Alzheimer's Disease: Genes, Proteins, and Therapy. Physiol. Rev. 2001, 81, 741–766. [DOI] [PubMed] [Google Scholar]
- Hardy J.; Selkoe D. J. The Amyloid Hypothesis of Alzheimer's Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Imaging Alzheimer's Amyloid. Nat. Biotechnol. 2000, 18, 823–824. [DOI] [PubMed] [Google Scholar]
- Mathis C. A.; Wang Y.; Klunk W. E. Imaging β-Amyloid Plaques and Neurofibrillary Tangles in the Aging Human Brain. Curr. Pharm. Des. 2004, 10, 1469–1492. [DOI] [PubMed] [Google Scholar]
- Nordberg A. PET Imaging of Amyloid in Alzheimer's Disease. Lancet Neurol. 2004, 3, 519–527. [DOI] [PubMed] [Google Scholar]
- Ono M.; Wilson A.; Nobrega J.; Westaway D.; Verhoeff P.; Zhuang Z. P. 11C-labeled Stilbene Derivatives as Aβ-Aggregate-Specific PET Imaging Agents for Alzheimer's Disease. Nucl. Med. Biol. 2003, 30, 565–571. [DOI] [PubMed] [Google Scholar]
- Mathis C. A.; Wang Y.; Holt D. P.; Huang G. F.; Debnath M. L.; Klunk W. E. Synthesis and Evaluation of 11C-Labeled 6-Substituted 2-Arylbenzothiazoles as Amyloid Imaging Agents. J. Med. Chem. 2003, 46, 2740–2754. [DOI] [PubMed] [Google Scholar]
- Klunk W. E.; Engler H.; Nordberg A.; Wang Y.; Blomqvist G.; Holt D. P. Imaging Brain Amyloid in Alzheimer's Disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [DOI] [PubMed] [Google Scholar]
- Kudo Y.; Okamura N.; Furumoto S.; Tashiro M.; Furukawa K.; Maruyama M.; Itoh M.; Iwata R.; Yanai K.; Arai H. 2-(2-[2-Dimethylaminothiazol-5-yl]ethenyl)-6-(2-[fluoro]ethoxy)benzoxazole: A Novel PET Agent for in vivo Detection of Dense Amyloid Plaques in Alzheimer’s Disease Patients. J. Nucl. Med. 2007, 48, 553–561. [DOI] [PubMed] [Google Scholar]
- Small G. W.; Kepe V.; Ercoli L. M.; Siddarth P.; Bookheimer S. Y.; Miller K. J.; Lavretsky H.; Burggren A. C.; Cole G. M.; Vinters H. V.; Thompson P. M.; Huang S. C.; Satyamurthy N.; Phelps M. E.; Barrio J. R. PET of Brain Amyloid and Tau in Mild Cognitive Impairment. N. Engl. J. Med. 2006, 355, 2652–2663. [DOI] [PubMed] [Google Scholar]
- Shoghi-Jadid K.; Small G. W.; Agdeppa E. D.; Kepe V.; Ercoli L. M.; Siddarth P.; Read S.; Satyamurthy N.; Petric A.; Huang S. C.; Barrio J. R. Localization of Neurofibrillary Tangles and β-Amyloid Plaques in the Brains of Living Patients with Alzheimer Disease. Am. J. Geriatr. Psychiatry 2002, 10, 24–35. [PubMed] [Google Scholar]
- Rowe C. C.; Ackerman U.; Browne W.; Mulligan R.; Pike K. L.; O’Keefe G.; Tochon-Danguy H.; Chan G.; Berlangieri S. U.; Jones G.; Dickinson-Rowe K. L.; Kung H. P.; Zhang W.; Kung M. P.; Skovronsky D.; Dyrks T.; Holl G.; Krause S.; Friebe M.; Lehman L.; Lindemann S.; Dinkelborg L. M.; Masters C. L.; Villemagne V. L. Imaging of Amyloid β in Alzheimer's Disease with 18F-BAY94-9172, A Novel PET Tracer: Proof of Mechanism. Lancet Neurol. 2008, 7, 129–135. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Oya S.; Kung M. P.; Hou C.; Maier D. L.; Kung H. F. F-18 Polyethyleneglycol Stilbenes as PET Imaging Agents Targeting Aβ aggregates in the Brain. Nucl. Med. Biol. 2005, 32, 799–809. [DOI] [PubMed] [Google Scholar]
