Abstract
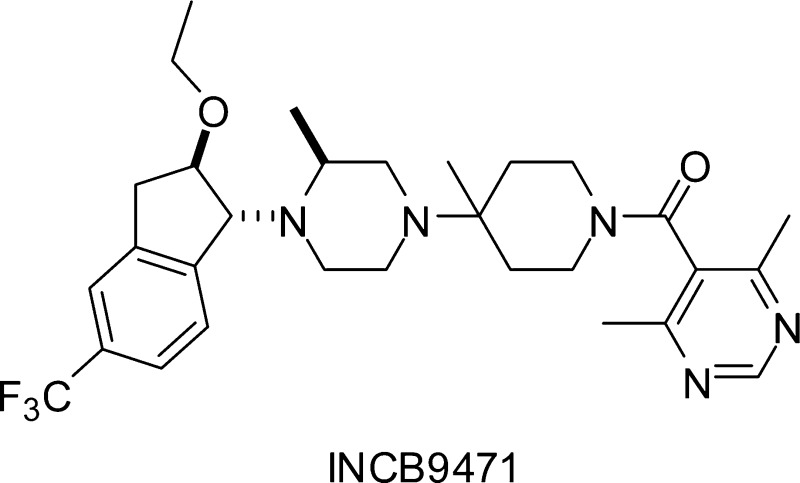
To identify a CCR5 antagonist as an HIV-1 entry inhibitor, we designed a novel series of indane derivatives based on conformational considerations. Modification on the indane ring led to the discovery of compound 22a (INCB9471) that exhibited high affinity for CCR5, potent anti-HIV-1 activity, high receptor selectivity, excellent oral bioavailability, and a tolerated safety profile. INCB9471 has entered human clinical trials.
Keywords: CCR5, antagonist, coreceptor, HIV-1, antiviral
CCR5 is a chemokine receptor, a member of the super family of seven-transmembrane G-protein-coupled receptors (GPCRs), and its natural ligands include the chemokines RANTES (regulated on activation, normal T cell expressed, and secreted), MIP-1α, and MIP-1β (macrophage inflammatory proteins-1α and -1β).1 In addition, CCR5 is a major coreceptor for the entry of human immunodeficiency virus type-1 (HIV-1) into host cells.2−4 HIV-1 binds to cellular membrane protein CD4 on T-cells and macrophages through an HIV envelope protein gp120. This binding induces a conformational change in gp120 to expose binding sites for CCR5. Binding of CCR5 to gp120 brings the viral envelope closer to the cell surface and allows interaction between glycoprotein gp41 on the viral envelope and a fusion domain on the host cell surface, leading to fusion of the viral envelope with the host cell membrane and entry of the viral core into the host cells. Genetic studies in populations at high risk for HIV exposure revealed that individuals homozygous for a 32-base pair deletion in CCR5 encoding genes (Δ32 CCR5) are highly resistant to HIV-1 infection and are without significant health problems attributable to a lack of functioning CCR5 receptors.5 HIV-1-infected individuals heterozygous for Δ32 CCR5 appear to have slower disease progression to AIDS and longer survival periods.6 These findings strongly suggest that CCR5 is an attractive target for potential therapeutic intervention of HIV-1 infection. Unlike reverse transcriptase and protease inhibitors that block viral replication at different stages in the viral life cycle after the HIV-1 virus has entered host cells, CCR5 antagonists target a much earlier stage by preventing the entry of the virus into cells. This represents a novel