Abstract
Busulfan liver metabolism depends on glutathione, a crucial mediator of cellular and systemic stress. Here we investigated 40 polymorphisms at 27 loci involved in hepatic glutathione homeostasis, with the aim of testing their impact on the clinical outcome of 185 busulfan-conditioned allogeneic transplants. GSTA2 S112T serine allele homozygosity is an independent prognostic factor for poorer survival (RR=2.388), for increased any time- and 100-day transplant-related mortality (RR=4.912 and RR=5.185, respectively). The genotype also predicts a wider busulfan area under the concentration-time curve (1214.36±570.06 vs. 838.10±282.40 mMol*min) and higher post-transplant bilirubin serum levels (3.280±0.422 vs. 1.874+0.197 mg/dL). In vitro, busulfan elicits pro-inflammatory activation (increased NF-KappaB activity and interleukin-8 expression) in human hepatoma cells. At the same time, the drug down-regulates a variety of genes involved in bilirubin liver clearance: constitutive androstane receptor, multidrug resistance-associated protein, solute carrier organic anion transporters, and even GSTA2. It is worthy of note that GSTA2 also acts as an intra-hepatic bilirubin binding protein. These data underline the prognostic value of GSTA2 genetic variability in busulfan-conditioned allotransplants and suggest a patho-physiological model in which busulfan-induced inflammation leads to the impairment of post-transplant bilirubin metabolism.
Introduction
Hematopoietic stem cell transplantation (HSCT) is a curative procedure for a variety of hematologic diseases.1 Transplant-related mortality (TRM) after HSCT depends mainly on graft-versus-host disease (GVHD), infections and preparative regimen toxicity. The assessment of polymorphisms at major histocompatibility complex (MHC) loci has long been proven to be crucial for successful allogeneic HSCT.2 Polymorphisms at non-MHC loci have also been associated with the HSCT outcome.3,4 Much attention has been paid to the relationship between genetic variation at innate immunity loci and GVHD.5 Until now, studies on genetic polymorphisms on HSCT have generated contrasting data, likely due to insufficient sample size, selection biases, patient and treatment heterogeneity.2
Hematopoietic stem cell transplantation is a systemic stress: the achievement of a favorable HSCT outcome is achieved via the successful functioning of multiple stress response systems, and is likely to be affected by genetic variability over a large array of loci.3
Busulfan, a bi-functional alkylating agent, is an alternative to total body irradiation, and it is one of the most widely used drugs in allogeneic HSCT conditioning regimen.1 Busulfan is used in association with cyclophosphamide1 and more recently with fludarabine.6 Systemic exposure to the drug is an impor tant parameter for monitoring therapeutic efficacy.7,8 Large inter-individual variability in busulfan pharmacokinetics has been observed:9 the therapeutic window of busulfan, expressed as area under the concentration-time curve (AUC), ranges from 900 to 1500 mMol*min. AUC levels lower than 900 mMol*min correlate with disease relapse and graft failure, while values higher than 1500 mMol*min are associated with extra-hematologic hepatic toxicity.10,11 The clearance of the drug occurs in the liver by direct conjugation with glutathione (GSH), as well as via enzymatic GSH conjugation by glutathione-S-transferases12,13 (GST). Liver GST activity, which is mainly due to GSTA1 and GSTA2 iso-enzymes, correlates with busulfan elimination.14 Depletion of liver GSH, as a consequence of busulfan conjugation, impairs the metabolism of other drugs such as cyclophosphamide.15 Plasma GST activity and GSH level associate with the variability in busulfan clearance.14,16
In this paper, 40 polymorphisms at 27 loci involved in hepatic glutathione balance17 were studied in a total of 185 patients who underwent allogeneic HSCT from 2005 to 2009, after a busulfan-based preparative regimen (busulfan cohort). The impact of such loci was tested on overall survival, TRM, busulfan AUC and serum liver function tests.18 The same polymorphisms were also tested on 146 consecutive patients not receiving busulfan in the preparative regimen, concomitantly undergoing transplantation at the same Institution (comparator cohort).
Methods
Patients
The busulfan cohort was made up of 185 consecutive patients (Table 1) who were transplanted at the Institute of Hematology “L. and A. Seràgnoli”, University of Bologna, Italy, between 2005 and 2009, using busulfan as conditioning. Myeloablative conditioning (0.8 mg/kg intravenous busulfan, 2-h infusions, four times a day, four consecutive days plus cyclophosphamide, 120 mg/kg) was administered to 167 patients. Lower (6 patients) or standard busulfan doses plus fludarabine 160 mg/m2 (12 patients) were also used. Ideal body weight (IBW) was calculated as 0.91 × (height in cm – 152) +50 (for men) or +45 (for women). IBW was used when lower than the actual weight and when body mass index (BMI) was lower/equal to 27. When BMI was greater than 27, IBW was adjusted as IBW+ 0.25 PPA1401D (actual weight – IBW).
Table 1.
Clinical characteristics of patients.
