The Philadelphia chromosome-negative myeloproliferative neoplasm (MPN) primary myelofibrosis (PMF) is characterized by progressive accumulation of connective tissue and endothelial proliferation in the bone marrow accompanied by extramedullary hematopoiesis with enlargement of the spleen and liver.1,2 Patients with PMF have a poor prognosis with a median survival of 6.5 years.3 When myelofibrosis develops during the course of polycythemia vera (PV) and essential thrombocythemia (ET), the conditions are termed post-PV myelofibrosis (PPV-MF) and post-ET MF (PET-MF), respectively;4 both have a dismal prognosis.
During recent years, a number of new agents have been studied in clinical phase II and III trials on MPNs. Histone deacetylase inhibition (HDACi) has shown a potent inhibitory activity on the autonomous proliferation of hematopoietic cells of PV and ET patients carrying the JAK2V617F mutation5 and reports have recently documented clinical efficacy of the HDACis vorinostat6 and panobinostat in MF.7,8 Therefore, this agent seems to be an obvious candidate drug to be evaluated in myelofibrosis and this prompted us to conduct a phase II study.
The primary objective of the study was to investigate whether vorinostat as monotherapy in patients with MF could induce a clinical response according to the International Working Group (IWG) response criteria9 at the end of an intervention and an observation period, respectively. A secondary objective was to investigate whether treatment with vorinostat influenced the JAK2V617F mutant allele burden and quality of life as assessed by the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF)10 and the EORTC QLQ-C30. Diagnostic criteria for PMF and PET-MF and PPV-MF were according to WHO criteria11 and the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT), respectively.4 Vorinostat 400 mg was administered once daily for 24 weeks after which patients were observed for an additional 12 weeks without vorinostat. Patients who required dose modification to less than 300 mg were withdrawn from the study. Hydroxyurea was permitted to control platelet count if the patient developed ischemic symptoms (e.g. transitory cerebral ischemia or completed stroke) or progression of microcirculatory ischemic symptoms at any platelet level. DNA purification and quantitative real-time polymerase chain reaction (qPCR) were performed as described12 and all patients (n=7) from one center (Roskilde) were given MPN-SAF and EORTC-QLQ-C30 questionnaires at baseline and again after 12 weeks of therapy.
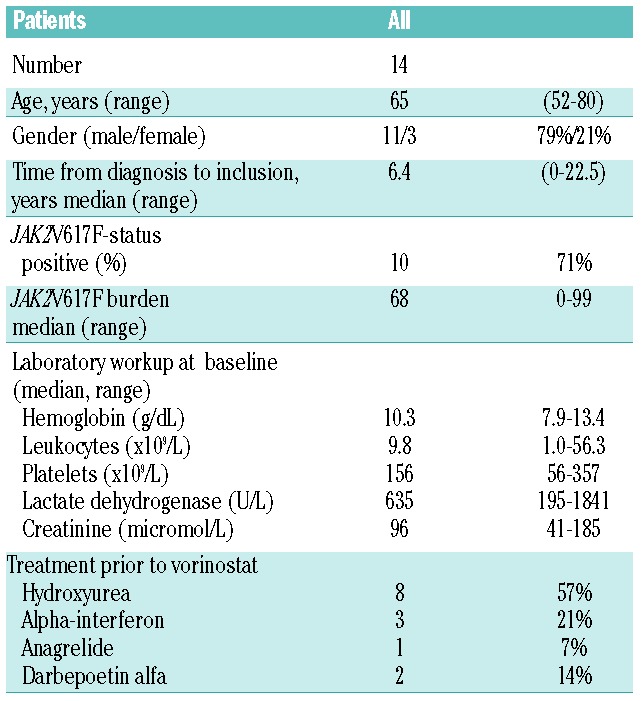
Fourteen patients (11 PMF and 3 PPV-MF) were included in the study after giving written informed consent. All patients had palpable splenomegaly (median 13.5 cm, range 2–28). With regard to symptoms, 43% reported fatigue and 29% reported weight loss during the six months prior to inclusion. Patients’ characteristics and laboratory data at baseline are listed in Table 1.