mechanism of action and may offer some advantages over the reverse transcriptase and protease inhibitors as it protects cells from irreversible damage by the virus. As a result, extensive searches for small molecule CCR5 inhibitors have been pursued, and in 2000, the first small molecule to inhibit HIV-1 growth by binding to CCR5−TAK779−was published in the scientific literature.7 Since then, a number of structurally diverse CCR5 antagonists with potent anti-HIV-1 activity have been reported, and several compounds have entered human clinical trials.8 In 2007, the first CCR5 antagonist maraviroc (Selzentry)9,10 was approved by the FDA and launched by Pfizer, demonstrating the validity of the target. Development of new CCR5 antagonists has continued to be pursued for antiviral utility and also for potential application in a variety of autoimmune diseases.11 The latter has been demonstrated by a primate model study in which a small molecule CCR5 antagonist exhibited efficacy in collagen-induced joint inflammation.12
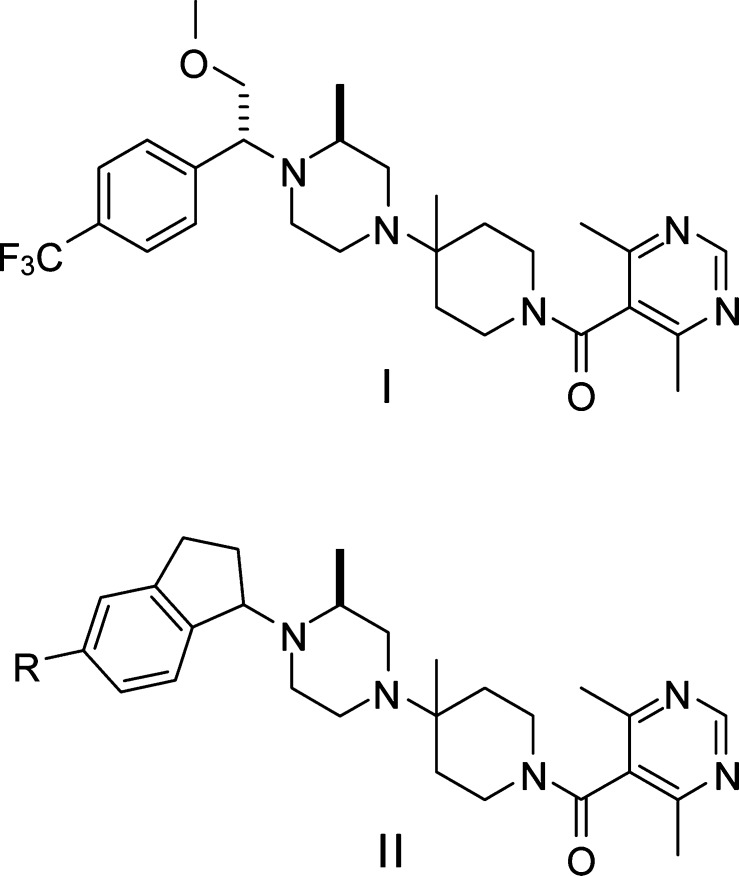
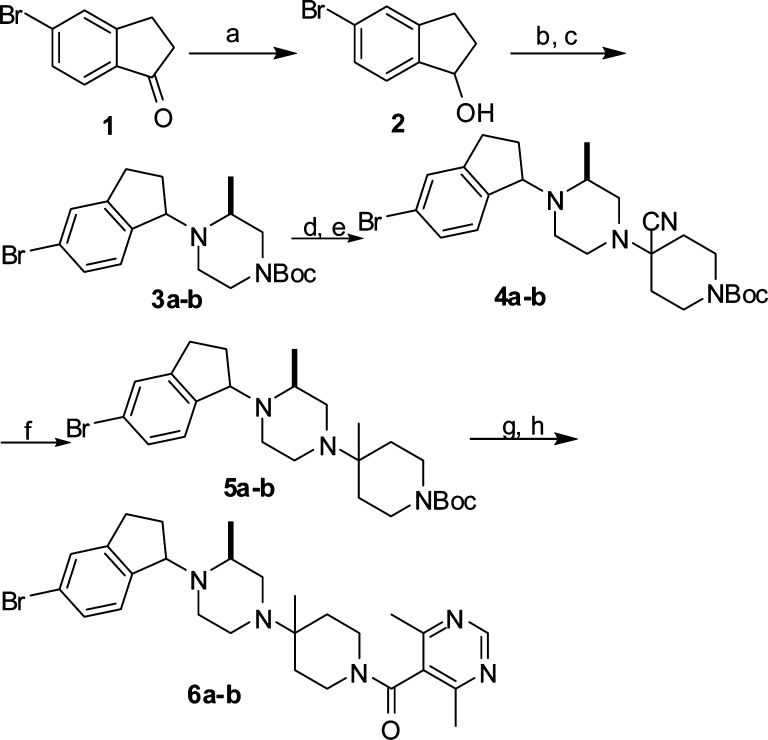
We were interested in small molecule CCR5 antagonists as HIV-1 entry inhibitors and as potential therapeutics for autoimmune diseases. Our goal was to identify a lead compound for optimization based on literature lead compounds through rational design, with special interest in the Schering-Plough's CCR5 antagonists as this series of compounds exhibited potent antiviral activity and good pharmacokinetic properties.13,14 Because the phenyl ring on the left-hand side of Schering-Plough's CCR5 antagonist Sch-D13 (I in Figure 1) is one of the key pharmacophore elements for interaction with the CCR5 receptor, we wondered whether the benzyl moiety on the left-hand side could be rigidified to enhance the binding affinity and potentially improve the physical properties. Conformational analysis revealed that a benzene-fused bicyclic system such as an indane could provide the desired rigidity with retained orientation of the phenyl ring if the connection of the bicyclic system to the piperazine nitrogen is at the benzylic position (α-position). On the basis of these considerations, an indane ring was chosen as the bicyclic system for our initial exploration (II in Figure 1). To quickly test this hypothesis, we