Abstract
In this study, we analyzed the influence of mesenchymal stromal cells derived from lymph nodes of non-Hodgkin’s lymphomas, on effector functions and differentiation of Vdelta (δ)2 T lymphocytes. We show that: i) lymph-node mesenchymal stromal cells of non-Hodgkin’s lymphoma inhibit NKG2D-mediated lymphoid cell killing, but not rituximab-induced antibody-dependent cell-mediated cytotoxicity, exerted by Vδ2 T lymphocytes; ii) pre-treatment of mesenchymal stromal cells with the aminobisphosphonates pamidronate or zoledronate can rescue lymphoma cell killing via NKG2D; iii) this is due to inhibition of transforming growth factor-β and increase in interleukin-15 production by mesenchymal stromal cells; iv) aminobisphosphonate-treated mesenchymal stromal cells drive Vδ2 T-lymphocyte differentiation into effector memory T cells, expressing the Thelper1 cytokines tumor necrosis factor-α and interferon-γ. In non-Hodgkin’s lymphoma lymph nodes, Vδ2 T cells were mostly naïve; upon co-culture with autologous lymph-node mesenchymal stromal cells exposed to zoledronate, the percentage of terminal differentiated effector memory Vδ2 T lymphocytes increased. In all non-Hodgkin’s lymphomas, low or undetectable transcription of Thelper1 cytokines was found. In diffused large B-cell lymphomas and in a group of follicular lymphoma, transcription of transforming growth factor β and interleukin-10 was enhanced compared to non-neoplastic lymph nodes. Thus, in non-Hodgkin lymphomas mesenchymal stromal cells interfere with Vδ2 T-lymphocyte cytolytic function and differentiation to Thelper1 and/or effector memory cells, depending on the prominent in situ cytokine milieu. Aminobisphosphonates, acting on lymph-node mesenchymal stromal cells, can push the balance towards Thelper1/effector memory and rescue the recognition and killing of lymphoma cells through NKG2D, sparing rituximab-induced antibody-dependent cell-mediated cytotoxicity.
Introduction
Gammadelta (γδ) T cells are unconventional T lymphocytes involved in stress response to injured, infected or transformed tissues.1,2 The majority of circulating γδ T lymphocytes belong to the Vδ2 subset and are able to recognize unprocessed non-peptide molecules, namely phosphoantigens (PAg) derived via the mevalonate or the 1-deoxy-D-xylolose-5-phosphate pathway in mammalian or bacterial cells, respectively1–5 γδ T cells also bind to stress-inducible MHC-class I related MICA and MICB molecules, and UL16-binding proteins (ULBPs) induced or up-regulated at the cell surface by viral infections or tumor transformation.6–8 Recognition of these molecules, also called NKG2D ligands (NKG2D-L), is mediated by the NKG2D receptor, expressed on both αβ and γδ T cells.7,9–11 Another type of γδ T-cell activation is represented by antibody-opsonized cells or micro-organisms through the binding of IgG Fc by the Fcγ receptor III CD16, which mediates the so-called antibody-dependent cell-mediated cytotoxicity (ADCC).2,12 Upon activation, γδ T cells also secrete pro-inflammatory and anti-tumor Th1 cytokines, including interferon (IFN)γ and tumor necrosis factor (TNF)α.1,2 Due to their peculiar antigen recognition and mechanism of activation, all γδ T cells are thought to participate in anti-tumor surveillance in several cancer types, including hematologic malignancies.6,8,13–18 Moreover, different drugs can be exploited to enhance each mechanism of γδ T-cell activation. First, aminobisphosphonates (N-BPs) commonly used to treat bone diseases and hypercalcemia in myeloma patients, have been shown to activate Vδ2 T cells by blocking protein prenylation along the cholesterol synthesis pathway and accumulating phosphorylated metabolites.3–5,19–22 Second, transretinoic acid and sodium valproate, employed in the treatment of acute myeloid leukemias, can induce surface expression of MICA/B and some ULBPs.1,8,23–25 Third, the anti-CD20 monoclonal antibody (mAb) rituximab, included in recent years in the therapeutic schemes for chronic lymphocytic leukemias (CLL) and B-cell lymphomas, can trigger ADCC in Vδ2 T cells.12,21,26 In addition, stimulation by PAg, accumulated in dendritic and also in cancer cells upon exposure to N-BPs, drives Vδ2 T-cell maturation from naive to effector-memory (EM) cells, many of which express CD16 at the cell surface.12,19,27 In this regard, we and others have described that γδ T lymphocytes are involved in the surveillance against acute myeloid leukemias, multiple myeloma, CLL, Hodgkin’s (HL) and non-Hodgkin’s lymphomas (NHL)13–26 by the means of one or another of the abovementioned mechanisms (i.e. PAg recognition, cytotoxicity of targets expressing stress-related molecules, ADCC). In turn, the tumor microenvironment can inhibit the development of an efficient anti-tumor response.12,28–30 In particular, we have recently described that γδ T cells from the lymph nodes (LN) of HL patients co-cultured in vitro with autologous lymph-node derived mesenchymal stromal cells (LNMSC) strongly reduced their cytolytic activity against NKG2D-L+ HL target cells.31
Here, LNMSC from NHL lymph nodes have been derived to study their impact on effector functions and differentiation of Vδ2 anti-tumor T lymphocytes. Furthermore, we have analyzed whether N-BPs can affect the LNMSC-mediated influence on Vδ2 T cells. We found that: i) LNMSC selectively inhibit NKG2D-mediated lymphoid cell killing, but not rituximab-mediated ADCC, exerted by Vδ2 T cells; ii) NKG2D-dependent killing is rescued upon pre-treatment of LNMSC with the N-BPs pamidronate or zoledronate; ii) the recovery is due to inhibition of TGFβ and increase in interleukin (IL)15 produced by LNMSC; iii) N-BPs-treated LNMSC drive Vδ2 T-lymphocyte differentiation into EM cells, producing Th1-type cytokines in vitro.
In all NHL LN specimens, low or undetectable transcription of Th1 cytokines (TNFα, IFNγ) was found whereas transcription of TGFβ and IL10 was enhanced, compared to non-neoplastic LN. Moreover, at the tumor site, Vδ2 T cells were mostly naïve. Upon co-culture with autologous LNMSC exposed to zoledronate, the percentage of Vδ2 T cells increased and displayed terminal differentiated EM phenotype.
Methods
Patient
Forty-eight patients with NHL were analyzed at the Department of Hematology University of Genoa, Italy: 30 follicular lymphomas (FL), 18 diffuse large B-cell lymphomas (DLCL) (Online Supplementary Table S1).32 LN bioptic specimens were obtained under diagnostic procedures, after informed consent and IRB approval (n. 0026910/07, 03/2009). Paraffin-embedded LN samples were processed at the Pathology Department. Fifteen non-neoplastic LN were also studied.