Table 1.
Demographic data for included patients.
Eight patients (57%) were followed to hospital visit n. 8 (i.e. end of intervention period). Five of these patients had been treated with vorinostat as monotherapy and the other 3 had also received concomitant HU. An intention-to-treat (ITT) response rate of 14% was observed: 2 of 14 patients, one in complete remission (CR) and one with clinical improvement (CI). None of the concomitantly treated patients experienced a clinical response at the end of intervention. In patients completing the intervention period on vorinostat only, we were able to compare spleen sizes to base-line values for 4 patients. In 4 of 4 patients, we observed a decrease (median 4.5 cm, range 1–14) from a median of 12 cm (range 4–28) at baseline to a median of 5 cm (0–23) after 24 weeks of therapy (P=0.12). We did not observe any significant changes in body weight during treatment. Six patients (43% of total) were evaluable for clinico-hematologic response after hospital visit n. 11 (completion of intervention and observation period). Of the patients evaluable for response assessment who had only been treated with vorinostat in the intervention period, ITT response was 0%. Unfortunately, the ability of vorinostat to increase hemoglobin and/or alter transfusion requirements was not assessable due to frequent dose adjustments in these patients; of the 14 patients included, only one patient did not have a dose reduction during the 24 weeks of therapy and, furthermore, 7 of 14 patients received blood transfusions during the intervention period.
Quantitative JAK2 analyses after 12 weeks of therapy were compared with base-line data for 8 patients. Eighty-eight percent of patients (7 of 8) experienced an increase in JAK2V617F tumor allele burden (P=0.04). Numerical median increase was 3.2%.
We did not observe any significant differences in MPN-SAF total symptom scores or EORTC QLQ-C30 functional scales or symptom scales combined. However, patients reported statistically significant increases in the severity of both “nausea and vomiting” and “abdominal discomfort” when analyzing single items from the EORTC-QLQ-C30 and MPN-SAF, respectively (P=0.03).
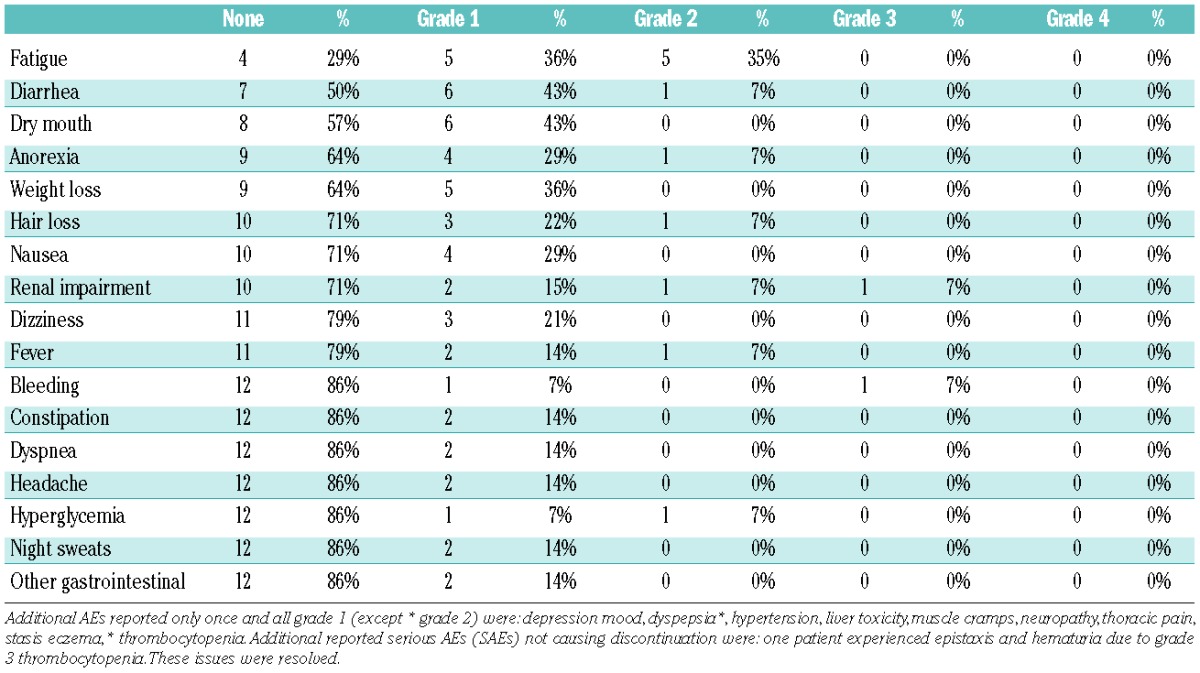
An overview of all reported adverse events (AEs) that did not lead to discontinuation of therapy is provided in Table 2. Seventy-one percent of all patients reported fatigue at least once during the intervention period (maximum grade 2) and 50% reported diarrhea (maximum grade 2).