initiated the synthesis of a 5-bromoindane derivative as 5-bromoindan-1-one (1) is commercially available. As outlined in Scheme 1, reduction of 1 with NaBH4 in THF converted the ketone to an alcohol. Following treatment of alcohol 2 with thionyl chloride, the resulting chloride was displaced with (S)-4-Boc-2-methylpiperazine to give a mixture of two diastereomers, which were separated by flash chromatography to provide the diastereomerically pure 3a (fast moving isomer) and 3b (slow moving isomer). Removal of Boc group in 3a or 3b was followed by condensation with 1-Boc-4-piperidinone in the presence of Ti(OiPr)4 and subsequently by addition of Et2AlCN to give rise to 4a or 4b. Displacement of the cyano in 4a or 4b with MeMgBr yielded 5a or 5b. Removal of Boc group in 5a or 5b followed by coupling with 4,6-dimethylpyrimidine-5-carboxylic acid afforded target compounds 6a or 6b.
Figure 1.
Scheme 1. Synthesis of Compounds 6a and 6b.
Reagents and conditions: (a) NaBH4, THF, 100%. (b) SOCl2. (c) tert-Butyl (3S)-3-methylpiperazine-1-carboxylate, DMF, NaI, Et3N, 70 °C, 19% (4a) and 18% (4b) for two steps. (d) 4 N HCl/dioxane. (e) N-Boc-piperidin-4-one, Ti(OiPr)4, Et3N, CH2Cl2, overnight, and then Et2AlCN, THF, 30 °C, 5 h. (f) MeMgBr, THF, overnight. (g) 4 N HCl/dioxane. (h) 4,6-Dimethylpyrimidine-5-carboxylic acid, BOP, Et3N, DMF, room temperature, 5 h.
Diastereomer 6a is a potent CCR5 antagonist with IC50 values of 3.9 and 1.1 nM in MIP-1β binding assay and CCR5 chemotaxis assay, respectively, similarly potent to Sch-D, while diastereomer 6b is much less active with an IC50 of 190 nM in MIP-1β assay (Table 1). Despite its high affinity for CCR5, 6a is a weak HIV-1 entry inhibitor with an IC50 of 334 nM against BaL strain, being 1450-fold less active than Sch-D. The discrepancy between the CCR5 inhibitory activity and the antiviral activity of 6a probably reflects a differential response of the two ligands−MIP-1β and gp120−to the conformational change caused by the binding of the small molecule to CCR5. We reasoned that the weak antiviral activity of 6a might be due to the bromine substituent at the 5-position of the indane ring, as in Sch-D the corresponding substituent is a trifluoromethyl group. This consideration led us to replace the bromine in 6a with a trifluoromethyl group.