- Choi S. R.; Golding G.; Zhuang Z.; Zhang W.; Lim N.; Hefti F.; Benedum T. E.; Kilbourn M. R.; Skovronsky D.; Kung H. F. Preclinical Properties of 18F-AV-45: A PET Imaging Agent for Aβ Plaques in the Brain. J. Nucl. Med. 2009, 50, 1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. F.; Choi S. R.; Qu W.; Zhang W.; Skovronsky D. 18F Stilbenes and Styrylpyridines for PET Imaging of Aβ Plaques in Alzheimer's Disease. J. Med. Chem. 2010, 53, 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole M.; Lewis D. M.; Buckley C.; Nelissen N.; Vandenbulcke M.; Brooks D. J.; Vandenberghe R.; Van Laere K. Whole-Body Biodistribution and Radiation Dosimetry of 18F-GE067: A Radioligand for in vivo Brain Amyloid Imaging. J. Nucl. Med. 2009, 505818–822. [DOI] [PubMed] [Google Scholar]
- Ono M.; Kung M. P.; Hou C.; Kung H. F. Benzofuran Derivatives as Aβ Aggregate Specific Imaging Agents for Alzheimer's Disease. Nucl. Med. Biol. 2002, 29, 633–642. [DOI] [PubMed] [Google Scholar]
- Ono M.; Kawashima H.; Nonaka A.; Kawai T.; Haratake M.; Mori H.; Kung M. P.; Kung H. F.; Saji H.; Nakayama M. Novel Benzofuran Derivatives for PET Imaging of β-Amyloid Plaques in Alzheimer's Disease Brains. J. Med. Chem. 2006, 49, 2725–2730. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Oya S.; Kung M. P.; Hou C.; Maier D. L.; Kung H. F. F-18 Stilbenes as PET Imaging Agents for Detecting β-Amyloid Plaques in the Brain. J. Med. Chem. 2005, 48, 5980–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W.; Berman R. J.; Gelb M. H. Synthesis and Evaluation of Phospholipid Analogues as Inhibitors of Cobra Venom Phospholipase A2. J. Am. Chem. Soc. 1987, 109, 8071–8081. [Google Scholar]
- Cox D. P.; Terpinski J.; Lawrynowicz W. “Anhydrous” Tetrabutylammonium Fluoride: A Mild but Highly Efficient Source of Nucleophilic Fluoride Ion. J. Org. Chem. 1984, 49, 3216–3219. [Google Scholar]
- Xu B.; Stephens A.; Kirschenheuter G.; Greslin A. F.; Cheng X.; Sennelo J.; Cattaneo M.; Zighetti M. L.; Chen A.; Kim S. A.; Kim H. S.; Bischofberger N.; Cook G.; Jacobson K. A. Acyclic Analogues of Aaenosine Bisphosphates as P2Y Receptor Antagonists: Phosphate Substitution Leads to Multiple Pathways of Inhibition of Platelet Aggregation. J. Med. Chem. 2002, 45, 5694–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M.; Watanabe R.; Kawashima H.; Cheng Y.; Kimura H.; Watanabe H.; Haratake M.; Saji H.; Nakayama M. Fluoro-Pegylated Chalcones as Positron Emission Tomography Probes for in vivo Imaging of β-amyloid Plaques in Alzheimer's Disease. J. Med. Chem. 2009, 52, 6394–6401. [DOI] [PubMed] [Google Scholar]
- Kung M. P.; Hou C.; Zhuang Z. P.; Skovronsky D.; Kung H. F. Binding of Two Potential Imaging Agents Targeting Amyloid Plaques in Postmortem Brain Tissues of Patients with Alzheimer's Disease. Brain Res. 2004, 1025, 98–105. [DOI] [PubMed] [Google Scholar]
- Kung M. P.; Hou C.; Zhuang Z. P.; Cross A. J.; Maier D. L.; Kung H. F. Characterization of IMPY as a Potential Imaging Agent for β-Amyloid Plaques in Double Transgenic PSAPP Mice. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1136–1145. [DOI] [PubMed] [Google Scholar]
- Stephenson K. A.; Chandra R.; Zhuang Z. P.; Hou C.; Oya S.; Kung M. P.; Kung H. F. Fluoro-Pegylated (FPEG) Imaging Agents Targeting Aβ Aggregates. Bioconjugate Chem. 2007, 18, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K.; Chapman P.; Nilsen S.; Eckman C.; Harigaya Y.; Younkin S.; Yang F.; Cole G. Correlative Memory Deficits, Aβ Elevation, and Amyloid Plaques in Transgenic Mice. Science 1996, 274, 99–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.