Methods
Zoledronic acid was provided (Zol) by Novartis Pharma (MTA 37318, Basel, Switzerland,). Pamidronate (Pam), mevastatin (Meva) and mevalonate were from Sigma Chemical Co. Vδ2T cells were isolated using anti-Vδ2 (BB3) mAb and EasySep kit (Stemcell Technologies). Purity of Vδ2T cells was over 99%. LNMSC were isolated and cultured as described (Online Supplementary Appendix).30,31 Co-cultures of LNMSC and Vδ2T lymphocytes, or LNMSC and autologous LN cell suspensions were perfomed at the ratio of 1:10.31 LNMSC were used either untreated or pre-treated for 12 h with Pam 5 μM or Zol 1 μM, or 48 h with Meva 10 μM, and N-BPs added during the last 12 h. Cells were washed prior to co-cultures. N-PBs doses have been chosen as effective on γδ T-cell proliferation and absence of toxic effects, according to the literature,5,20 determined in preliminary experiments. The morphological effects of mevastatin on LNMSC were evaluated by confocal analysis of actin rearrangement (Online Supplementary Appendix). 30 The effects of N-BPs on LNMSC viability were assessed using MTT assay. In some experiments, the anti-TGFβ mAb (1D11, R&D System) was added to co-cultures. Supernatants (SN) were recovered for cytokine measurement after 48 h. Vδ2 T cells were evaluated for cytotoxicity (Day 5), cell proliferation or phenotype (Days 5, 10 and 14). After labeling of Vδ2T cells with CFSE, proliferation was measured by FACS analysis calculating the decrease in green fluorescence intensity. Data were analyzed with Modfit 3.1 computer program (Verity Software House). Immunofluorescence was performed as described.31 Samples were run on CyAnADP flowcytometer (Beckman Coulter). Results are expressed as log mean fluorescence intensity (MFI) or percentage of positive cells. TGFβ, IL15, IL10, TNFα or IFNγ were quantified by ELISA (eBioscience or PeproTech), compared to a standard curve. Cytolytic activity of Vδ2T cells was analyzed in a 4-h51Cr-release assay against the C1R or the MICA-transfected C1R-MICA (provided by A. Steinle, Frankfurt, Germany) or LYB8 or KARPAS human cell lines, or untreated or N-PBs treated LNMSC. In some samples, effector cells were exposed to anti-NKG2D mAb prior to the cytotoxicity assay.23 For redirected killing, the P815 murine target cell line was used in the presence of anti-NKG2D or anti-CD16 mAb.33 ADCC assay was performed using the anti-CD20 antibody rituximab and the CD20+ cell lines C1R or C1R-MICA+ as targets. Also, Vδ2 T cells were used as effector cells after five days of co-culture with either untreated or N-BPs-treated LNMSC. RNA extraction and cDNA synthesis were performed as described.31 Q-RT-PCR for TGFβ, IL4, IL12, IL15, IL10, TNFα, IFNγ, GATA3, TXB21, STAT1, STAT5, STAT6 was performed on the 7900HT FastRT-PCR system (Applied Biosystems) with fluorescent Taqman method. mRNAs were normalized to RPLP0, referred to a standard curve and results were expressed as ΔCT.34,35 Statistical analysis was performed using a two-tailed Student’s t-test. Further details are available in the Online Supplementary Appendix.
Results
LNMSC inhibit NKG2D-mediated lymphoid cell killing but not ADCC
Data from 48 NHL patients (30 FL, 18 DLCL), who had been classified according to the WHO classification, were analyzed (Online Supplementary Table S1).32
Co-culture of Vδ2 T lymphocytes with LNMSC (Online Supplementary Appendix and Online Supplementary Figure S1) was performed as described (Online Supplementary Appendix).31 On Day 5, Vδ2 T cells were recovered and used in a redirected killing assay against the FcγR+ P815 cell line (Figure 1A), or in ADCC assay against the C1R NKG2D-L+ CD20+ lymphoid cell line (Figure 1B and C). The redirected killing assay was used to explore the efficiency of a given receptor expressed by T cells in delivering an activation signal. When an antibody to a triggering receptor (such as NKG2D) is added, cell lysis can be activated due to the bridge occurring between target (through FcγR) and effector cells (re-directed killing). The C1R NKG2D-L+CD20+ cell line was used to mimic lymphoid cells recognized by Vδ2 T cells through NKG2D activating receptor and to check the effectiveness in ADCC assay to the therapeutic anti-CD20 antibody rituximab. As Vδ2 T cells were sorted by positive anti-TCR selection, this procedure might have provided a stimulus making cells more responsive to NKG2D stimulation. Nevertheless, Vδ2T cells were used five days after the separation and cultured (in the presence or not of untreated or N-BPs-treated LNMSC) in IL2-containing medium (Online Supplementary Appendix). We found that basal cytolytic activity exerted by Vδ2 T cells against P815 was extremely low (approx. 5%) (Figure 1A) as expected, since the murine P815 cell line is not a good target for human T cells. Interestingly, in the presence of anti-NKG2D or anti-CD16 mAb, cytotoxicity rose up to 25% and 55%, respectively (Figure 1A). An unrelated mAb matched for isotype did not trigger P815 cell lysis (data not shown).
Figure 1.
