Elevated tricuspid regurgitant velocity (TRV) measured by echocardiogram predicts death in adult sickle cell anemia (SCA)1–4 and occurs in children,5–9 although etiology and association with survival and complications requiring hospitalization are unknown. In children, hemolysis is associated with TRV in some5,6 but not all7,9 studies. We studied TRV prevalence and predictors in African children with no hydroxyurea and limited blood transfusion exposure in a region of low malaria prevalence.10
Ethical clearance was granted by Muhimbili University of Health and Allied Sciences (MU/RP/AEC/Vol.XIIV/01) and the Children’s Hospital Colorado (COMIRB 10-0030). Written informed consent was obtained from all participants. Inclusion criteria were age 9–19 years and HbSS by hemoglobin electrophoresis and high performance liquid chromatography for SCA cases. Non-SCA controls were HbAS or HbAA by electrophoresis. Exclusion criteria included blood transfusion within the previous two months, sickle crisis within the previous two weeks, febrile illness within the previous seven days, fever or signs of acute illness on the day of the echocardiogram or echocardiographically identified hemodynamically significant congenital heart disease.
Echocardiography was undertaken in April 2010. Blood pressure (BP) was measured twice after 5 min rest using an automated digital device (PRO 400 V2 Dinamap) and the mean was calculated. Resting hemoglobin oxygen saturation (SpO2) was obtained over 5 min using pulse oximetry (Masimo Rad-57). Axial temperature and heights and weights were recorded and clinical examination performed to exclude acute complications. Two-dimensional and Doppler echocardiography was performed using a standard pediatric protocol. TRV was assessed in the parasternal long- and short-axis and apical 4-chamber views. All studies were performed on a GE Vivid Q portable ultrasound system using a 5S or M4S transducer as appropriate by one of 3 experienced sonographers/pediatric cardiologists from the Children’s Hospital, Colorado, USA. TRV was not quantified if no measurable regurgitation was present or if the quality of the Doppler tracing precluded accurate TRV measurement; these patients were excluded from further analysis. All measurements were later over-read by a different sonographer and agreement reached. Elevated TRV was categorized as the mean plus 2xSD TRV in controls.
Laboratory results were available from previous steady-state samples for SCA cases (defined as absence of pain or fever, negative malaria rapid test, and no recorded hospital admission or blood transfusion within 30 days) and at screening for the non-SCA controls. Full blood counts were performed using an automated cell counter (Pentra 60, Horiba ABX, Kyoto, Japan). Biochemical tests were performed using an automated chemistry analyzer (Roche Cobas Mira, New York, USA or Abbott Architect, New York, USA). NT pro-BNP was measured using a cardiac reader (Roche Diagnostics, Basel, Switzerland) in fresh lithium heparin plasma collected in 2007/2008.
Continuous variables were compared between SCA-cases and non-SCA controls using Student’s t-tests or Wilcoxon’s rank sum tests. Tests for trend were used for differences across TRV categories. Multivariable linear regression was used to test for independent associations between explanatory variables and TRV in which all variables significant at P≤0.1 were included and then non-significant variables were dropped simultaneously. Risk of prospective hospitalization according to TRV category was assessed using Cox’s regression and the Cnaan and Ryan approach, modeling the effect of age at start of surveillance.11 Hospital admissions less than seven days apart were treated as a single event and previous number of admissions included as a co-factor. Repeated events within individuals were accounted for using robust standard errors. The end date for observation for hospitalization was 31st March 2012 for all subjects; none were lost to follow up.
A total of 265 subjects were included in this analysis (215 SCA cases and 50 non-SCA controls); none had received hydroxyurea or chronic blood transfusions. Table 1 summarizes the demographic, clinical, laboratory and echocardiographic variables in the cases and controls. Cases were non-significantly older but were more anemic and significantly more wasted and stunted than controls. Systolic, diastolic, and mean blood pressures and SpO2 were also significantly lower in SCA cases. Although only limited data were available for controls, hemolytic and liver function markers were lower than in the cases (Table 1)
Table 1.
Characteristics of SCA cases and non-SCA controls.