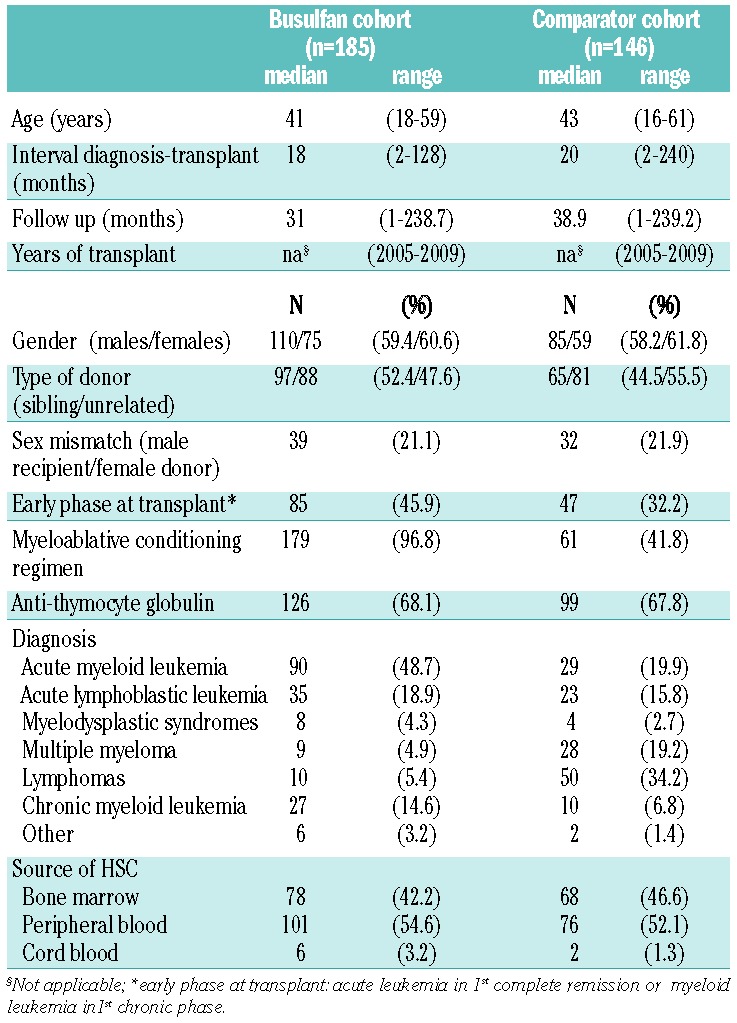
A total of 146 consecutive patients (comparator cohort, Table 1) transplanted at the same Institution were also studied. In this cohort, the myeloablative conditioning was cyclophosphamide (120 mg/kg) plus unfractionated total body irradiation (8 Gy).
Graft-versus-host disease prophylaxis was cyclosporin-A and short-term methotrexate plus rabbit anti-thymocyte globulin (3–5 mg/kg/die, Fresenius, Bad-Homburg, Germany), from Day -6 to Day -2. Patients with acute leukemia in first complete remission or with chronic myeloid leukemia in first chronic phase were classified as being in early phase at transplant; the remaining patients were classified as being in advanced phase. Written informed con sent for the study was obtained from all patients. The study was approved by the Ethics Committee of Saint Orsola-Malpighi University Hospital, Bologna, Italy.
Busulfan pharmacokinetics
Plasma busulfan kinetics (64 patients) was assessed before administration, and at 15, 60, 120, 180, 240 min, after dose 9 (Day 3).19 Plasma busulfan concentrations were determined by HPLC (Perkin Elmer, USA).20 AUC was calculated by Kinetica software (Thermo Scientific, Waltham, MA, USA).
Genetic analysis
Forty polymorphisms at 27 loci were analyzed (Online Supplementary Tables S1 and S2). Thirty-six were genotyped using MassARRAY high-throughput DNA analysis (Sequenom Inc., San Diego, CA, US). Three (CBS rs72958776-68bpIns, GST-T1 and GST-M1 null alleles) were genotyped by PCR; GPX1 SNP rs1050450 was assessed by tetra-ARMS PCR.
In vitro study
Human hepatoma HepG2 cells were grown in DMEM 10% FBS (Life Technologies, Carlsbad, CA, USA). GSTA2-specific siRNA and GC-matched control siRNA were transfected by Lipofectamine 2000 (Life Technologies) to HepG2 cells (104/3 cm2 well, 72 h). Cells were exposed to busulafan (2.5–400 uM, 96 h) and cell death was assessed by Trypan blue.
Real-time PCR
mRNA was extracted by Trizol (Life Technologies). Real-time PCR primers and probes for GSTA2, interleukin-8 (IL8), constitutive androstane receptor (CAR), multidrug resistance-associated protein ABCC2, solute carrier organic anion transporters SLCO1B1 and SLCO1B3 and β-glucuronidase mRNAs were from Life Technologies. The amount of the target genes was calculated by the 2-ddcT method.
Luciferase assay
NF-kappaB luciferase21 reporter activity was assessed in HepG2 (1 mg/well, 24 h) by Dual-Luciferase® Reporter Assay System (Promega, Madison, WI, USA).