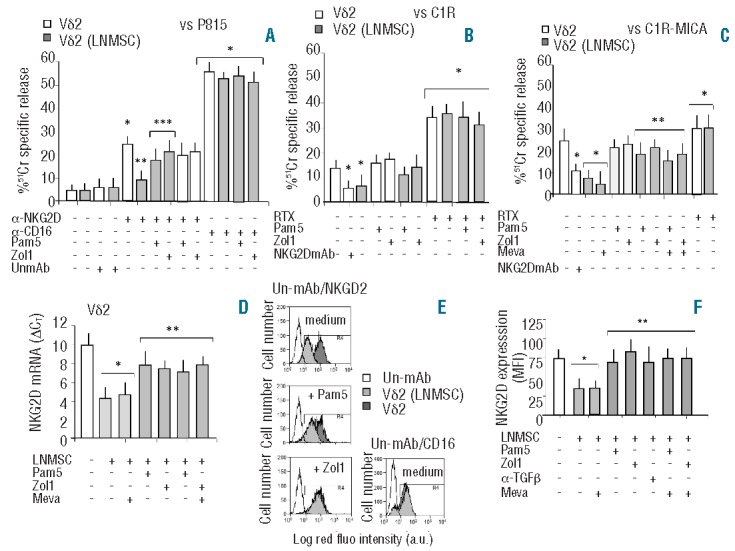
N-BPs prevent the inhibition of NKG2D-mediated lymphoid cell killing and NKG2D downregulation induced by LNMSC on Vδ2 T cells. Vδ2 T cells isolated from PBMC were cultured with IL2 alone (white columns) or with IL2 and either untreated LNMSC or LNMSC pre-treated for 12 h with Pam (5 μg/mL) or Zol (1 μg/mL) at the 1:10 LNMSC:Vδ2 T ratio (gray columns). Some samples were treated for 48 h with mevastatin (Meva, 10 μM) and Zol added during the last 12 h. In some experiments, Vδ2 T cells were pre-treated with Pam or Zol in order to exclude any possible direct effect of NBPs on these cells. On Day 5, Vδ2 T cells were harvested and used in a redirected killing assay against the P815 cell line in the presence of the anti-NKG2D or the anti-CD16 mAb or an unrelated mAb (UnmAb) matched for the isotype (all at 5 μg/mL) (A), or in an ADCC assay against the C1R (B), or the C1R-MICA+ lymphoid cell lines (C), in the presence of the anti-CD20 therapeutic antibody rituximab (20 μg/mL, RTX). In some experiments, Vδ2 T cells were exposed to saturating amounts (5 μg/mL) of anti-NKG2D mAb before adding the C1R or the C1R-MICA+ targets The effector-target (E:T) ratio was 10:1. Target cells were labeled with 51Cr and used in a standard 4-h cytolytic assay. Results are shown as percentage of 51Cr specific release and are the mean±SD from 6 experiments. (A) *P<0.005 versus Vδ2 T cells without mAb or with UnmAb; **P<0.001 versus NKG2D-triggered Vδ2 T cells not co-cultured with LNMSC; ***P<0.001 versus Vδ2 T cells co-cultured with untreated LNMSC. (B and C) *P<0.001 versus Vδ2 T cells not co-cultured with LNMSC or in the absence of mAbs; **P<0.001 versus Vδ2 T cells after co-culture with LNMSC. (D) Transcription of NKG2D in Vδ2 T cells before (white column) or after (gray columns) co-culture with LNMSC either untreated or treated with Pam (5 μg/mL) or Zol (1 μg/mL) or Meva (10 μM), as indicated. Q-RT-PCR was performed with the fluorescent Taqman method. mRNAs were normalized to RPLP0 as a control gene and referred to a standard curve. After subtracting the threshold cycle (CT) value for RPLP0 from the CT values of the target genes, results were expressed as ΔCT. *P<0.001 versus Vδ2 T cells not co-cultured with LNMSC (white columns); **P<0.001 versus Vδ2 T cells co-cultured with LNMSC, with or without Meva (light gray columns). (E) FACS analysis of expression of NKG2D or CD16 by immunofluorescence using the specific mAbs followed by isotype specific PE-GAM before (dark gray) or after (gray) co-culture with LNMSC either untreated (medium) or treated with Pam (5 μg/mL) or Zol (1 μg/mL). Empty histograms show Vδ2 cells stained with an unrelated mAb (un-mAb) followed by PE-GAM (histograms of Vδ2 T cells from LNMSC co-cultures stained with un-mAb were exactly superimposable; data not shown). Results are expressed as log red fluorescence intensity (a.u.) versus number of cells and are from one representative experiment out of 6. (F) Vδ2 T cells harvested from the indicated culture conditions were analyzed for NKG2D expression. In some experiments cultures were performed with an anti-TGFβ mAb (5 μg/mL) as indicated. Results are shown as mean fluorescence intensity (MFI) of NKG2D expression and are the mean±SD from 6 experiments *P<0.005 versus Vδ2 T cells not co-cultured with LNMSC (white column); **P<0.001 versus Vδ2 T cells after co-culture with LNMSC. Statistical analysis: two-tailed Student’s t-test.
Interestingly, upon co-culture with LNMSC, Vδ2 T lymphocytes strongly reduced their capability for re-directed killing when the anti-NKG2D mAb was used, whereas P815 cytotoxicity triggered by the anti-CD16 mAb was preserved (Figure 1A). Co-culture with LNMSC led to a significant decrease in cultured Vδ2 T-cell killing of lymphoid cell line C1R (approx. 50% of inhibition) (Figure 1B) or C1R expressing higher levels of the NKG2D-L MICA (Figure 1C for C1R-MICA, approx. 70% of inhibition). The covering of NKG2D with specific anti-NKG2D mAb strongly decreased (by 50%) cytolysis of C1R or C1R-MICA (Figure 1B and C) indicating that NKG2D-NKG2D-L interaction is involved in cell killing. On the other hand, it is worthy of note that ADCC triggered by rituximab was not affected by LNMSC co-culture (Figure 1B for C1R and Figure 1C for C1R-MICA). These data suggest that LNMSC affect the recognition of NKG2D-L, sparing the ability of Vδ2 effector cells to exert ADCC.
Further experiments showed that LNMSC induce the downregulation of transcription (Figure 1D) and expression (Figure 1E and F) of the NKG2D receptor in Vδ2 T cells, at variance with CD16 (Figure 1E). This is likely to be due to TGFβ production by LNMSC as an anti-TGFβ was able to neutralize NKG2D downregulation (Figure 1F).
N-BPs can rescue the ability of Vδ2 T cells to recognize lymphoid cells via NKG2D
When LNMSC were exposed overnight to pamidronate (Pam 5μM) or zoledronate (Zol 1μM), the inhibitory effect exerted by LNMSC on NKG2D-mediated cytotoxicity was not observed, either in a redirected killing (Figure 1A) or in a cytolytic assay against C1R or C1R-MICA+ lymphoid targets (Figure 1B and C). N-BPs doses and time points were selected on the basis of preliminary experiments, showing that N-BPs did not change LNMSC phenotype (Online Supplementary Figure S1) and were not toxic for these cells, as demonstrated by the MTT assay (Online Supplementary Figure S2).
