Abstract
During the course of our research efforts to develop a potent and selective γ-secretase inhibitor for the treatment of Alzheimer's disease, we investigated a series of carboxamide-substituted sulfonamides. Optimization based on potency, Notch/amyloid-β precursor protein selectivity, and brain efficacy after oral dosing led to the discovery of 4 (BMS-708163). Compound 4 is a potent inhibitor of γ-secretase (Aβ40 IC50 = 0.30 nM), demonstrating a 193-fold selectivity against Notch. Oral administration of 4 significantly reduced Aβ40 levels for sustained periods in brain, plasma, and cerebrospinal fluid in rats and dogs.
Keywords: γ-Secretase, brain penetrant, oxadiazole, Alzheimer's, amyloid, clinical candidate
Histopathological and genetic evidence suggest that the amyloid-β (Aβ) peptide plays a key causative role in Alzheimer's disease (AD).1 Aβ is generated by the proteolytic processing of the amyloid-β precursor protein (APP) through consecutive cleavages by β-site APP-cleaving enzyme (BACE) and γ-secretase. APP is cleaved by BACE to form a β-secretase-derived C-terminal fragment of APP (βCTF), which undergoes further cleavage by γ-secretase to form Aβ.2 Most Aβ isoforms contain a common N terminus, formed by BACE cleavage, while heterologous cleavage by γ-secretase creates Aβ isoforms ranging from 37 (Aβ37) to 42 (Aβ42) amino acid residues. Aβ40 is the most abundant isoform, while Aβ42 is most tightly linked with AD pathogenesis.
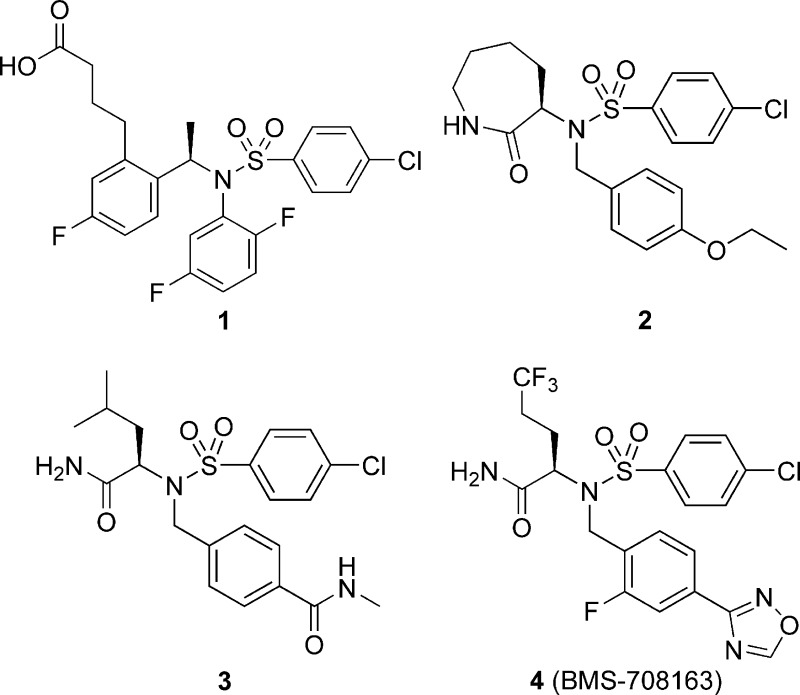
In our laboratories, one approach for the treatment of AD has been to identify potent and selective γ-secretase inhibitors to lower the production of Aβ in the brains of affected and at-risk patients. Because of the role of γ-secretase in Notch processing, the identity of secretase inhibitors with minimal impact on Notch signaling was also an important feature of our strategy. Our first γ-secretase clinical candidate, the 4-chloro-N-(2,5-difluorophenyl)phenylsulfonamide 1 (Figure 1),3 progressed to phase I clinical trials. However, clinical progression of 1 was halted due to significant pharmacokinetic liabilities and lack of significant Aβ lowering in both single-ascending and multiple-ascending dose studies in man.4 In addition to our efforts in the clinic, Wyeth recently reported preclinical5 results with the γ-secretase inhibitor begacestat, and Eli Lilly presented clinical evidence indicating reductions in plasma Aβ40 with the small molecule γ-secretase inhibitor LY450139.6 The phase I clinical trials with 1 provided specific information to guide our efforts to develop an improved second-generation compound for clinical evaluation. Compound 1 demonstrated significant human Pregnane-X Receptor (PXR) transactivation (EC60 = 2.4 uM), indicating potential for autoinduction in man. In addition, 1 had a modest brain to plasma ratio (B/P = 0.2) in Tg2576 mice. We reasoned that improvement in the brain to plasma ratio should increase central exposure and efficacy and decrease side effects associated with peripheral exposure such as autoinduction and Notch-related toxicities. Furthermore, 1 showed limited ability to lower brain Aβ40 in rats, which may be related to lower levels of APP-derived secretase substrate in this model.7
Figure 1.