Statistical analysis
Cox proportional model and general linear model (GLM) for repeated measures were employed. P=0.00125 was considered significant in multiple comparisons. The analyses were performed by PASW18 (SPSS-IBM, NY). Haplotypes were estimated by Thesias (http://genecanvas.ecgene.net).22
Results
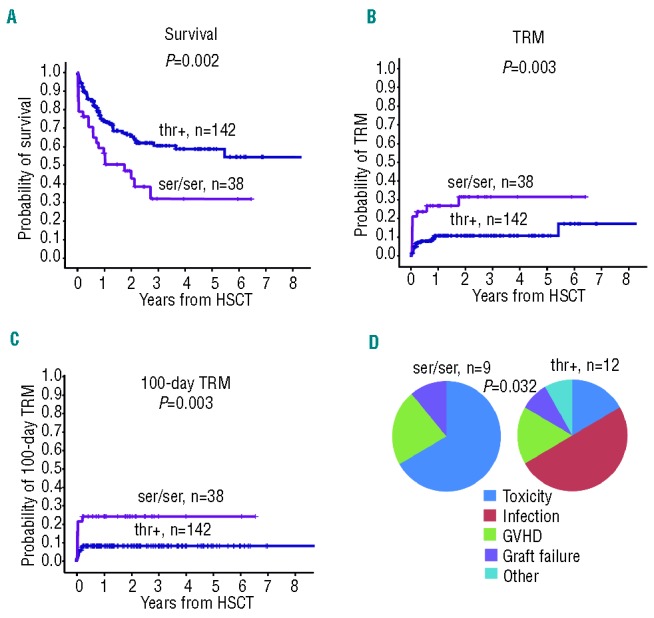
GSTA2 S112T locus impacts TRM and survival, but not GVHD and relapse, in patients receiving busulfan
Clinical characteristics of transplanted patients are reported in Table 1. Multivariate Cox regression was performed using the five clinical variables included in the EBMT score (age, sex mismatch, disease phase, type of donor, interval diagnosis-transplant) plus intensity of conditioning. The analysis revealed that disease phase at transplant (advanced vs. early) is a significant prognostic factor for both TRM (advanced vs. early, RR=2.689, 95%CI: 1.078–6.703; P=0.034) and overall survival (OS, advanced vs. early, RR=3.975, 95%CI: 2.262–6.986; P=1.61*10−6). All the genotypes investigated (Online Supplementary Tables S1 and S2) were singularly tested in a Cox model implementing clinical variables as covariates (Online Supplementary Table S3). The serine (ser) to threonine (thr) amino acid substitution at GSTA2 Codon 112 (S112T) was the only locus which remained significant for TRM after adjustment for multiple comparisons (n=40; P=0.001), while the effect on OS was only marginally significant (P=0.005) (Online Supplementary Table S3). GSTA2 is a glutathione transferase, which is expressed in the liver and metabolizes busulfan in cooperation with GSTA1.13 GSTA2 S112T genotypes were then recoded as dichotomous variables, namely serine homozygotes (ser/ser, n=38) and threonine carriers (thr+, n=142). We observed that GSTA2 S112T locus was predictive of OS (ser/ser vs. thr+, RR=2.388, 95%CI: 1.407–4.502; P=0.001), TRM (ser/ser vs. thr+, RR=4.912, 95%CI: 2.083–11.563; P=0.0002) and 100-day TRM (ser/ser vs. thr+, RR=5.185, 95%CI: 1.971–13.641; P=0.001) (Table 2). The Kaplan and Meyer plot showed the poorer survival and higher TRM of GSTA2 S112T ser/ser patients (Figure 1A–C). Furthermore, because deaths mostly occurred in the first post-transplant weeks, we also calculated 100-day TRM (Figure 1C). In line with expectations, the analysis of causes of 100-day TRM death revealed a significantly higher number of deaths due to conditioning-related toxicity23 in GSTA2 S112T ser/ser patients (6 of 9, 66.6%), compared to thr+ (2 of 12, 16.6%; P=0.032, Fisher exact test) (Figure 1D).
Table 2.
Multivariate Cox analysis of overall survival (OS), transplant-related mortality (TRM) and 100-day TRM.
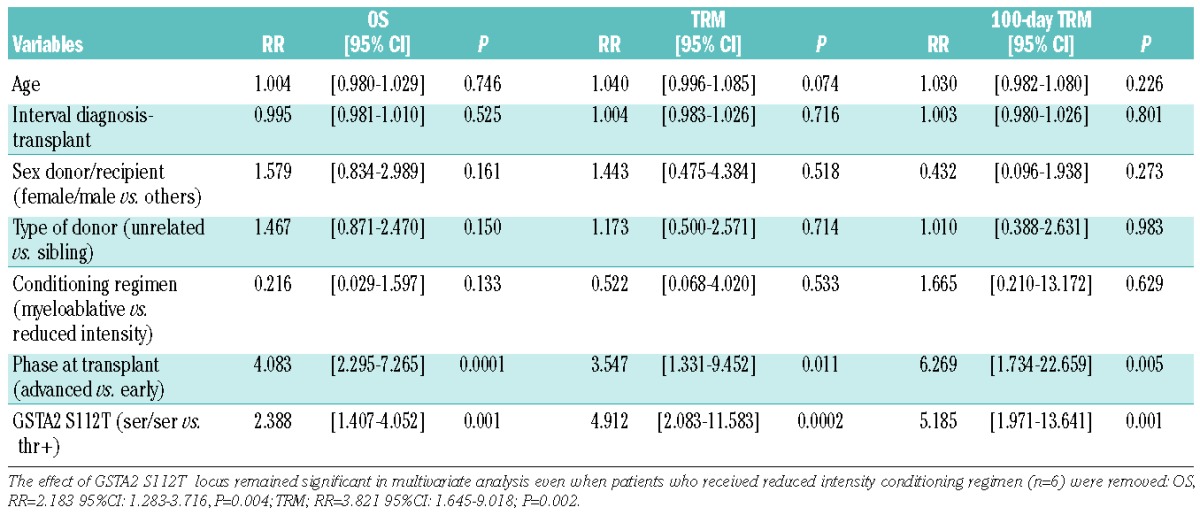
Figure 1.
GSTA2 S112T locus affects OS and TRM in patients prepared with busulfan. (A) Kaplan-Meyer analysis of OS, (B) TRM at any time and (C), 100-day TRM according to GSTA2 S112T genotypes (ser/ser, n=38; thr+, n=142); P values refer to log rank test; (D) pie-chart representation of 100-day TRM causes of death in ser/ser (n=9) and thr+ (n=12) patients. In particular, in serine homozygotes the organ toxicity was: liver (2 patients), liver and renal (1 patient), gut (2 patients), heart (1 patient). In threonine carriers the organ involvement was liver (1 patient), heart (1 patient).
Interestingly, GSTA2 S112T locus did not significantly affect either TRM nor OS in the comparator cohort (Online Supplementary Table S3), even when the analysis was performed separately on the subset of patients receiving myeloablative conditioning regimen (ser/ser, n=9 vs. thr+, n=52, OS: RR=0.361 95%CI: 0.115–2.201; P=0.361; TRM: RR=0.558 95%CI: 0.070–4.412; P=0.580) or reduced-intensity conditioning regimen (ser/ser, n=21 vs. thr+, n=64; OS: RR=0.891 95%CI: 0.493–2.237, P=0.891; TRM: RR=0.797 95%CI: 0.245–2.593; P=0.706).