Interestingly, the inhibitory effect of LNMSC on Vδ2 T-cell cytotoxic activity was also found when two lymphoma cell lines (LYB8 and KARPAS) were used as targets (Online Supplementary Figure S3A and B). Also in this case, the inhibition could be prevented by pre-treatment of LNMSC with Zol, whereas Pam did not display any effect (Online Supplementary Figure S3A and B). Interestingly, treatment of LNMSC with N-BPs prevented the downregulation of transcription (Figure 1D) and expression (Figure 1E and F) of the NKG2D receptor in Vδ2 T cells. The inhibitor of synthesis of mevalonate, which is a precursor of endogeneous pyrophosphates, mevastatin at doses (10 μM) and timing (48 h) efficient in inducing marked morphological changes in LNMSC related to actin cytoskeleton rearrangement (Online Supplementary Figure S4), did not counteract the action of N-BPs on LNMSC related to either cytotoxicity (Figure 1C and Online Supplementary Figure S4B) or NKG2D expression (Figure 1D and F). As mevalonate reverted the mevastatin-mediated effects on LNMSC (Online Supplementary Figure S4), this may suggest that pyrophosphates may not be involved in the N-BPs-mediated short-term effects. Accordingly, the effect of N-BPs was conceivably due to the prevention of TGFβ-mediated downregulation of NKG2D in Vδ2 effector T cells. Indeed, zoledronate could decrease the transcription of TGFβ in LNMSC (Figure 2A). Pre-treatment with N-BPs prevented also the increase of TGFβ in LNMSC that follows co-culture with Vδ2 T cells (Figure 2A). Since TGFβ may be expressed also by Vδ2 T cells, we determined that the fraction of contaminating Vδ2 T cells in LNMSC samples used for evaluating TGFβ transcription was always less than 2%, as assessed by immunofluorescence (data not shown). On the other hand, IL15 transcription was up-regulated in LNMSC by exposure to Pam or Zol (Figure 2B). Although not statistically significant, due to the heterogeneity in TGFβ basal production among the different LNMSC used (n=6), TGFβ measured in the SN decreased upon treatment of LNMSC with Pam or Zol (Figure 2C), while IL15 significantly increased in the SN of LNMSC treated with Pam or Zol (Figure 2D). In Vδ2 T cells, IL10 transcription increased upon co-culture with untreated LNMSC but not when LNMSC were pre-treated with Pam or Zol (Figure 2E). No significant variations in IL10 content in the SN (ranging from 8 to 12 pg/mL) was found (data not shown), possibly due to the rapid turnover of the cytokine once secreted. In turn, LNMSC exposed to N-BPs induced TNFα and IFNγ transcription (Figure 2F and G, respectively) and secretion (Figure 2H and I, respectively) in Vδ2 T cells. The treatment of LNMSC with mevastatin for 48 h and N-BPs added during the last 12 h of incubation, did not affect the ability of LNMSC to stimulate TNFα and IFNγ production in Vδ2 T cells (Online Supplementary Figure 3C and D). Thus, Vδ2 T cells co-cultured with N-BPs pre-treated LNMSC display a Th1 cytokine pattern, whereas untreated LNMSC induce the transcription of the regulatory cytokine TGFβ.
Figure 2.
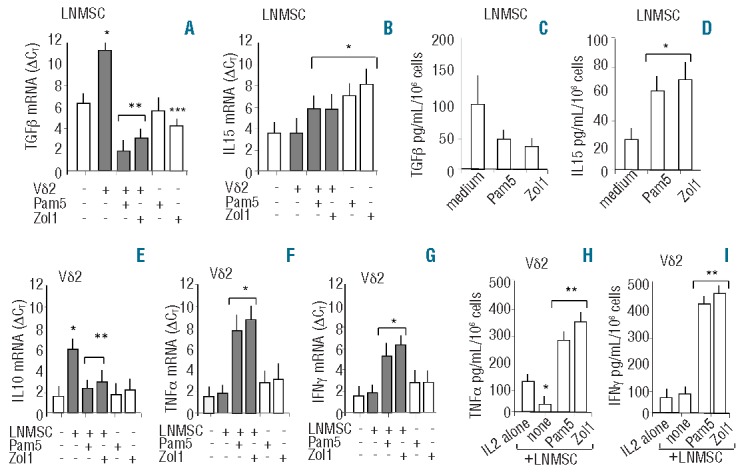
Effects of N-BPs on TGFβ or IL15 expression in LNMSC and IL10, TNFα, IFNγ production in Vδ2 T cells. Cultures of LNMSC or Vδ2 T cells alone or co-cultures of LNMSC and Vδ2 T cells (at 1:10 LNMSC: Vδ2 T-cell ratio were performed in medium with IL2. In some experiments, LNMSC were pre-treated for 12 h with Pam (5 μg/mL) or Zol (1 μg/mL), as indicated On Day 5, LNMSC (adherent cells) (A and B) or Vδ2 T cells (non-adherent cells) (E–G) were recovered and evaluation of mRNA transcription levels for TGFβ (A), IL15 (B), IL10 (E), TNFα (F) or IFNγ (G) was performed by Q-RT-PCR using specific probes with the fluorescent Taqman method. mRNAs were normalized to RPLP0 as a control gene and referred to a standard curve. After subtracting the threshold cycle (CT) value for RPLP0 from the CT values of the target genes, results were expressed as ΔCT. White columns: LNMSC (A and B) or Vδ2 T cells (E–G) cultured alone; gray columns LNMSC (A and B) or Vδ2 T cells (E–G) from LNMSC-Vδ2 T-cell co-cultures. Results are the mean±SD from 6 experiments. (A and B) *P<0.005 versus LNMSC alone; **P<0.001 versus LNMSC alone or versus untreated LNMSC co-cultured with Vδ2 T cells; ***P<0.005 versus untreated LNMSC. (E–G) *P<0.001 versus Vδ2 T cells alone. **P<0.001 versus LNMSC-Vδ2 T-cell co-cultures. TGFβ (C), IL-15 (D) measured by ELISA in the SN of LNMSC untreated (medium) or pre-treated with Pam (5 μg/mL) or Zol (1 μg/mL), as indicated. *P<0.001 versus untreated LNMSC (medium). TNFα (H) or IFNγ (I) measured by ELISA in the SN of Vδ2 T cells cultured alone (IL2) or co-cultured with untreated LNMSC (none) or LNMSC pre-treated with Pam or Zol. Results are expressed as pg/mL/106 cells and are the mean±SD from 6 experiments. *P<0.001 versus Vδ2 T cells alone (IL2); ** P<0.001 versus untreated LNMSC-Vδ2 T-cell co-cultures (none). Statistical analysis: two-tailed Student’s t-test.
N-BPs-treated LNMSC drive Vδ2 T-lymphocyte differentiation into effector memory cells
We further analyzed whether untreated or N-BPs-treated LNMSC affect the proliferation and/or differentiation of different Vδ2 T-cell subsets. As shown in Figure 3A, when CFSE-labeled T cells were cultured with Pam- or Zol-pre-treated LNMSC, a significant growth of Vδ2 T cells was observed, at variance with co-cultures with untreated LNMSC. This was also observed using purified Vδ2 T cells evaluating the increase in proliferating generations compared to parental cell population (Figure 3B).
Figure 3.