Table 1. CCR5 and HIV-1 Activities.
| CCR5 IC50 (nM) |
HIV-1 IC50 (nM)c |
|||
|---|---|---|---|---|
| compd | MIP-1βa | CTXb | ADA | BaL |
| Sch-D | 3.0 | 1.2 | 0.56 | 0.23 |
| 6a | 3.9 | 1.1 | 334 | |
| 6b | 190 | |||
| 13a | 5 | 1.2 | 19 | |
| 20a | 211 | |||
| 21a | 4.6 | 3.5 | 12 | 11 |
| 22a | 6.5 | 3 | 0.36 | 0.16 |
Inhibition of binding of 250 pM 125I-labeled MIP-1β to IL-10-stimulated human monocytes.
Inhibition of migration of IL-10-stimulated human monocytes in response to 30 nM MIP-1β.
Inhibition of HIV-1 (ADA and BaL strains) infection of PHA-activated human PBMC. Infectivity was determined by measuring viral p24 core protein in culture supernatants.
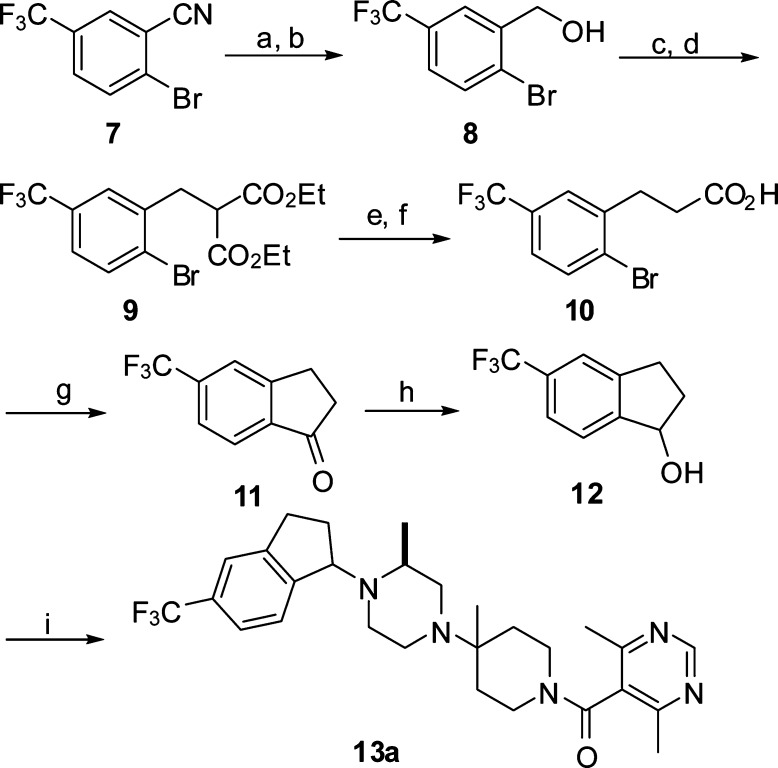
The synthesis of the trifluoromethyl counterpart of 6a is illustrated in Scheme 2. 2-Bromo-5-(trifluoromethyl)benzonitrile 7 was converted to an alcohol 8 by sequential reductions using DIBAL and NaBH4. Treatment of 8 with thionyl chloride followed by displacement of the resulting chloride with diethyl malonate provided the diester 9. Saponification and subsequent decarboxylation yielded the monocarboxylic acid 10. Ring closure by treatment with n-butyl lithium gave the indanone 11. Reduction of 11 with NaBH4 afforded the alcohol 12, which was processed to the final product 13a (less polar diastereomer) in a manner analogous to that described in Scheme 1. As shown in Table 1, replacement of the bromine in 6a with trifluoromethyl retained the CCR5 potency in both MIP-1β and chemotaxis assays but, as expected, improved the antiviral IC50 from 334 to 19 nM against BaL virus strain. Despite the 18-fold improvement over 6a, however, 13a is still 82-fold less active than Sch-D in the antiviral activity. By analogy with Sch-D, we decided to introduce an alkoxy residue at the 2-position of the indane ring.
Scheme 2. Synthesis of Compounds 13a.