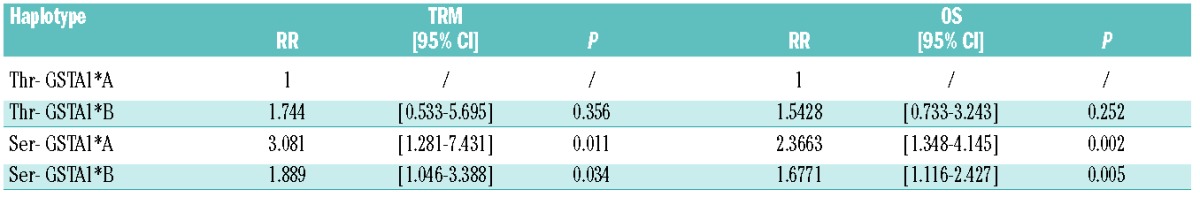
We also observed a weak association between TRM and other polymorphisms located at the GSTA1-GSTA2 genomic region, namely GSTA1 SNPs (rs1051775, rs3957356, rs4715332) (Online Supplementary Table S3). As previously reported,24,25 owing to the significant linkage disequilibrium among polymorphisms at GSTA2 and GSTA1 loci (Online Supplementary Table S4), the two loci haplotype frequency distribution of GSTA2 S112T and GSTA1 loci was estimated: the ser allele was highly associated with the so-called GSTA1*B rs3957356-rs4715332 functional haplotype25,26 (Online Supplementary Table S5). Nevertheless, survival analysis of GSTA2 S112T-GSTA1*A/B haplotypes reinforced the conclusion that GSTA1*B haplotype does not significantly modify the impact of the GSTA2 S112T locus on TRM and survival in our case set (Table 3 and Online Supplementary Table S6). Moreover, GSTA2 S112T locus does not affect relapse rate, which was instead associated with the phase at transplant (Online Supplementary Table S7). Furthermore, incidence of aGVHD grades III–IV and hepatic acute GVHD stages III–IV were similar between GSTA2 S112T ser/ser and thr+ patients (18.9% vs. 19.1%; P=0.591 and 5.4% vs. 5.6%; P=0.472, respectively).
Table 3.
Multivariate Cox analysis of TRM and OS according to GSTA2 S112T- GSTA1*A/B haplotypes.
Impact of the GSTA2 S112T locus on busulfan-AUC and the post transplant serum bilirubin level
GSTA2 S112T polymorphism is not located in the enzyme catalytic site but it has been supposed to affect protein stability.27 Busulfan is almost exclusively metabolized in the liver, where GSTA2 is expressed.15,28 Anova analysis using age, gender, BMI and source of HSC as covariates, revealed higher systemic exposure to busulfan (Day -3 AUC) in GSTA2 S112T ser/ser patients compared to thr+ (1214.36+570.06 vs. 838.10+282.40 mMol*min, F=9.185; P=0.001) (Figure 2A). Notably, BMI was not affected by S112T GSTA2 polymorphism, given that ser/ser patients show the same BMI distribution as thr+ patients, in each gender group (males: 27.70+2.62 vs. 26.5+3.85, females: 21.13+1.51 vs. 24,21+4.55; P=0.85, GLM analysis). Moreover, there was no difference in busulfan AUC between patients with BMI lower than/equal to 27 versus those with BMI greater than 27 (mean values: 871.00+343.09 vs. 903.96+355.21 mMol*min; P=0.29, adjusted for gender).
Figure 2.
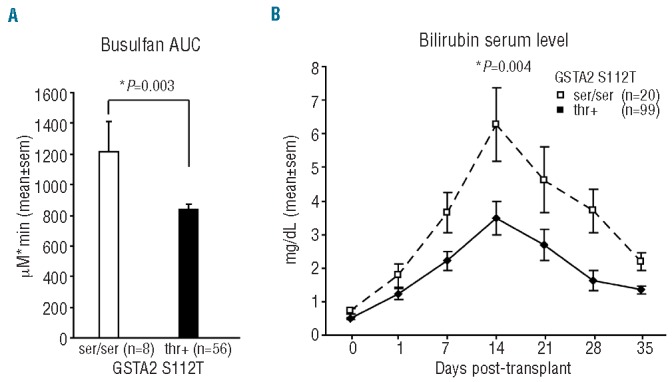
GSTA2 S112T locus impacts serum bilirubin and plasma busulfan levels. (A) Busulfan AUC in patients receiving busulfan according to GSTA2 S112T genotypes (ser/ser, n=8, thr+, n=56); P values refer to multivariate GLM adjusted for age, gender, BMI and source of HSC. (B) Bilirubin serum levels according to GSTA2 S112T genotypes (ser/ser, n=20, thr+, n=99) from pre-transplant to 35 days post-transplant; P value is referred to GLM Anova for repeated measures, adjusted for age, sex mismatch, interval from diagnosis to transplant, intensity of conditioning and disease phase at transplant.
Because glutathione depletion causes liver damage,18 we then evaluated the association between GSTA2 S112T SNP and liver function tests: total bilirubin, alanine transaminase, aspartate transaminase, cholinesterase, alkaline phosphatase and gamma-glutamyl transferase were examined weekly from the day of conditioning, up to Day 35 post transplant. Higher unfractioned plasma bilirubin levels for the first five weeks after transplant were found in GSTA2 S122T ser/ser patients compared to thr+ (GLM-estimated marginal means: 3.280+0.422 vs. 1.874+0.197 mg/dL, F=8.728; P=0.004) (Figure 2B). Interestingly, no association was found between GSTA2 S112T locus and all the other liver functional tests (Online Supplementary Table S8).