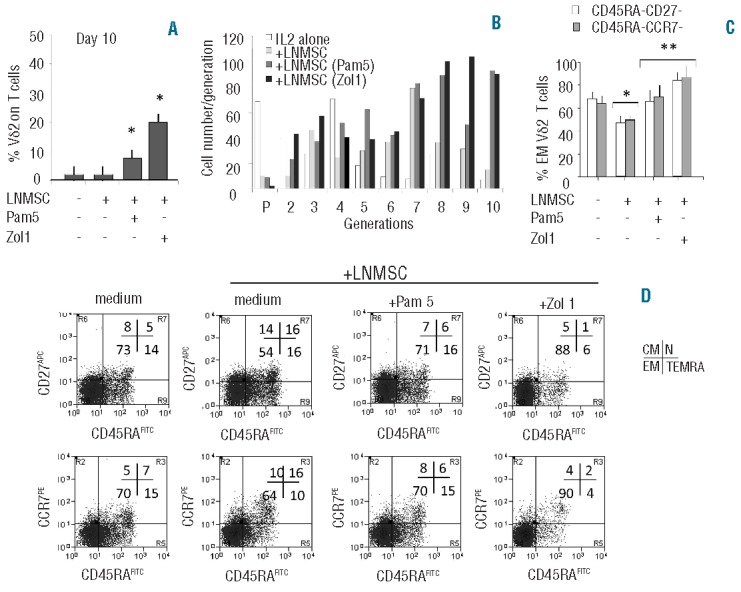
N-BPs-treated LNMSC drive Vδ2 T lymphocyte to effector memory T cells. (A and B) Peripheral blood T lymphocytes (A) or CFSE-labeled purified Vδ2 T cells (B) were co-cultured with LNMSC, either untreated or pre-treated for 12 h with Pam (5 μM) or Zol (1 μM), as indicated. On Day 10, percentage of Vδ2 T cells was evaluated by immunofluorescence using the anti-Vδ2 mAb PE-BB3 and FACS analysis (A). Mean±SD from 4 experiments. *P<0.001 versus T or Vδ2 cells cultured on untreated LNMSC. (B) Proliferation was assessed on the basis of the decreased green (CFSEdull) fluorescence of dividing cells. Data were analyzed by the Modfit 3.1 computer program and expressed as cell number/generation of proliferating cells. (C and D) Immunophenotype of Vδ2 T cells cultured alone or co-cultured for 10 days with untreated or Pam or Zol pre-treated LNMSC, as indicated, stained with APC-anti-CD27 or PE-anti-CCR7 and FITC-anti-CD45RA. (C) Results are expressed as percentage of effector memory (EM) T cells, i.e. CD45RA-CD27- (white columns) or CD45RA-CCR7- cells (gray columns). Mean±SD from 6 experiments *P<0.001 versus Vδ2 T cells in the absence of LNMSC **P<0.001 versus Vδ2 T cells co-cultured with untreated LNMSC. (D) Vδ2 T cells alone (medium, left dot plots) or Vδ2 T cells co-cultured with untreated or treated LNMSC, as indicated, were stained as in (C). Results are expressed as log green fluorescence intensity (x-axis, MFI, a.u.) versus either log far red (first row) or red (second row) fluorescence intensity (y-axis, MFI, a.u.). One representative experiment out of 6 is shown. Percentages of positive cells in each quadrant show the naive (N, upper right, double positive), central memory (CM, upper left, CD45RA− and CD27+ or CCR7+), effector memory (EM, lower left, double negative) and terminally differentiated effector memory (TEMRA, lower right, CD45RA+ but CD27− or CCR7−) cells. Statistical analysis: two-tailed Student’s t-test.
Given that treatment of LNMSC with either Pam or Zol induced on one hand the decrease in TGFβ and IL10 (regulatory cytokines)36,37 production together with increased expression of TNFα and IFNγ (Th1 type cytokines),36 and on the other hand stimulated Vδ2 T-cell proliferation, we asked whether variations in Vδ2 T-cell subsets related to their effector functions occurred during co-culture. On the basis of immunophenotyping, four effector cell subsets can be recognized: naive (N, CD45RA+CD27+CCR7+) and central memory (CM, CD45RA−CD27+CCR7+) both with proliferative capacity but low effector functions, effector memory (EM, CD45RA−CD27−CCR7−), and terminally differentiated effector memory (TEMRA, CD45RA+CD27−CCR7−), displaying efficient cytotoxic activity and cytokine production.27 Vδ2 T cells isolated from peripheral blood of healthy donors were 10±2% N, 10±3% CM, 50±5% EM and 30±2% TEMRA (data not shown). Immunophenotyping on Day 10 of culture showed that the percentage of EM Vδ2 T cells decreased upon co-culture with LNMSC (from 70±5% in IL2 alone to 58±6%, Figure 3C and D). In contrast, when LNMSC were pre-treated with Pam 5 μM or Zol 1 μM, the percentage of EM Vδ2 T cells remained stable in the case of Pam treatment (from 70±5% to 70±4%) or significantly increased in the case of Zol exposure (from 70±5% to 88±3%) (Figure 3C and D). On the other hand, after co-culture with LNMSC, the N-cell population increased from approximately 5% to 16%, whereas it was almost undetectable (1–2%) when LNMSC were pre-treated with Zol (Figure 3D).
Prevalence of naive Vδ2 T lymphocytes and of regulatory cytokine pattern in NHL
To characterize which Vδ2 T-cell population (in particular N vs. EM) was present in situ in NHL and define the role of autologous LNMSC in the differentiation of the different Vδ2 T-cell subsets, samples of LN cell suspensions obtained from DLCL (n=5), FL NHL (n=5) and non-neoplastic LN (n=3) were examined for Vδ2 T-cell phenotype by FACS analysis. In 4 cases (2 FL and 2 DLCL) co-cultures with autologous LNMSC, either untreated or exposed to Zol, were assessed. First, the percentage of Vδ2 T cells among T lymphocytes of NHL LN cell suspensions was approximately 11±5% in FL versus 3±1% in DLCL and versus 6±2% in non-neoplastic LN (data not shown). As shown in Figure 4 (A and B), Vδ2 T cells were mostly naive (CD45RA+CD27+) in FL and in DLCL, compared to non-neoplastic LN where more than one-third of these cells displayed a central memory (CD45RA−CD27+) phenotype. Upon co-culture with autologous LNMSC, the percentage of Vδ2 T cells increased when LNMSC were pre-treated with Zol, while untreated LNMSC did not induce a rise in Vδ2 T cells (Figure 4C). Among Vδ2 T cells cultured in IL2 alone, approximately 60% were naive, with 40% of TEMRA cells (Figure 4D and E). Interestingly, the percentage of TEMRA cells decreased (approx. 30%) upon co-culture with autologous LNMSC. In turn, this subset was rescued when LNMSC were pre-treated with Zol (>80%) (Figure 4D and E).
Figure 4.