Reagents and conditions: (a) DIBAL, CH2Cl2, room temperature, 1 h, 50%. (b) NaBH4, THF, 0 °C, 1 h, 88%. (c) SOCl2, 1 h. (d) Ethyl malonate, NaH, DMF, room temperature, 3 h, 64% for two steps. (e) NaOH, EtOH/H2O, reflux, 2 h. (f) 180 °C, 1 h, 67% for two steps. (g) n-BuLi (2×), THF/hexanes, −78 °C, 37%. (h) NaBH4, THF/MeOH, 0 °C, 30 min, 92%. (i) Similar procedures as described in Scheme 1.
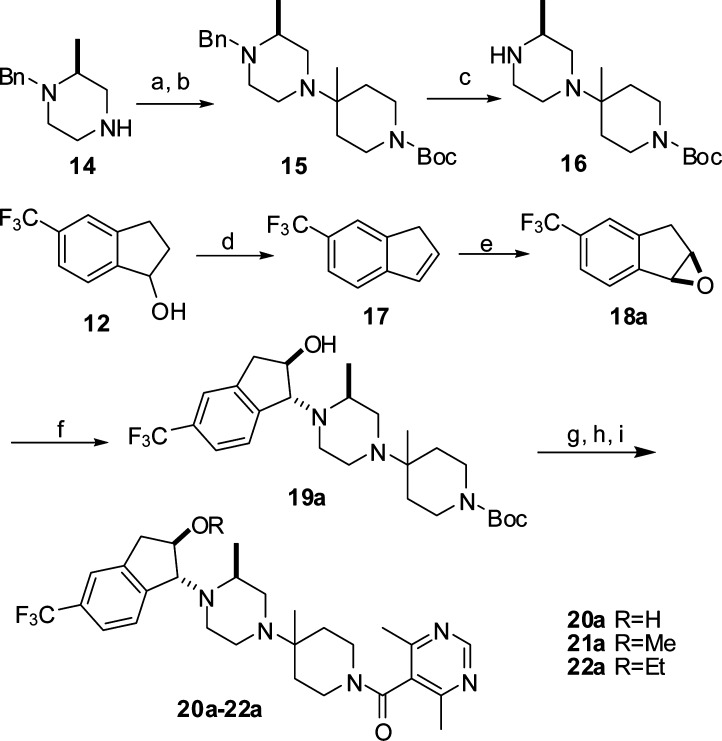
Unlike the synthesis of 6a and 13a, the alkoxy analogues of 13a were synthesized through a nonlinear sequence by assembly of the middle piperazinyl-piperidine core (16) prior to attachment of the indane ring to the piperazine (Scheme 3). This approach allowed us to exploit the structure−activity relationship at the 2-position of the indane ring more efficiently. Intermediate 16 was prepared by reaction of (S)-1-benzyl-2-methylpiperazine 14 with 1-Boc-4-piperidinone in an analogous manner as depicted in Scheme 1 and subsequent debenzylation by hydrogenolysis. A hydroxyl group was introduced at the 2-position of the indane ring through regioselective ring opening with 16 of (1S,2R)-indane epoxide 18, which was obtained with 84% ee by dehydration of 12 followed by asymmetric epoxidation using a Jacobson's catalyst.15 Without purification, ring opening of the crude 18 generated a mixture of two diastereomers 19a and 19b with a ratio of ∼9:1, which was separated by flash chromatography. The absolute stereochemistry of the major (1R,2R)-indanyl diastereomer 19a was confirmed by X-ray crystallography. Alkylation at the hydroxyl, Boc deprotection, and coupling with 4,6-dimethylpyrimidine-5-carboxylic acid converted 19a to target compounds 20a−22a that were determined to be the active diastereomers by MIP-1β assay (Table 1). The minor (1S,2S)-indanyl diastereomer 19b was elaborated to provide the inactive isomers 20b−22b, which showed IC50 values of >1000 nM in MIP-1β assay.
Scheme 3. Synthesis of Compounds 20a−22a.