Exposure of human hepatoma cells to busulfan induces a pro-inflammatory response and reduces the expression of genes involved in bilirubin clearance
The data above suggest that, in patients receiving busulfan, the difference in post-transplant plasma bilirubin level between ser/ser versus thr+ patients is not correlated with the extent of liver necrosis. In this regard, negligible cell death was observed in human hepatoma cells HepG2 exposed to busulfan at concentrations comparable to those occurring in vivo, even when such cells were knocked down by a GSTA2-specific short interfering RNA oligonucleotide (siGSTA2) (Figure 3A). In HepG2 cells, busulfan administration elicited the upregulation of NF-kappaB, the master controller of the inflammatory response29 (Figure 3B). Moreover, siGSTA2 transfected HepG2 cells disclosed the upregulation of NF-kappaB activity and of the pro-inflammatory cytokine interleukin-8, compared to controls (Figure 3C).
Figure 3.
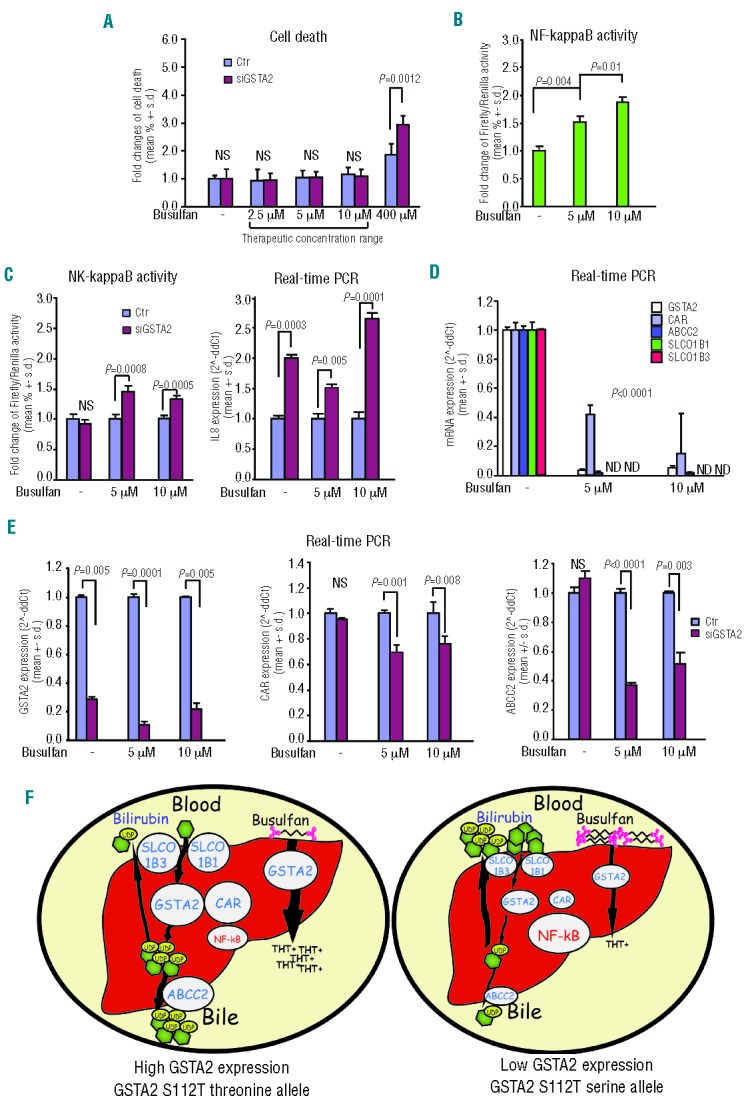
Exposure to busulfan and GSTA2 knockdown induce a pro-inflammatory response and reduce the expression of CAR, ABCC2, SLCO1B1 and SLCO1B3 in human hepatoma cells (HepG2). (A) Cell death analysis of HepG2 cells transfected with control (Ctr) or GSTA2 specific siRNA (siGSTA2) exposed to increasing busulfan concentrations; (B) NF-kappaB luciferase assay in HepG2 cells exposed to increasing busulfan concentrations; (C) NF-kappaB luciferase assay and Interleukin-8 (IL-8) real-time PCR analysis in Ctr/siGSTA2 transfected HepG2 cells exposed to increasing busulfan concentrations; (D) real-time PCR analysis of GSTA2, CAR, ABCC2 SLCO1B1 and SLCO1B3 mRNA level in Ctr/siGSTA2 transfected HepG2 cells exposed to increasing busulfan concentrations; (E) real-time PCR analysis of GSTA2, CAR and ABCC2 mRNA level in HepG2 cells exposed to increasing busulfan concentrations; (F) descriptive picture of the proposed mechanism: busulfan exposure triggers hepatic inflammatory activation which associates with the downregulation of genes involved in bilirubin metabolism, namely GSTA2, CAR, ABCC2, SLCO1B1, SCLO1B3. This phenomenon is amplified by decreased levels of GSTA2 and, speculatively, in serine GSTA2S112T allele carriers.
At cellular and systemic levels, inflammation reduces the expression of a variety of genes involved in liver bilirubin metabolism,29,30 namely constitutive androstane receptor31 (CAR), multidrug resistance associated protein MRP2/ABCC232 and solute organic carriers SLCO1B1 and SCLO1B3.32 We also included GSTA2 in this gene set, since it is the intra-hepatic bilirubin ligandin, a function which is independent of its enzymatic activity.33 We found that exposure of HepG2 cells to non-cytotoxic concentrations of busulfan elicited a dramatic decrease in GSTA2, CAR, ABCC2, SLCO1B1 and SCLO1B3 expression (Figure 3D). Notably, SLCO1B1 and SCLO1B3 became almost undetectable upon busulfan exposure. Moreover, in siGSTA2-transfected HepG2, we observed a further decrease in CAR and ABCC2 expression compared to controls (Figure 3E). These data led us to argue that the pro-inflammatory activation of busulfan-exposed hepatocytes causes the downregulation of GSTA2 and of bilirubin-metabolizing enzymes (Figure 3F). This metabolic reshaping is expected to impair bilirubin clearance in the post-transplant phase and to be dependent on the individual genetic landscape.