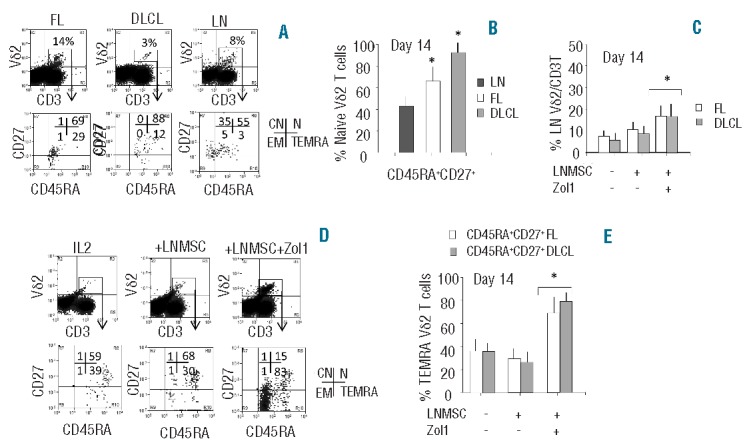
Vδ2 T-lymphocyte phenotype in NHL in situ. (A and B) Cell suspensions obtained from 5 DLCL, 5 FL NHL and 3 non-neoplastic LN were analyzed for phenotype by FACS analysis. (A) Upper dot plots: cells stained with the VioBlue anti-CD3 and the FITC anti-Vδ2 mAbs. Lower dot plots: gate on Vδ2 T cells, double staining with PE-anti-CD45RA and APC anti-CD27 mAbs. One representative experiment; results are expressed as log violet (x-axis, MFI, a.u.) versus log green fluorescence intensity (first row) or log red (x-axis, MFI, a.u.) versus log far red (second row) fluorescence intensity (y-axis, MFI, a.u.). Percentages of positive cells in each quadrant of the lower row show the naive (N, upper right, double positive), central memory (CM, upper left, CD45RA−CD27+), effector memory (EM, lower left, double negative) and terminally differentiated effector memory (TEMRA, lower right, CD45RA+ CD27−) cells. (B) Results are expressed as percentage of naive (double positive) Vδ2 T cells; mean±SD from 3 non-neoplastic LN (dark gray columns), 5 FL (white columns) and 5 DLCL (gray columns) cell suspensions. *P<0.001 versus LN. (C) Cell suspensions from FL (white columns) or DLCL (gray columns) were co-cultured with autologous LNMSC, either untreated or pre-treated for 12 h with Zol (1 μM), as indicated. On Day 14, percentage of Vδ2 T cells was evaluated by immunofluorescence using the anti-Vδ2 mAb FITC-γδ123 and the VioBlue anti-CD3 and FACS analysis; mean±SD from 5 FL and 5 DLCL. *P<0.001 versus cell suspensions cultured with IL2 alone, or on untreated or Zol-treated LNMSC. (D and E) Immunophenotype of cells co-cultured for 14 days alone (IL2) or with untreated or Zol pre-treated LNMSC, stained with VioBlue anti-CD3, FITC anti-Vδ2 mAbs, APC-anti-CD27 and PE-anti-CD45RA. (D) One representative experiment, gate on Vδ2 T cells. Results are expressed as log red fluorescence intensity (x-axis, MFI, a.u.) versus log far red fluorescence intensity (y-axis, MFI, a.u.); percentage of CM, N, EM and TEMRA in each panel of the lower row. (E) Results are expressed as percentage of TEMRA Vδ2 T cells, i.e. CD45RA+CD27−. Mean±SD from 2 experiments with FL (white columns) and 2 with DLCL (gray columns) cell suspensions. *P<0.001 versus cells versus cells co-cultured with untreated LNMSC. Statistical analysis: two-tailed Student’s t-test.
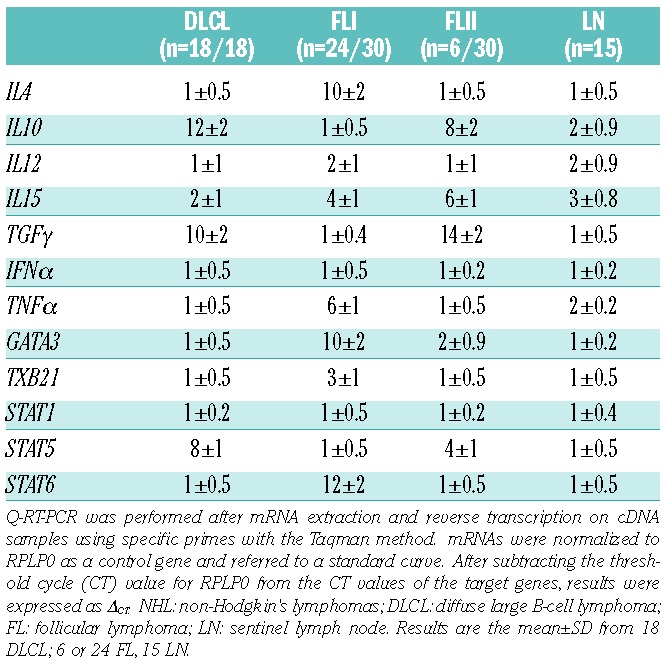
When LN sections (18 DLCL, 30 FL, 15 non-neoplastic LN) were analyzed by Q-RT-PCR for the expression of genes related to Th1/Th2 cytokines, we found low or undetectable transcription of Th1 cytokines (TNFα, IFNγ)36 in all NHL. Moreover, in all DLCL studied, transcription of TGFβ, IL10 and STAT5, related to TGFβ,37 was increased, compared to non-neoplastic LN (Table 1). On the other hand, most FL (two-thirds, FLI) displayed high levels of transcription of IL4, GATA3, and STAT6, that is a Th2 pattern,36 and low TGFβ and IL10 (Table 1). A small fraction (one-third) of FL (FLII) showed the same pattern of DLCL, that is overexpression of TGFβ and IL10. No differences in IL12 expression were found in DLCL or FL compared to LN (Table 1).
Table 1.
Expression of genes characterizing the different Th subsets in NHL lymph nodes.
Discussion
Results show that: i) LNMSC derived from NHL inhibit NKG2D-mediated lymphoid cell killing but not rituximab-induced ADCC exerted by peripheral blood Vδ2 T cells; ii) pre-treatment of LNMSC with N-BPs can rescue the ability of Vδ2 T cells to kill lymphoid cells via NKG2D; iii) this is related to inhibition of TGFβ and increase in IL15 production by LNMSC; iv) N-BPs-treated LNMSC drive Vδ2 T-lymphocyte differentiation into EM T cells, expressing a Th1-type cytokine pattern; v) at the tumor site, Vδ2 T cells were mostly naive both in DLCL and FL; iv) upon co-culture with autologous LNMSC exposed to zoledronate, the percentage of Vδ2 T cells with terminal differentiated EM phenotype increased.
It is commonly accepted that γδ T cells have innate-like characteristics that allow their rapid activation through NKG2D, following recognition of stress-induced ligands on lymphoma cells.1,15,24,31 Upon activation, γδ T cells become cytotoxic and secrete pro-inflammatory and anti-tumor Th1 cytokines, including IFNγ and TNFα.1,2 However, here we provide evidence that LNMSC, within the NHL microenvironment, may impede this activation by two ways: the former, through the downregulation of NKG2D expression following TGFβ production, the latter by inhibiting the Vδ2 T-lymphocyte differentiation to EM cells (CD45RA−CD27−CCR7−). Indeed, at the tumor site, we found a predominance of naive Vδ2 T lymphocytes, together with a deficient Th1 (IFNγ, TNFα) cytokine pattern. Moreover, in all DLCL and in a fraction of FL, a regulatory (TGFβ, IL10) cytokine pattern was predominant. Naive Vδ2 T cells were also present in the group of FL expressing the Th2 cytokine IL4 and the related genes GATA3 and STAT6, with low TGFβ and IL10. In all cases, low levels of transcription of IL12, a cytokine involved in Th1 differentiation,36 was observed.