Novel sulfonamide-based γ-secretase inhibitors.
The discovery of a second-generation compound with improved pharmacokinetic properties commenced with the identification of the novel 2-oxoazepan-3-yl-benzenesulfonamide 2 (Aβ42 IC50 = 9.0 nM) from a high-throughput screen of the BMS corporate compound deck.8
Early structure−activity relationship (SAR) exploration identified an improvement in Aβ40 in vitro potency by deannulation of the caprolactam ring of 2 (Table 1), which afforded a variety of acyclic amino acid amide analogues that were initially optimized based on potency, selectivity, and desirable physicochemical properties. The (R)-derived amino acid sulfonamides consistently provided higher potency. Further exploration into the SAR of the acyclic carboxamide series led to N-methyl-substituted benzenecarboxamide 3, which was identified as potent inhibitor of Aβ40 formation (IC50 = 0.14 nM).
Table 1. Summary of GS H4-8 SW1-40 IC50 Values and Notch (mNotch1-ΔE) IC50 Values for Selected Sulfonamide-Based γ-Secretase Inhibitorsa.
| IC50 ± SD (nM) |
|||
|---|---|---|---|
| compound | Aβ | Notch | Notch/APP ratio |
| 1 | 1.4 ± 1.2 | 540 ± 230 | 390 |
| 2 | 4.1 ± 1.2b | 960 ± 420 | 230 |
| 3 | 0.14 ± 0.05 | 26 ± 9.1 | 190 |
| 4 | 0.30 ± 0.15 | 58 ± 23 | 193 |
IC50 and standard deviation values were calculated on the basis of a minimum of three independent experiments.
IC50 values measured using a GS H4-8 SW1-42 assay (n = 3).
Compound 3 was tested in female wild-type rats to determine central and peripheral efficacy following oral administration at 1, 3, 10, and 30 mg/kg. Animals were sacrificed at 5 h postdose, and brain and plasma samples were collected for analyses of both drug exposure and Aβ40 levels.9 A single dose of compound 3 at 30 mg/kg significantly reduced both plasma Aβ40 and brain Aβ40 to levels of 14 ± 21 and 59 ± 12% (% of vehicle control ± SEM), respectively. However, no significant reductions in brain Aβ40 were observed at 1, 3, and 10 mg/kg. Although we were encouraged by this preliminary finding, this series required further optimization to reduce the dose necessary for Aβ40 reduction in the brain. Consistent with the discrepancy between central and peripheral efficacy, analysis of plasma and brain exposures of 3 revealed that the brain to plasma ratio was very low (brainconcn/plasmaconcn = 0.01). In addition, 3 had poor in vitro microsomal stability [% remaining after 10 min of incubation in liver microsomes = 19% (rat) and 44% (human)],10 indicating the potential for rapid clearance in vivo. In an effort to improve brain penetration and metabolic stability, a variety of substituents on the benzenecarboxamide nitrogen were tested.11
1,2,4-Oxadiazoles have been successfully employed as amide and ester bioisosteres for the optimization of brain-penetrant molecules.12−14 We utilized this approach for optimizing 3 and prepared a number of oxadiazole analogues of 3. From this effort, the novel oxadiazole (R)-2-(4-chloro-N-(2-fluoro-4-(1,2,4-oxadiazol-3-yl)benzyl)phenylsulfonamido)-5,5,5-trifluoropentanamide (4; BMS-708163)15 was synthesized and subsequently evaluated in vitro and in vivo. The Cytochrome P-450 (CYP) inhibition in human recombinant CYP enzymes for compound 2 was reported to be in the nanomolar range for CYP isoforms 3A4 and 2C19.8 SAR was not performed on CYP inhibition per se; however, this liability was continually monitored in subsequent analogues. Compound 4 was evaluated in vitro as an inhibitor of human recombinant CYPs. Weak inhibition (IC50 = 20 μM) was observed for CYP2C19. No inhibition was observed for the other isoforms up to the maximum tested concentration of 40 μM.