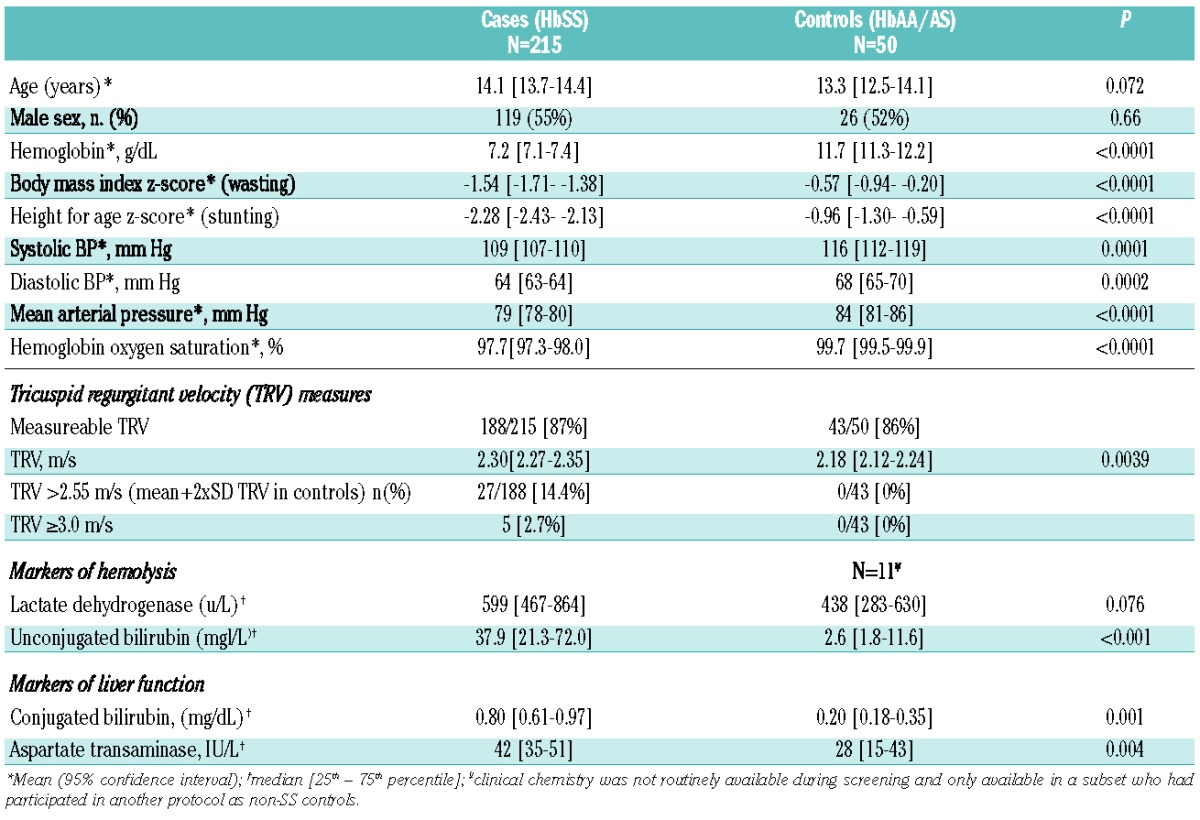
The proportion of subjects with measureable TRV was similar between cases and controls. Mean TRV was significantly higher in cases versus controls (P=0.0039). A cut off TRV of over 2.55 m/s was considered elevated. The prevalence of elevated TRV was 14.4% in cases and 0% in controls, and the prevalence in cases of TRV ≥3.0 m/s was 2.7% (Table 1).