Also others have demonstrated that TGFβ interferes with γδ T-cell functions. Indeed, it is a regulatory cytokine known to inhibit effector T-cell functions and delay PAg-induced maturation of TCRVγ9 effector T lymphocytes. It can also drive the differentiation of a subset of Vδ2T cells with suppressor functions.37–39 Of note, we show that pre-treatment of LNMSC with N-BPs (zoledronate being more potent than pamidronate) rescues the ability to kill lymphoma target cells and induces proliferation of Vδ2 T cells that express the EM phenotype. The N-BPs-mediated rescue of cell killing was due, at least in part, to the decrease in TGFβ and simultaneous increase in IL15 production in N-BPs-treated LNMSC.
Possibly due to the accumulation of phosphate metabolites, upon N-BPs treatment, LNMSC become susceptible targets for Vδ2T cells; but this is evident only when these effectors are cultured with IL2. This would suggest that also in vivo at least a fraction of LNMSC undergo elimination by activated Vδ2 effector cells. Nevertheless, the cytokine switch in LNMSC from TGFβ to IL15, detectable by the first 24–48 h of N-BP treatment, occurs before LNMSC death.
The N-BPs-mediated rescue of cell killing appeared to be insensitive to short-time incubation of LNMSC with efficient doses and timing of mevastatin. Moreover, in co-cultures of Vδ2 T cells with N-BPs-treated LNMSC, an increase in Th1-type cytokine transcription and secretion (TNFα and IFNγ) was observed which was not affected by mevastatin. This would suggest that pyrophosphates, downstream mevalonate, may not be essential in short-time N-BPs-mediated effects. It still remains to be seen whether pyrophosphates produced in N-BPs-treated LNMSC play a role in proliferation of Vδ2 T cells. Indeed, this effect is evident after a longer period of incubation between LNMSC and lymphocytes. Since the blocking of mevalonate synthesis by mevastatin is conceivably limited in time, we cannot exclude the relevance of pyrophosphates.
It should be noted that LNMSC derived from FL did not differ significantly from DLCL-derived LNMSC in modulating Vδ2 T lymphocytes, suggesting that immunosuppression is an intrinsic functional behavior of these cells, as previously reported for bone marrow MSC.29,30 N-BPs seems to act on LNMSC interfering with their immunosuppressive effect, favoring Th1 development and potentiating the growth and differentiation of effector Vδ2 T cells. Other authors reported that bone marrow-derived mesenchymal cells decrease the response of γδ T cells to isopentenyl-pyrophosphate (IPP), whereas in our experiments N-PBs induce proliferation of Vδ2 T cells.40,41 However, in the reported papers, mesenchymal cells were derived from the bone marrow and IPP was added directly to the proliferation assay, while we used LNMSC pre-treated with N-BPs so that phosphate metabolites deriving from the mevalonate cycle could accumulate, being more efficient in trigger cell proliferation.42 In any case, we show that, in the presence of N-BPs, a subset of Vδ2 T cells, with EM/TEMRA characteristics, is able to expand and recover its functional capabilities, such as NKG2D-mediated antitumor cytotoxicity.
Since in our experiments, Vδ2 T cells were sorted by positive anti-TCR selection, this procedure might have provided a stimulus making cells more responsive to NKG2D stimulation and more effective in anti-lymphoma cell killing. In addition, Vδ2T cells were used after culture in IL2-containing medium, so that it is conceivable that also IL2 provides Vδ2 T cells with an activating signal. Nevertheless, N-BPs can prevent the down-regulating effect of LNMSC on Vδ2 T-cell-effector functions, whatever activating stimulus they may receive. Also, positively selected Vδ2 T cells were used in all co-culture experiments so that any activating stimulus due to separating and culture procedures was superimposable in our experimental conditions. Moreover, we have chosen to show experiments obtained with positively selected Vδ2 T-cell populations as positive separation allows an optimal purity and recovery, at variance with negative selection that might lead to the loss of a part of the Vδ2 effector T cells, in particular those expressing molecules (such as CD8 and CD16), important in exerting the cytolytic functions.
Cytolytic activity of γδ T cells can also be activated by the binding of CD16 to the Fc region of IgG, triggering ADCC, that is exploited for anti-lymphoma therapy with monoclonal antibodies such as the anti-CD20 rituximab.17,18,21,26 In our system, we found that rituximab-mediated ADCC is spared by LNMSC and is also maintained in the presence of N-BPs, thus suggesting that the two lines of therapy may be associated. We can hypothesize that, in vivo, in areas relatively free of Vδ2 T cells, N-BPs modulate the cytokine microenvironment making it unfavorable to cancer cells. In areas where Vδ2 T cells, tumor cells and LNMSC are present together, effector T cells might on the one hand eliminate stromal cells that may contribute to lymphoma cell growth, and on the other hand become effective in lymphoma cell killing. The action of Vδ2 effector T cells may be polarized towards cancer cell killing by rituximab administration. Therapeutic strategies based on the combination of N-BPs, mAbs and conventional chemotherapy or therapies aimed to act on MSC, including RNA interference targeting TGFβ, have been envisaged.1,2,17,18,42,43 Here we provide evidence that N-BPs can directly modulate the effects exerted by LNMSC on γδ T cells, possibly contributing to the normalization of LN microenvironment.