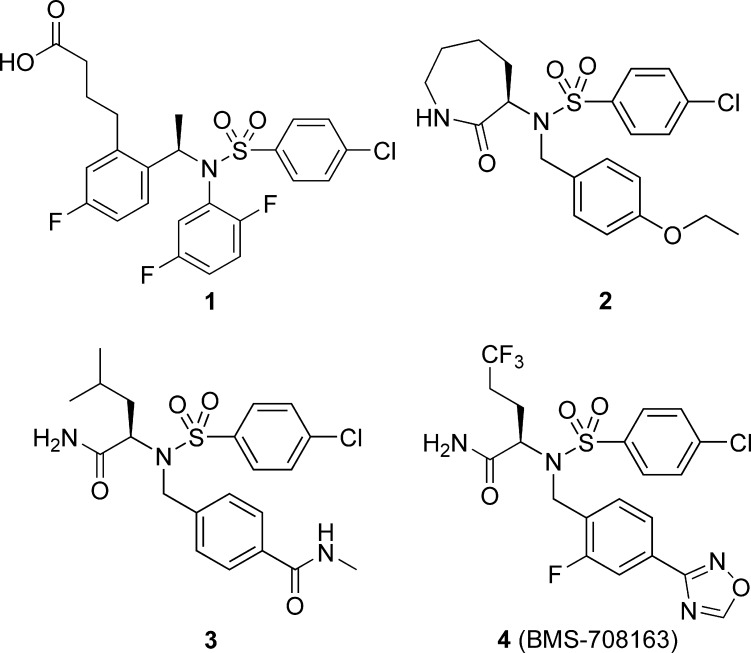
The synthesis of 4 was initiated via an asymmetric Strecker reaction with trifluorobutyraldehyde 5 and (R)-α-methylbenzylamine,16 affording nitrile 6 as a 3.65:1 mixture of diastereomers (Scheme 1). Subsequent hydrolysis of nitrile 6 directly to the carboxamide 7, followed by fractional crystallization of the HCl salt, afforded 8 in a >99:1 ratio of the desired (R,R) vs the (S,R) diastereomer. Removal of the chiral auxiliary under hydrogenolysis conditions with catalytic palladium hydroxide gave the amine hydrochloride salt 9, followed by conversion of the amine to the corresponding sulfonamide under standard conditions afforded 10 in 84% yield over the two steps. Alkylation of 10 with cesium carbonate and 4-cyano-2-fluorobenzyl bromide 11 afforded 12 in 68% yield with no loss of stereochemical purity as determined by chiral high-performance liquid chromatography. Lastly, conversion of 12 to oxadiazole 4 was accomplished via a two-step procedure starting with the addition of hydroxylamine to the arylnitrile, affording the intermediate amide-oxime 13. Annulation of the amide-oxime 13 with triethyl orthoformate and a catalytic amount of boron trifluoride etherate gave the oxadiazole 4 in 69% yield over two steps.17 The overall conversion of aldehyde 5 to oxadiazole 4 was amenable to scale-up as needed for in vivo testing.