Discussion
In this paper, we report that the GSTA2 S112T polymorphism affects survival of HSCT patients receiving a busulfan-based conditioning regimen, serine allele homozygotes being more prone to higher transplant-related and overall mortality compared to threonine allele carriers. The frequency of GSTA2 S112T serine homozygotes reported here (21%) is close to that found in the Italian population34 (19.8%) and in Caucasians24 (18.5%), suggesting that the locus does not represent a risk factor for developing hematopoietic malignancies. Interestingly, the association of GSTA2 S112T with TRM and survival is not significant in the cohort of HSCT patients not receiving busulfan (comparator cohort). Therefore, the data suggest that the polymorphism changes the individual response to the drug. In support of this hypothesis, we report that GSTA2 S112T serine homozygotes show higher busulfan plasma levels compared to threonine carriers, indicating that such a group of patients displays a reduced busulfan clearance capability. In fact, busulfan metabolism mainly involves its conjugation with GSH in the liver, via alpha class cytosolic GST.12,13 Busulfan administration depletes the content of hepatocyte GSH, the co-factor of GST enzymes.18,35 Plasma GSH correlates with busulfan clearance capacity16 and hepatic GST activ ity negatively correlates with plasma busulfan and positively associates with its clearance.14 In line with these data, the association between high busulfan plasma levels and post-transplant mortality has already been described.7,8,10 Consistent with this, we found that the proportion of deaths due to drug toxicity in GSTA2 S112T serine homozygotes is higher than in other patients. Hence, the GSTA2 S112T locus may be regarded as a reliable predictor for individual susceptibility to the adverse effects of busulfan. It might also be argued that GSTA2 S112T locus can be used to tailor busulfan dosages. However, our series is based on the standard association of busulfan and cyclophosphamide, the GSTA2 S112T locus should be assessed in patients receiving other busulfan-based drugs combinations.
Our data do not confirm the previously reported association between busulfan AUC and GVHD.9 Insufficient power or heterogeneity of patients’ GVHD risk (i.e. type of donor, source of HSC, HLA distance) may explain these findings.
The GSTA2 S112T amino acid change is not located at the enzyme catalytic site.36 Moreover, serine and threonine residues are both polar non-charged amino acids and their reciprocal substitution is not expected to yield any predictable change in the protein structure.36 Nevertheless, in the liver, the GSTA2 S112T locus has been found to impact the GSTA2 protein level and themostability.24,27 These data suggest that the GSTA2 S112T locus affects the individual capability to metabolize busulfan, independently of the alteration of GSTA2 enzyme activity.
GSTA2 and GSTA1 share 95% amino acid identity but GSTA2 is endowed with lower busulfan-GSH conjugation activity.13,28 The GSTA2 S112T locus is closely associated with the functional (rs395376)-52bp GSTA1 polymorphism, which modifies the gene transcription rate.34 Consequently, the tight linkage disequilibrium among GSTA1 and GSTA2 polymorphisms24,25,37 might explain the functional association reported here. The GSTA1 promoter hosts functional *A and *B haplotypes:25,26,37 the GSTA1*B haplotype has been associated with 4-fold reduction of GSTA1 enzyme activity and higher busulfan plasma concentration.8,26,37–39 Though the GSTA2 S112T serine allele is in linkages with the GSTA1 *B haplotype,24–26 it seems to be the sole cause for the results here obtained. Even the GSTA2 thr-*B haplotype, which is expected to combine the low activity of both isoenzymes,24,25 seems not to impact patients’ survival. Intriguingly, the association between the GSTA1*B haplotype and reduced busulfan clearance was observed with oral but not intravenous administration.40,41 On the contrary, other authors failed to find any association between GSTA1 polymorphisms and busulfan metabolism.42,43 Nevertheless, our data support the role of GSTA1-GSTA2 genomic region in the inter-individual variability in busulfan metabolism.
Here we show that 35-day post-transplant serum bilirubin levels are increased in GSTA2 S112T serine homozygotes patients receiving a preparative regimen containing busulfan. Since GSTA2 S112T serine homozygotes do not display significantly different alterations in other liver function tests, we speculate that such patients might undergo specific alterations of bilirubin clearance. In this regard, busulfan is not hepatotoxic itself, at least within the therapeutic concentration range.44 According to this, we did not find cell death in human hepatoma cells exposed to therapeutic concentrations of busulfan, even upon siRNA-mediated GSTA2 knockdown. We observed that busulfan triggers the pro-inflammatory activation of human hepatoma cells. At cellular and systemic levels,29,30 inflammation has been linked to the downregulation of a variety of genes involved in bilirubin metabolism. In particular, we show here that busulfan down-regulates: i) CAR, the nuclear transcription factor for several liver bilirubin clearance genes;32 ii) MRP2/ABCC2, a member of the ATP-binding cassette family of membrane transporters localized at the apical border of hepatocytes, which is involved in the transport of conjugated bilirubin and glutathione trafficking to the bile;29 iii) SLCO1B1 and SLCO1B3, trans-membrane transporters at hepatocytes’ baso-lateral border, which control the uptake of conjugated and/or unconjugated serum bilirubin.32 Intriguingly, busulfan also down-regulates GSTA2 which is part of the bilirubin metabolism, owing to its ability to act as intra-hepatocytic ligandin, which prevents the backflow of hepatic bilirubin into blood circulation.31 In addition, we found that the exposure of GSTA2 knockdown cells elicits higher inflammatory activation and lower expression of CAR and ABCC2 compared to Controls. Since it has been previously reported that post-transplant interleukin-8 serum level is highly correlated with serum bilirubin,45 it can be hypothesized that the pro-inflammatory status, which develops in patients prepared with busulfan, might be linked to the reduced clearance of unconjugated bilirubin, as well as to the increase in serum bilirubin. Given this, these findings lead us to speculate that post transplant hyper-bilirubinemia in GSTA2 S112T serine homozygotes may be the consequence of their peculiar liability to the development of busulfan-dependent pro-inflammatory liver injury (Figure 3F).