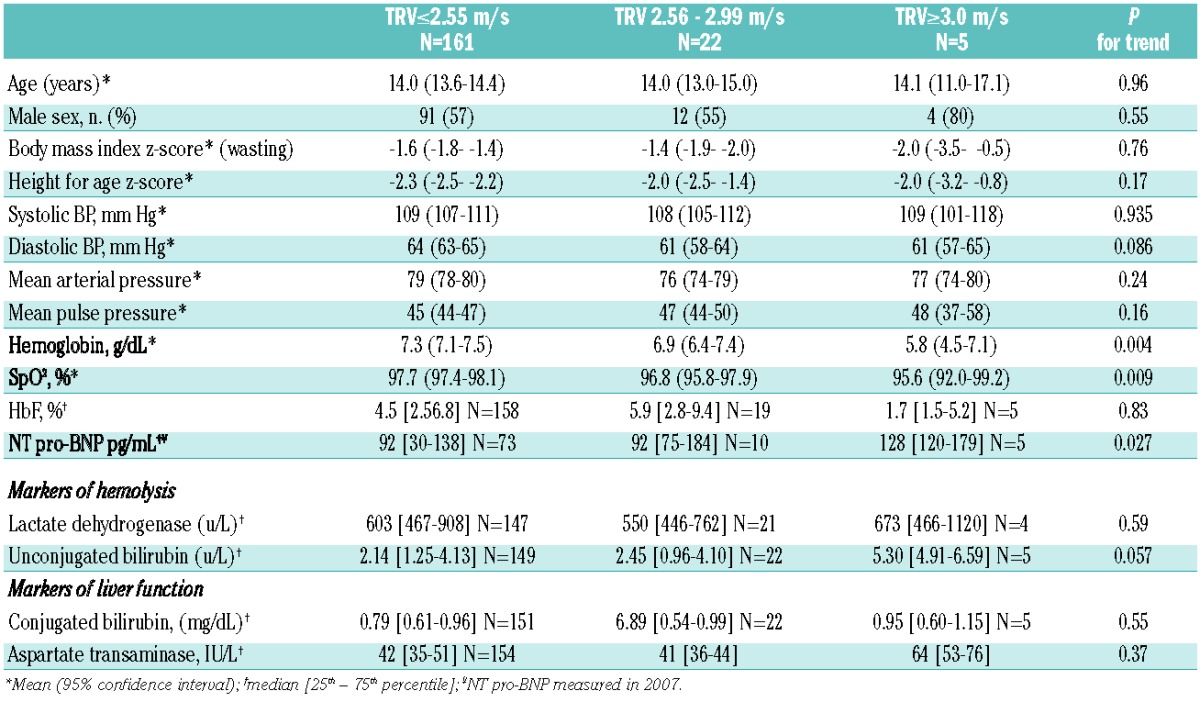
We prospectively hypothesized that hemoglobin, SpO2, markers of hemolysis and systolic blood pressure would differ according to TRV category (Table 2). Hemoglobin and SpO2 demonstrated an inverse association with TRV (P<0.01 for trend). Unconjugated bilirubin concentrations were higher in children with TRV over 3 m/s (P=0.059 for trend) but there was no evidence of any difference in LDH across the TRV categories. NT pro-BNP measured at steady state between September 2007 and January 2008 weakly predicted higher subsequent TRV category (P=0.027). In multivariable analysis, only hemoglobin (β-coefficient −0.48; P=0.006) and SpO2 (β-coefficient −0.023, P=0.004) independently associated with TRV, but only explained 11% of TRV variation. There was no evidence that any of the tested variables differed in the 27 SCA cases in whom TRV could not be measured compared to cases with a non-elevated TRV.
Table 2.
Hematologic measures, markers of hemolysis and liver function and daytime hemoglobin oxygen saturation by tricuspid regurgitant jet velocity category.
Thirty-seven hospitalizations occurred among 27 patients from a total of 370 person years observation (PYO) with 10.0 (95%CI: 7.2–13.8) hospitalizations per 100 PYO. Independent of age, TRV over 2.55 m/s was borderline associated with an increased risk of hospitalization (2.44 [1.04/5.76]; P=0.04). However, no hospitalizations occurred in the 5 children with a TRV of over 3 m/s. The primary cause of hospitalization was pain in 23 of 37 (62%) and for the remainder were infections (14%), severe anemia (8%) or “other” (16%). There was no evidence of any difference according to TRV for cause classified as pain versus non-pain causes.