Acknowledgments
We thank D. Zennaro and L. Grasso for preparation of tissue samples.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was partially supported by the Italian Association for Cancer Research (AIRC Grant n. IG-8727 to MRZ) and by the Compagnia di San Paolo (Grant n.2007.2065 and 2010.0625). AM is a fellow granted by the Italian Foundation for Cancer Research (FIRC).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org
References
- 1.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31(2):184–96 [DOI] [PubMed] [Google Scholar]
- 2.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10(7):467–78 [DOI] [PubMed] [Google Scholar]
- 3.Das H, Wang L, Kamath A, Bukowski JF.V 9V 2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98(5):1616–8 [DOI] [PubMed] [Google Scholar]
- 4.Gober HJ, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197(2):163–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Sarikonda G, Puan K-J, Tanaka Y, Fenq J, Giner JL, et al. Indirect stimulation of human V 2V 2 T cells through alterations in isoprenoid metabolism. J Immunol. 2011;187(10):5099–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci. USA 1999;96(12):6879–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rael and HL60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413(6852):165–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gamma delta T cells. Science. 2001;294(5542):605–9 [DOI] [PubMed] [Google Scholar]
- 9.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, et al. ULBPs, novel MHC class-I-related molecules, bind to CMV glicoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14(2):123–33 [DOI] [PubMed] [Google Scholar]
- 10.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102(4):1389–96 [DOI] [PubMed] [Google Scholar]
- 11.Rincon-Orozoco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V 9V 2 T cells by NKG2D. J Immunol. 2005;175(4):2144–51 [DOI] [PubMed] [Google Scholar]
- 12.Lafont V, Liautard J, Liautard JP, Favero J. Production of TNF-alpha by human V gamma 9V delta 2 T cells via engagement of Fc gamma RIIIA, the low affinity type 3 receptor for the Fc portion of IgG, expressed upon TCR activation by nonpeptidic antigen. J Immunol. 2001;166 (12):7190–9 [DOI] [PubMed] [Google Scholar]
- 13.Kunzman V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of T cells by aminobiphosphonates and induction of anti-plasma cell activity in multiple myeloma. Blood. 2000;96(2):384–92 [PubMed] [Google Scholar]
- 14.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human γ T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol. 2002;23(1):14–7 [DOI] [PubMed] [Google Scholar]
- 15.Street SE, Hayakawa Y, Zhan Y, Lew AM, Mac Gregor D, Jamieson AM, et al. Innate immune surveillance of spontaneous B cell lymphoma by natural killer and gammadelta T cells. J Exp Med. 2004;199(6):879–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilhelm M, Kunzman V, Eckstein S, Reimer P, Weissinger F, Ruediger T, et al. T cells for immune therapy of patients. Blood. 2003;102(1):200–6 [DOI] [PubMed] [Google Scholar]
- 17.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T lymphocytes in tumor immunology. Cancer Res. 2007;67(1):5–8 [DOI] [PubMed] [Google Scholar]
- 18.Hannani D, Ma Y, Yamazaki T, De’Chanet-Merville J, Kroemer G, Zitvogel L. Harnessing. γ T cells in anticancer immunotherapy. Trends Immunol. 2012;33(5):199–206 [DOI] [PubMed] [Google Scholar]
- 19.Mariani S, Muraro M, Pantaleoni F, Fiore F, Nuschak B, Peola S, et al. Effector T cells and tumor cells as immune targets of zolendronic acid in multiple myeloma. Leukemia. 2005;19(4):664–70 [DOI] [PubMed] [Google Scholar]
- 20.Fiore F, Castella B, Nuschak B, Bertieri R, Mariani S, Bruno B, et al. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood. 2007;110(3):921–7 [DOI] [PubMed] [Google Scholar]
- 21.Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S, et al. Bromohydrin pyrophosphate enhances antibody-dependent cellmediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113(20):4875–84 [DOI] [PubMed] [Google Scholar]
- 22.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, et al. Induction of T lymphocyte effector functions by bisphosphonate zolendronic acid in cancer patients in vivo. Blood. 2003;102(6):2310–1 [DOI] [PubMed] [Google Scholar]
- 23.Poggi A, Venturino C, Catellani S, Clavio M, Miglino M, Gobbi M, et al. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 2004;64(24):9172–9 [DOI] [PubMed] [Google Scholar]
- 24.Catellani S, Poggi A, Bruzzone A, Caligaris-Cappio F, Gobbi M, Zocchi MR. Expansion of Vdelta1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing UL-16-binding proteins. Blood. 2007;109(5):2078–85 [DOI] [PubMed] [Google Scholar]
- 25.Poggi A, Catellani S, Garuti A, Pierri I, Gobbi M, Zocchi MR. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia. 2009;23(4):641–8 [DOI] [PubMed] [Google Scholar]
- 26.Tokuyama H, Hagi T, Mattarollo SR, Morley J, Wang Q, So HF, et al. V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs-rituximab and trastuzumab. Int J Cancer. 2008;122(11):2526–34 [DOI] [PubMed] [Google Scholar]
- 27.Angelini DF, Borsellino G, Poupot M, Diamantini A, Poupot R, Bernardi G, et al. Fc RIII discriminates between 2 subsets of V 9V 2 effector cells with different responses and activation pathways. Blood. 2004;104(6):1801–7 [DOI] [PubMed] [Google Scholar]
- 28.Carbone A, Gloghini A, Cabras A, Elia G. The Germinal centre-derived lymphomas seen through their cellular microenvironment. Br J Haematol. 2009;145(4):468–80 [DOI] [PubMed] [Google Scholar]
- 29.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–506 [DOI] [PubMed] [Google Scholar]
- 30.Musso A, Zocchi MR, Poggi A. Relevance of the mevalonate biosynthetic pathway in the regulation of bone marrow mesenchymal stromal cell-mediated effects on T-cell proliferation and B-cell survival. Haematologica. 2011;96(1):16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zocchi MR, Catellani S, Canevali P, Tavella S, Garuti A, Villaggio B, et al. High ERp5/ADAM10 expression in lymph node microenvironment and impaired NKG2D-ligands recognition in Hodgkin lymphomas. Blood. 2012;119(6):1479–89 [DOI] [PubMed] [Google Scholar]
- 32.Turner JJ, Morton LM, Linet MS, Clarke CA, Kladin ME, Vajdic CM, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116(20):e90–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghio M, Contini P, Negrini S, Boero S, Musso A, Poggi A. Soluble HLA-I-mediated secretion of TGF-beta1 by human NK cells and consequent down-regulation of antitumor cytolytic activity. Eur J Immunol. 2009;39(12):3459–68 [DOI] [PubMed] [Google Scholar]
- 34.Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia-a Europe Against Cancer program. Leukemia. 2003;17(12):2318–57 [DOI] [PubMed] [Google Scholar]
- 36.Mosmann TR, Coffmann RL. TH1 and TH2 cells: different patterns of lymphocyte secretion lead to different functional properties Annu Rev Immunol. 1989;7:145–73 [DOI] [PubMed] [Google Scholar]
- 37.Cua DJ, Kastelein RA. TGF-beta, a ‘double agent’ in the immune pathology war. Nat Immunol. 2006;7(6):557–9 [DOI] [PubMed] [Google Scholar]
- 38.Capietto A-H, Martinet L, Cendron D, Fruchon S, Pont F, Fournié JJ. Phosphoantigen overcome human TCRV 9+ cell immunosuppression by TGF-: relevance for cancer immunotherapy. J Immunol. 2010;184(12):6680–7 [DOI] [PubMed] [Google Scholar]
- 39.Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, et al. TGF- 1 and IL15 induce FoxP3+ regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183(6):3574–7 [DOI] [PubMed] [Google Scholar]
- 40.Prigione I, Benvenuto FG, Bocca P, Battistini L, Uccelli A, Pistoia V. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells. 2009;27(3):693–702 [DOI] [PubMed] [Google Scholar]
- 41.Martinet L, Fleury-Cappellesso S, Gadelorge M, Dietrich G, Bourin F, Fournié JJ, et al. A regulatory cross-talk between Vgamma9Vdelta2 T lymphocytes and mesenchymal stem cells. Eur J Immunol. 2009;39(3):752–62 [DOI] [PubMed] [Google Scholar]
- 42.Chiplunkar S, Dhar S, Wesh D, Kabelitz D. T cells in cancer immunotherapy: current status and future prospects. Immunotherapy. 2009;1(4):663–78 [DOI] [PubMed] [Google Scholar]
- 43.Friese MA, Wischhusen J, Wick W, Weiler M, Eisele G, Steinle A, et al. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004;64(20):7596–603 [DOI] [PubMed] [Google Scholar]