Reagents and conditions: (a) N-Boc-piperidin-4-one, Ti(OiPr)4, Et3N, CH2Cl2, room temperature, 20 h, and then Et2AlCN, room temperature, 20 h, 89%. (b) MeMgBr, THF, overnight, 93%. (c) Pd(OH)2/C, H2, MeOH/AcOH, 60 psi, 18 h, 100%. (d) Toluenesulfonic acid, toluene, reflux, 3 h, 96%. (e) (S,S)-(+)-N,N′-bis(3,5-ditert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, NaClO, 4-(3-phenylpropyl)pyridine N-oxide, NaOH, CH2Cl2/H2O, 88%, 84% ee. (f) (1) Compound 16, EtOH, 70 °C, 2 days; (2) [(1R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl]methanesulfonic acid, MeCN, 70% for two steps; (3) 1 N NaOH, 99%. (g) NaH, RI, DMF. (h and i) Similar procedures as in Scheme 1.
Substitution at the 2-position of the indane ring in 13a with a hydroxyl group resulted in a 40-fold loss in CCR5 activity (20a, Table 1), which was completely recovered once the hydroxyl was methylated (21a), suggesting that a hydroxyl is too polar to be tolerated at this position for CCR5 binding. Disappointingly, introducing a methoxy residue to the 2-position of the indane ring was accompanied only by a minor improvement in antiviral activity (11 nM for 21a vs 19 nM for 13a). Remarkably, replacement of the methyl group in 21a with ethyl provided a very potent entry inhibitor 22a (INCB9471) with subnanomolar potency in both ADA and BaL strains and, therefore, is as potent as Sch-D. The 69-fold enhancement in antiviral potency by a one-carbon homologation in going from methyl in 21a to ethyl in 22a may be ascribed to a better interaction of the latter molecule with the CCR5 receptor that leads to a more profound conformational change of the receptor required for optimally blocking binding of gp120.
INCB9471 exists as a 1:1 mixture of two rotamers in solution. Rotation around the amide bond between the pyrimidinecarbonyl and the piperidine nitrogen is slow enough to be detected by chiral high-performance liquid chromatography and NMR. When crystallized with an acid, the di-HCl crystalline salt form predominantly exists as one rotamer while the bistoluenesulfonic acid crystalline salt form predominantly exists as the other rotamer. Once dissolved in water, the predominant rotamer rapidly converts to the other rotamer, resulting in an equilibrium of a 1:1 ratio within 1 h at 37 °C.
INCB9471 was further evaluated in vitro. Kinetic studies indicated that INCB9471 rapidly associates with human CCR5 but dissociates slowly with a Kd of 3.1 nM in human peripheral blood mononuclear cells (PBMCs). It inhibited CCR5-mediated signaling events such as intracellular calcium mobilization, ERK phosphorylation, and CCR5 receptor internalization with IC50 values of 16, 3, and 1.5 nM, respectively. It is a selective CCR5 antagonist, showing no significant inhibitory activity when tested against a panel of >50 ion channels, transporters, chemokine receptors, and additional GPCRs. Studies using incremental amounts of INCB9471 demonstrated reductions in maximal ligand binding and ligand-induced Ca2+ responses without concurrent changes in the affinity of CCR5 ligands, indicating that INCB9471 is an allosteric noncompetitive CCR5 inhibitor. In anti-HIV-1 activity, INCB9471 is potent against R5 HIV-1 strains representing the major clades including A, B, C, D, E, F, G, and J, with a geometric mean IC90 for all viruses tested to date of 9 nM in PBMCs, whereas it is inactive against cells infected with X4 HIV-1 strains. More importantly, INCB9471 is a potent inhibitor of mutant HIV-1 variants that are resistant to other drugs including NRTIs, NNRTIs, PIs, and the fusion inhibitor T20. The observed antiviral activity is not a reflection of general cytotoxicity as INCB9471 did not exhibit effects on cell viability at concentrations up to 25 μM when tested against a variety of human primary cell lines. In the hERG patch clamp assay, INCB9471 inhibited hERG potassium current with an IC50 of 4.5 μM, which is 500 times above its mean antiviral IC90 value.