In conclusion, our data suggest that the GSTA2 S112T polymorphism is predictive of transplant outcome in patients receiving busulfan in the preparative regimen, at least in association with cyclophosphamide. This was a single center retrospective study and this analysis warrants validation by a prospective clinical study to gauge the role of genotyping at GSTA S112T locus in the adjustment of busulfan dosages.
Acknowledgments
We thank Dr. Gabriele Grossi, Daniela Bastia and Dr. Chiara Fazio for technical assistance.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work has been supported by Progetto Università Regione 2008-2011, N. 1412: “HSCT transplant in the elderly” (G. Bandini and M. Bonafè), University of Bologna RFO funds, Cornelia Pallotti and Roberto Pallotti Foundation (M. Bonafè).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309(22):1347–53 [DOI] [PubMed] [Google Scholar]
- 2.Hansen JA, Chien JW, Warren EH, Zhao LP, Martin PJ. Defining genetic risk for graft-versus-host disease and mortality following allogeneic hematopoietic stem cell transplantation. Curr Opin Hematol. 2010;17(6):483–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien JW, Zhang XC, Fan W, Wang H, Zhao LP, Martin PJ, et al. Evaluation of published single nucleotide polymorphisms associated with acute graft versus host disease. Blood. 2012;119(22):5311–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullally A, Ritz J. Beyond HLA: the significance of genomic variation for allogeneic hematopoietic stem cell transplantation. Blood. 2007;109(4):1355–62 [DOI] [PubMed] [Google Scholar]
- 5.Hansen JA, Petersdorf EW, Lin MT, Wang S, Chien JW, Storer B, et al. Genetics of allogeneic hematopoietic cell transplantation. Role of HLA matching, functional variation in immune response genes. Immunol Res. 2008;41(1):56–78 [DOI] [PubMed] [Google Scholar]
- 6.Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(5):523–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89(9):3055–60 [PubMed] [Google Scholar]
- 8.Gaziev J, Nguyen L, Puozzo C, Mozzi AF, Casella M, Perrone Donnorso M, et al. Novel pharmacokinetic behaviour of intravenous busulfan in children with thalassemia undergoing hematopoietic stem cell transplantation: a prospective evaluation of pharmacokinetic and pharmacodynamic profile with therapeutic drug monitoring. Blood. 2010;115(22):4597–604 [DOI] [PubMed] [Google Scholar]
- 9.Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8(9):477–85 [DOI] [PubMed] [Google Scholar]
- 10.Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE, et al. Association of busulfan area under the curve with veno-occlusive disease following HSCT. Bone Marrow Transplant. 1996;17(2):225–30 [PubMed] [Google Scholar]
- 11.McCune JS, Gibbs JP, Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000;39(2):155–65 [DOI] [PubMed] [Google Scholar]
- 12.Gibbs JP, Czerwinski M, Slattery JT. Busulfan-glutathione conjugation catalyzed by human liver cytosolic glutathione S-transferases. Cancer Res. 1996;56(16):3678–81 [PubMed] [Google Scholar]
- 13.Czerwinski M, Gibbs JP, Slattery JT. Busulfan conjugation by glutathione S-transferases alpha, mu, and pi. Drug Metab Dispos. 1996;24(9):1015–9 [PubMed] [Google Scholar]
- 14.Poonkuzhali B, Chandy M, Srivastava A, Dennison D, Krishnamoorthy R. Glutathione S-transferase activity influences busulfan pharmacokinetics in patients with beta thalassemia major undergoing bone marrow transplantation. Drug Metab Dispos. 2001;29(3):264–7 [PubMed] [Google Scholar]
- 15.Hassan M, Ljungman P, Ringdén O, Hassan Z, Oberg G, Nilsson C, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant. 2000;25(9):915–24 [DOI] [PubMed] [Google Scholar]
- 16.Almog S, Kurnik D, Shimoni A, Loebstein R, Hassoun E, Gopher A, et al. Linearity and stability of intravenous busulfan pharmacokinetics and the role of glutathione in busulfan elimination. Biol Blood Marrow Transplant. 2011;17(1):117–23 [DOI] [PubMed] [Google Scholar]
- 17.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30(1–2):42–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis. 2002;22(1):27–42 [DOI] [PubMed] [Google Scholar]
- 19.Vaughan WP, Carey D, Perry S, Westfall AO, Salzman DE. A limited sampling strategy for pharmacokinetic directed therapy with intravenous busulfan. Biol Blood Marrow Transplant. 2002;8(11):619–24 [DOI] [PubMed] [Google Scholar]
- 20.Rifai N, Sakamoto M, Lafi M, Guinan E. Measurement of plasma busulfan concentration by high-performance liquid chromatography with ultraviolet detection. Therapeutic Drug Monitoring. 1997;19(2):169–74 [DOI] [PubMed] [Google Scholar]
- 21.Storci G, Sansone P, Mari S, D’Uva G, Tavolari S, Guarnieri T, et al. TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype. J Cell Physio. 2010;225(3):682–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tregouet DA, Garelle V. A new JAVA interface implementation of THESIAS: Testing haplotype Effects In Association Studies. Bioinformatics. 2007;23(8):1038–9 [DOI] [PubMed] [Google Scholar]
- 23.Bearman SI. Toxicities of stem cell transplantation regimens. In: Atkinson K, Champlin R, Ritz J, Fibbe WE, Ljungman P and Brenner MK, eds. Clinical bone marrow and blood stem cell transplantation. Cambridge: Cambridge University Press; 2004. p. 1301–56 [Google Scholar]