In this first report of a large population of children with SCA resident in Africa, the prevalence of high TRV is similar to that observed in the US and Europe,5–9,12 despite the fact that study subjects had had no hydroxyurea exposure, fewer blood transfusions (cross-sectional data from same aged children in the Muhumbili Sickle Cohort: 80% none, 13% 1, 6% 2–5; Makani and Cox, unpublished data, 2013) and higher burden of infectious diseases. The high TRV cut off generated from our controls was similar to that from American age-matched controls (>=2.6 m/s).5 Unconjugated bilirubin, a robust measure of hemolysis in those without Gilbert’s syndrome, was increased in cases versus controls and was higher in cases with the most elevated TRV. LDH may not be specific to hemolysis,13 and was similar between the groups and not associated with TRV category. This is in contrast to the PUSH study5,14,15 in which all assessed hemolytic markers were associated with TRV over 2.6 m/s (unconjugated bilirubin not reported) but in line with two smaller studies.7,9 In a study of adolescent and adult Nigerian patients, combined16 LDH, but not total bilirubin (unconjugated bilirubin not reported) was associated with elevated TRV. We did not have reticulocyte counts available and could not calculate a hemolysis index.5 However, it remains a possibility that the association between hemolytic markers and elevated TRV in SCA may not be directly causal,17 but an effect of increased cardiac load from increased anemia14 rather than increased vascular resistance.12
Reduced SpO2 in children with elevated TRV has been previously reported, including in the largest dataset yet18 in which elevated TRV and poor 6-min walk test performance were not explained by impaired lung function. In a study of TRV and markers of endothelial activation, SpO2 was also associated with TRV, but not independent of its association with the markers of endothelial activation, particularly vascular endothelial growth factor (VEGF),19 suggesting a mechanism by which low SpO2 may contribute to vascular changes suspected to underlie the development of elevated TRV. Limited expertise and resources to conduct echocardiographic screening in most African countries mean that a biomarker like NT-pro BNP, which predicts high-risk individuals in adult SCA patients in the USA,20,21 could be particularly useful in these settings. We found some evidence that high NT-pro BNP is associated with the development over time of high TRV (>=3 m/s). This is in contrast to Nigerian adult patients in whom NT-pro BNP was associated with worse functional outcomes (6-min walk test) but was not associated with TRV or left ventricular diastolic dysfunction.16 The utility of NT-pro BNP in predicting death in African patients has not so far been reported.
This is the first large-scale study in children with SCA to conduct a prospective evaluation of clinical outcomes. We are currently unable to reliably determine whether the increased hospitalizations in children with elevated TRV resulted from cardio-pulmonary consequences of elevated TRV. However, within this age group, given the apparent lack of increased risk of death, it is more probable that elevated TRV is a marker of more general disease severity. Adult Nigerian patients with elevated estimated pulmonary artery pressure had a history of increased crisis rate, but prospective follow up has not yet been reported,22 whilst in American children, elevated TRV was associated with more than 3 annual hospitalizations for pain.23
The strengths of our study include the large number of HbSS patients with echo-cardiograms conducted by highly experienced staff within a short time frame and to a tightly controlled research protocol with a strict definition of steady-state used in the inclusion criteria, thus ensuring measurements were not affected by acute conditions. The interpretation of the NT-pro BNP with TRV is limited by the time difference between the measurements and its predictive value for risk of death, its specificity for cardiac dysfunction or its intra-individual variation in children with SCA are not known. Our other laboratory markers were measured at steady state before the echocardiography; a longer confirmed fever-free period before echocardiography and venepuncture on the same day might yield different results.
In summary, the prevalence of elevated TRV is similar in Tanzanian and US children with SCA. TRV predicts hospitalization and is independently associated with anemia and low hemoglobin oxygen saturation but only the highest TRV values are associated with unconjugated hyperbilrubinemia, a marker of hemolysis. Long-term follow up is required to determine changes in TRV over time, response to interventions and association with mortality.
Acknowledgments
The authors would like to thank Jeanine Gruenwald (RCDS) and Scott Kirby (RCDS) for their time and expertise in conducting the echocardiograms and associated data preparation. We warmly thank the patients and staff of MNH and MUHAS, Dar-es-Salaam, Tanzani, who made this work possible. We also thank the staff in the clinical chemistry unit at MNH central pathology laboratory for conducting the clinical chemistry analyses and Josephine Mgaya and Harvest Mariki for the sickle diagnostics, hematology analyses and sample archiving.