Table 2.
All reported adverse events (AEs) listed in regard to prevalence.
The causes of discontinuation in the intervention period included “progressive disease” (PD, n=1) and non-compliance (n=1) whereas 4 patients discontinued due to a combination of grade 1–3 non-hematologic AEs. Two patients discontinued therapy in the observation period; one transformed to AML and one died due to klebsiella pneumonia.
In conclusion, vorinostat monotherapy in myelofibrosis at a dosage of 400 mg is associated with significant adverse effects. These do not allow long-term administration of therapy despite the fact that splenomegaly partially regressed in a proportion of patients. Based upon our findings, future studies of HDACi should exploit a lower dosage design that might allow for prolonged treatment.8 In addition, combination therapies (e.g. HDACi and JAK-inhibitor or HDACi and DNA-hypomethylating agents) might allow lower dosages of each agent to be used for longer intervention periods. Gene expression profiling and epigenome studies during treatment with vorinostat are ongoing in an attempt to clarify particular patterns of gene and epigenome deregulation which might contribute to a better understanding of the pathogenetic mechanisms accounting for the observed clinical and biochemical effects of vorinostat in our patient cohort.
Acknowledgments
The authors wish to express their gratitude and warmest appreciation to the following research nurses who were indispensable in carrying out this study: Hanne Bagger Christiansen (Roskilde), Trine Løw (Roskilde), Carsten Larsen (Odense) and Julie Schønemann (Copenhagen). The authors also wish to thank molecular biologist Karin de Stricker, (Odense) for skillful technical assistance in sample handling and quantitative JAK2 analysis.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355(23):2452–66 [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342(17):1255–65 [DOI] [PubMed] [Google Scholar]
- 3.Cervantes F, Dupriez B, Passamonti F, Vannucchi AM, Morra E, Reilly JT, et al. Improving survival trends in primary myelofibrosis: an international study. J Clin Oncol. 2012;30(24):2981–7 [DOI] [PubMed] [Google Scholar]
- 4.Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22(2):437–8 [DOI] [PubMed] [Google Scholar]
- 5.Guerini V, Barbui V, Spinelli O, Salvi A, Dellacasa C, Carobbio A, et al. The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2(V617F). Leukemia. 2008;22(4):740–7 [DOI] [PubMed] [Google Scholar]
- 6.Lee J. Clinical efficacy of vorinostat in a patient with essential thrombocytosis and subsequent myelofibrosis. Ann Hematol. 2009;88(7):699–700 [DOI] [PubMed] [Google Scholar]
- 7.Deangelo DJ, Mesa RA, Fiskus W, Tefferi A, Paley C, Wadleigh M, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol. 2013;162(3):326–35 [DOI] [PubMed] [Google Scholar]
- 8.Mascarenhas J, Lu M, Li T, Petersen B, Hochman T, Najfeld V, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF). Br J Haematol. 2013;161(1):68–75 [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood. 2006;108(5):1497–503 [DOI] [PubMed] [Google Scholar]
- 10.Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–8 [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22 [DOI] [PubMed] [Google Scholar]
- 12.Andersen CL, McMullin MF, Ejerblad E, Zweegman S, Harrison C, Fernandes S, et al. A phase II study of vorinostat (MK-0683) in patients with polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2013;162(4):498–508 [DOI] [PubMed] [Google Scholar]