- 24.Ning B, Wang C, Morel F, Nowell S, Ratnasinghe DL, Carter W, et al. Human glutathione S-transferase A2 polymorphisms: variant expression, distribution in prostate cancer cases/controls and a novel form. Pharmacogenetics. 2004;14(1):35–44 [DOI] [PubMed] [Google Scholar]
- 25.Maekawa K, Hamaguchi T, Saito Y, Tatewaki N, Kurose K, Kaniwa N, et al. Genetic variation and haplotype structures of the glutathione S-transferase genes GSTA1 and GSTA2 in Japanese colorectal cancer patients. Drug Metab Pharmacokinet. 2011;26(6):646–58 [DOI] [PubMed] [Google Scholar]
- 26.Coles BF, Morel F, Rauch C, Huber WW, Yang M, Teitel CH, et al. Effect of polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and GSTA2 expression. Pharmacogenetics. 2001;11(8):663–9 [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Modén O, Mannervik B. Differences among allelic variants of human glutathione transferase A2-2 in the activation of azathioprine. Chem Biol Interact. 2010;186(2):110–7 [DOI] [PubMed] [Google Scholar]
- 28.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88 [DOI] [PubMed] [Google Scholar]
- 29.Pasparakis M. Regulation of tissue home-ostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9(11):778–88 [DOI] [PubMed] [Google Scholar]
- 30.Kawase A, Tsunokuni Y, Iwaki M. Effects of Alterations in CAR on Bilirubin Detoxification in Mouse Collagen-Induced Arthritis. Drug Metab Dispos. 2007;35(2):256–61 [DOI] [PubMed] [Google Scholar]
- 31.Assenat E, Gerbal-Chaloin S, Larrey D, Saric J, Fabre JM, Maurel P, et al. Interleukin 1beta inhibits CAR-induced expression of hepatic genes involved in drug and bilirubin clearance. Hepatology. 2004;40(4):951–60 [DOI] [PubMed] [Google Scholar]
- 32.van de Steeg E, Stránecký V, Hartmannová H, Nosková L, Hřebíček M, Wagenaar E, et al. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest. 2012;122(2):519–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang W, Zhang J, Chua SS, Qatanani M, Han Y, Granata R, et al. Induction of bilirubin clearance by the constitutive androstane receptor (CAR). Proc Natl Acad Sci USA. 2003;100(7):4156–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landi S, Gemignani F, Neri M, Barale R, Bonassi S, Bottari F, et al. Polymorphisms of glutathione-S-transferase M1 and manganese superoxide dismutase are associated with the risk of malignant pleural mesothelioma. Int J Cancer. 2007;120(12):2739–43 [DOI] [PubMed] [Google Scholar]
- 35.Goekkurt E, Stoehlmacher J, Stueber C, Wolschke C, Eiermann T, Iacobelli S, et al. Pharmacogenetic analysis of liver toxicity after busulfan/cyclophosphamide-based allogeneic hematopoietic stem cell transplantation. Anticancer Res. 2007;27(6C):4377–80 [PubMed] [Google Scholar]
- 36.Tetlow N, Board PG. Functional polymorphism of human glutathione transferase A2. Pharmacogenetics. 2004;14(2):111–6 [DOI] [PubMed] [Google Scholar]
- 37.Morel F, Rauch C, Coles B, Le Ferrec E, Guillouzo A. The human glutathione transferase alpha locus: genomic organization of the gene cluster and functional characterization of the genetic polymorphism in the hGSTA1 promoter. Pharmacogenetics. 2002;12(4):277–86 [DOI] [PubMed] [Google Scholar]
- 38.Kusama M, Kubota T, Matsukura Y, Matsuno K, Ogawa S, Kanda Y, et al. Influence of glutathione S-transferase A1 polymorphism on the pharmacokinetics of busulfan. Clin Chim Acta. 2006;368(1–2):93–8 [DOI] [PubMed] [Google Scholar]
- 39.Johnson L, Orchard PJ, Baker KS, Brundage R, Cao Q, Wang X, et al. Glutathione S-transferase A1 genetic variants reduce busulfan clearance in children undergoing hematopoietic cell transplantation. J Clin Pharmacol. 2008;48(9):1052–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SD, Lee JH, Hur EH, Lee JH, Kim DY, Lim SN, et al. Influence of GST gene polymorphisms on the clearance of intravenous busulfan in adult patients undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(8):1222–30 [DOI] [PubMed] [Google Scholar]
- 41.Abassi N, Vadnais B, Knutson JA, Blough DK, Kelly EJ, O’Donnell PV, et al. Pharmacogenetics of intravenous and oral busulfan in hematopoietic cell transplant recipients. J Clin Pharmacol. 2011;51(10):1429–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ansari M, Lauzon-Joset JF, Vachon MF, Duval M, Théoret Y, Champagne MA, et al. Influence of GST gene polymorphisms on busulfan pharmacokinetics in children. Bone Marrow Transplant. 2010;45(2):261–7 [DOI] [PubMed] [Google Scholar]
- 43.Zwaveling J, Press RR, Bredius RG, van Derstraaten TR, den Hartigh J, Bartelink IH, et al. Glutathione S-transferase polymorphisms are not associated with population pharmacokinetic parameters of busulfan in pediatric patients. Ther Drug Monit. 2008; 30(4):504–10 [DOI] [PubMed] [Google Scholar]
- 44.DeLeve LD, Wang X. Role of oxidative stress and glutathione in busulfan toxicity in cultured murine hepatocytes. Pharmacology. 2000;60(3):143–54 [DOI] [PubMed] [Google Scholar]
- 45.Ferrà C, de Sanjosé S, Gallardo D, Berlanga JJ, Rueda F, Marìn D, et al. IL-6 and IL-8 levels in plasma during hematopoietic progenitor transplantation. Haematologica. 1998;83(12):1082–7 [PubMed] [Google Scholar]