Footnotes
Funding: This research was supported by Wellcome Trust, UK, project grant 080025 (SEC), personal fellowship 072064, (JM) and strategic award (085438) (CN, JM and SC) and funding from the Jayden DeLuca Foundation, the Leah Bult Foundation, UL1 RR025780 Colorado Clinical Translational Science Institute, National Center for Research Resources, and National Institutes of Health to support staff time and travel to Tanzania (AY and technicians).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, et al. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134(1):109–15 [DOI] [PubMed] [Google Scholar]
- 2.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–95 [DOI] [PubMed] [Google Scholar]
- 3.Sutton LL, Castro O, Cross DJ, Spencer JE, Lewis JF. Pulmonary hypertension in sickle cell disease. Am J Cardiol. 1994;74(6):626–8 [DOI] [PubMed] [Google Scholar]
- 4.Lorch D, Spevack D, Little J. An elevated estimated pulmonary arterial systolic pressure, whenever measured, is associated with excess mortality in adults with sickle cell disease. Acta Haematol. 2011;125(4):225–9 [DOI] [PubMed] [Google Scholar]
- 5.Minniti CP, Sable C, Campbell A, Rana S, Ensing G, Dham N, et al. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94(3):340–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liem RI, Young LT, Thompson AA. Tricuspid regurgitant jet velocity is associated with hemolysis in children and young adults with sickle cell disease evaluated for pulmonary hypertension. Haematologica. 2007;92(11):1549–52 [DOI] [PubMed] [Google Scholar]
- 7.Colombatti R, Maschietto N, Varotto E, Grison A, Grazzina N, Meneghello L, et al. Pulmonary hypertension in sickle cell disease children under 10 years of age. Br J Haematol. 2010;150(5):601–9 [DOI] [PubMed] [Google Scholar]
- 8.Johnson MC, Kirkham FJ, Redline S, Rosen CL, Yan Y, Roberts I, et al. Left ventricular hypertrophy and diastolic dysfunction in children with sickle cell disease are related to asleep and waking oxygen desaturation. Blood. 2010;116(1):16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Prevalence and risk factors of elevated pulmonary artery pressures in children with sickle cell disease. Pediatrics. 2008;121(4):777–82 [DOI] [PubMed] [Google Scholar]
- 10.Makani JK, Komba AN, Cox SE, Oruo J, Mwamtemi K, Kitundu J, et al. Malaria in patients with sickle cell anemia: burden, risk factors and outcome at outpatient clinic and during hospitalization. Blood. 2010;115(2):215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cnaan A, Ryan L. Survival analysis in natural history studies of disease. Stat Med. 1989;8(10):1255–68 [DOI] [PubMed] [Google Scholar]
- 12.Chaudry RA, Cikes M, Karu T, Hutchinson C, Ball S, Sutherland G, et al. Paediatric sickle cell disease: pulmonary hypertension but normal vascular resistance. Arch Dis Child. 2011;96(2):131–6 [DOI] [PubMed] [Google Scholar]
- 13.Ballas SK. Lactate dehydrogenase and hemolysis in sickle cell disease. Blood. 2013;121(1):243–4 [DOI] [PubMed] [Google Scholar]
- 14.Dham N, Ensing G, Minniti C, Campbell A, Arteta M, Rana S, et al. Prospective echocardiography assessment of pulmonary hypertension and its potential etiologies in children with sickle cell disease. Am J Cardiol. 2009;104(5):713–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordeuk VR, Campbell A, Rana S, Nouraie M, Niu X, Minniti CP, et al. Relationship of erythropoietin, fetal hemoglobin, and hydroxyurea treatment to tricuspid regurgitation velocity in children with sickle cell disease. Blood. 2009;114(21):4639–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aliyu ZY, Gordeuk V, Sachdev V, Babadoko A, Mamman AI, Akpanpe P, et al. Prevalence and risk factors for pulmonary artery systolic hypertension among sickle cell disease patients in Nigeria. Am J Hematol. 2008;83(6):485–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebbel RP. Reconstructing sickle cell disease: a data-based analysis of the “hyperhemolysis paradigm” for pulmonary hypertension from the perspective of evidence-based medicine. Am J Hematol. 2011;86(2):123–54 [DOI] [PubMed] [Google Scholar]
- 18.Campbell A, Minniti CP, Nouraie M, Arteta M, Rana S, Onyekwere O, et al. Prospective evaluation of haemoglobin oxygen saturation at rest and after exercise in paediatric sickle cell disease patients. Br J Haematol. 2009;147(3):352–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu X, Nouraie M, Campbell A, Rana S, Minniti CP, Sable C, et al. Angiogenic and inflammatory markers of cardiopulmonary changes in children and adolescents with sickle cell disease. PLoS ONE. 2009;4(11):e7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, et al. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296(3):310–8 [DOI] [PubMed] [Google Scholar]
- 21.Machado RF, Hildesheim M, Mendelsohn L, Remaley AT, Kato GJ, Gladwin MT. NT-pro brain natriuretic peptide levels and the risk of death in the cooperative study of sickle cell disease. Br J Haematol. 2011;154(4):512–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oguanobi NI, Ejim EC, Anisiuba BC, Onwubere BJ, Ike SO, Ibegbulam OG, et al. Clinical and electrocardiographic evaluation of sickle-cell anaemia patients with pulmonary hypertension. ISRN Hematol. 2012;2012:768718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darbari DS, Onyekwere O, Nouraie M, Minniti CP, Luchtman-Jones L, Rana S, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr. 2012;160(2):286–90 [DOI] [PMC free article] [PubMed] [Google Scholar]