In vitro ADMET profiling revealed that INCB9471 has a high permeability across Caco-2 monolayers with a value of 16.5 × 10−6 cm/s, suggesting a potential for high oral absorption. Bidirectional transport studies across Caco-2 cells indicated that this compound is not a substrate for P-gp or other efflux transporters. In protein binding, INCB9471 has a free fraction of 16% in human serum, resulting in a mean protein binding adjusted IC90 of ∼60 nM in antiviral activity. When incubated with human liver microsomes, INCB9471 exhibited low intrinsic clearance. However, studies with recombinant CYP isozymes showed that INCB9471 is a substrate for CYP3A4 but not other major CYPs. Recombinant CYP3A4 metabolized INCB9471 to its O-de-ethylated metabolite (compound 20a). No glutathione adducts of INCB9471 or its known metabolites were detected when INCB9471 was incubated with human S9, with or without NADPH and the cofactor glutathione. INCB9471 is not a CYP inhibitor, with IC50 values of >25 μM against five major CYP isozymes CYP3A4, CYP2D6, CYP1A2, CYP2C9, and CYP2C19 nor a CYP inducer at concentrations up to 30 μM.
In vivo, INCB9471 exhibited low systemic clearance and high volume of distribution when administered iv in rats and dogs, which resulted in a long half-life of 6 h in rats and 11 h in dogs (Table 2). Longer half-lives were observed when dosed orally in both species. The oral bioavailability was maximum in rats (F = 100%) and near maximum in dogs (F = 95%). The high level of plasma concentrations at 24 h (C24 h) relative to the peak concentrations (Cmax), coupled with the long oral half-life in both species, underscores a potential for once a day dosing for this compound.
Table 2. PK Parameters of INCB9471.
| rats | dogs | ||
|---|---|---|---|
| dose iv/po (mg/kg) | 1/20 | 1/20 | |
| iv | CL (L/h/kg) | 0.62 | 0.2 |
| Vdss (L/h) | 4.1 | 3.8 | |
| T1/2 (h) | 6 | 11 | |
| AUC (nM h) | 2898 | 7854 | |
| po | Tmax (h) | 3 | 0.5 |
| Cmax (nM) | 4071 | 14007 | |
| C24 h (nM) | 1088 | ||
| T1/2 (h) | 12 | 17 | |
| AUC (nM h) | 55160 | 149779 | |
| F (%) | 100 | 95 |
Given the excellent in vitro and in vivo profiles, INCB9471 was selected for preclinical evaluation. The safety profile from GLP toxicology studies in rodents and primates justified its advancement into human clinical trials. Phase I and phase II human clinical trials demonstrated that INCB9471 was safe and efficacious in viral load reduction and exhibited a better PK profile (T1/2 = 58−60 h in repeat dosing) than Sch-D (T1/2 = 28−33 h in repeat dosing).16
Acknowledgments
We thank Naiming Zhou, Kathy He Wang, Maryanne Covington, and Yanlong Li for technical assistance.
Supporting Information Available
Experimental procedures for synthesis of compounds 6a,b, 13a, and 20a−22a and assay protocols. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Samson M.; Labbe O.; Mollereau C.; Vassart G.; Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry 1996, 35, 3362–3367. [DOI] [PubMed] [Google Scholar]
- Alkhatib G.; Combadiere C.; Broder C. C.; Feng Y.; Kennedy P. E.; Murphy P. M.; Berger E. A. CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 1996, 272, 1955–1958. [DOI] [PubMed] [Google Scholar]
- Deng H.; Liu R.; Ellmeier W.; Choe S.; Unutmaz D.; Burkhart M.; DiMarzio P.; Marmon S.; Sutton R. E.; Hill C. M.; Littman D.; Landau N. R. Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996, 381, 661–666. [DOI] [PubMed] [Google Scholar]
- Dragic T.; Litwin V.; Allaway G. P.; Martin S. R.; Huang Y.; Nagashima K. A.; Cayanan C.; Maddon P. J.; Koup R. A.; Moore J. P.; Paxton W. A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC CKR-5. Nature 1996, 381, 667–673. [DOI] [PubMed] [Google Scholar]
- Liu R.; Paxton W. A.; Choe S.; Ceradini D.; Martin S. R.; Horuk R.; Macdonald M. E.; Stuhlmann H.; Koup R. A.; Landau N. R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiple-exposed individuals to HIV-1 infection. Cell 1996, 86, 367–377. [DOI] [PubMed] [Google Scholar]
- Michael N. L.; Chang G.; Louie L. G.; Mascola J. R.; Dondero D.; Birx D. L.; Sheppard H. W. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat. Med. 1997, 3, 338–340. [DOI] [PubMed] [Google Scholar]
- Shiraishi M.; Aramaki Y.; Seto M.; Imoto H.; Nishikawa Y.; Kanzaki N.; Okamoto M.; Sawada H.; Nishimura O.; Baba M.; Fujino M. Discovery of novel, potent, and selective small-molecule CCR5 antagonists as anti-HIV-1 agents: Synthesis and biological evaluation of anilide derivatives with a quaternary ammonium moiety. J. Med. Chem. 2000, 43, 2049–2063. [DOI] [PubMed] [Google Scholar]
- Palani A.; Tagat J. R. Discovery and development of small-molecule chemokine coreceptor CCR5 antagonists. J. Med. Chem. 2006, 49, 2851–2867. [DOI] [PubMed] [Google Scholar]
- Wood A.; Armour D. The discovery of the CCR5 receptor antagonist, UK-427,857, a new agent for the treatment of HIV infection and AIDS. Prog. Med. Chem. 2005, 43, 239–271. [DOI] [PubMed] [Google Scholar]
- Dorr P.; Westby M.; Dobbs S.; Griffin P.; Irvine B.; Macartney M.; Mori J.; Rickett G.; Smith-Burchnell C.; Napier C.; Webster R.; Armour D.; Price D.; Stammen B.; Wood A.; Perros M. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type-I activity. Antimicrob. Agents Chemother. 2005, 49, 4721–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. E.; Steinmetz O. M.; Stahl R. A.; Panzer U. Targeting of Th1-associated chemokine receptors CXCR3 and CCR5 as therapeutic strategy for inflammatory diseases. Mini-Rev. Med. Chem. 2007, 7, 1089–1096. [DOI] [PubMed] [Google Scholar]
- Vierboom M. P.; Zavodny P. J.; Chou C. C.; Tagat J. R.; Pugliese-Sivo C.; Strizki J.; Steensma R. W.; McCombie S. W.; Celebi-Paul L.; Remarque E.; Jonker M.; Narula S. K.; Hart B. Inhibition of the development of collagen-induced arthritis in rhesus monkeys by a small molecular weight antagonist of CCR5. Arthritis Rheum. 2005, 52, 627–636. [DOI] [PubMed] [Google Scholar]
- Tagat J. R.; McCombie S. W.; Nazareno D.; Labroli M. A.; Xiao Y.; Steensma R. W.; Strizki J. M.; Baroudy B. M.; Cox K.; Lachowicz J.; Varty G.; Watkins R. Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV: Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]-4-[4-{2-methoxy-1(R)-4-(trifluoromethyl)-phenyl}ethyl-3(S)-methyl-1-piperazinyl]-4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective and orally bioavailable CCR5 antagonist. J. Med. Chem. 2004, 47, 2405–2408. [DOI] [PubMed] [Google Scholar]
- Strizki J. M.; Tremblay C.; Xu S.; Wojcki L.; Wagner N.; Gonsiorek W.; Hipkin W.; Chou C.-C.; Pugliesie-Sivo C.; Xiao Y.; Tagat J. R.; Cox K.; Priestley T.; Sorota S.; Huang W.; Hirsch M.; Reyes G. R.; Baroudy B. M. Discovery and characterization of Vicriviroc (Sch-417690), a CCR5 antagonist with potent activity against human immunodeficiency virus type-1. Antimicrob. Agents Chemother. 2005, 49, 4911–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen E. N.; Zhang W.; Muci A. R.; Ecker J. R.; Deng L. Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane. J. Am. Chem. Soc. 1991, 113, 7063–7064. [Google Scholar]
- Schürmann D.; Fätkenheuer G.; Reynes J.; Michelet C.; Raffi F.; van Lier J.; Caceres M.; Keung A.; Sansone-Parsons A.; Dunkle L. M.; Hoffmann C. Antiviral activity, pharmacokinetics and safety of vicriviroc, an oral CCR5 antagonist, during 14-day monotherapy in HIV-infected adults. AIDS 2007, 21, 1293–1299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.