Scheme 1. Synthesis of Compound 4.
Reagents and conditions: (a) NaCN, AcOH, MeOH, 3.65:1 de, 89%. (b) H2SO4, CH2Cl2. (c) HCl, Et2O/MeOH recrystallization, >99% de, 23%. (d) Pd(OH)2, H2 (50 psi), EtOH/H2O. (e) 4-Chlorobenzene-1-sulfonyl chloride, DIPEA, CH2Cl2, 84% (two steps). (f) Cs2CO3, DMF, 68%. (g) NH2OH, EtOH. (h) BF3:OEt2, CH(OMe)3, CH2Cl2 69% (two steps).
In vitro, 4 demonstrated an improved metabolic stability profile relative to 3 across species [% remaining after 10 min of incubation = 32% (rat), 75% (dog), and 97% (human)], as well as a significantly diminished PXR transactivation profile (EC60 > 16.7uM) relative to compound 1. Compound 4 potently inhibited the formation of Aβ40 and Aβ42 in H4-8Sw cells. Analysis of multiple experiments yielded an IC50 = 0.30 ± 0.15 nM (mean ± SD, n = 50) for Aβ40 inhibition and an IC50 = 0.27 ± 0.12 nM (mean ± SD, n = 45) for Aβ42 inhibition.
In addition to APP processing, γ-secretase activity is required for signaling by the Notch family of transmembrane receptors.18,19 Because inhibition of Notch signaling causes undesired, mechanism-based side effects, a cellular assay for Notch1-ΔE signaling was used to counterscreen γ-secretase inhibitors.20−22 Compound 4 exhibited weaker potency for inhibition of Notch processing, IC50 = 58 ± 23 nM (mean ± SD, n = 58), as compared to its inhibition potency for APP cleavage. On the basis of the cellular potencies for inhibiting Aβ40 generation (0.30 nM) and Notch signaling (58 nM) in these assays, 4 demonstrated a Notch/APP selectivity ratio of 193X (95% CI = 163−232). With compound 4, there were no dose-limiting effects in dogs with QD/PO dosing for 6 months at 3 mg/kg.
Table 2 illustrates the pharmacokinetic parameters of 4 in female rats and male dogs.23 In both rats and dogs following an IV dose, plasma concentrations exhibited a multiexponential decline. The total body clearance of 4 was low in both species (rat =11.7 ± 2.48 mL/min/kg; dog = 4.20 ± 0.93 mL/min/kg). The volume of distribution at steady state (Vss) was high, indicating extensive extravascular distribution (rat = 5.34 L/kg; dog = 3.87 L/kg). Oral bioavailability from solution formulations of 4 was 105 and 42% in rats and dogs, respectively. In addition, 4 demonstrated a high brain to plasma ratio (brainconcn/plasmaconcn = 2.4) in dogs.
Table 2. Single-Dose Pharmacokinetic Parameters of 4 in Rats and Dogsa.
| species | route | dose (mg/kg) | Cmax (μM) | Tmax (h) | AUC (0−24 h) (μM h) | T1/2 (h) | CLTp (mL/min/kg) | Vss (L/kg) | F (%) |
|---|---|---|---|---|---|---|---|---|---|
| rat (female) | IV | 1 | 2.60 ± 0.42 | 6.90 ± 2.20 | 11.7 ± 2.48 | 5.34 ± 0.74 | |||
| PO | 10 | 2.29 ± 1.1 | 14.0 ± 9.2 | 27.2 ± 7.39 | 105 | ||||
| dog (male) | IV | 1 | 6.04 ± 1.12 | 11.4 ± 1.76 | 4.20 ± 0.93 | 3.87 ± 0.56 | |||
| PO | 2.5 | 0.58 ± 0.24 | 0.75 ± 0.25 | 6.35 ± 2.07 | 42 |
Mean ± SD. Compound 4 (10 μM) was 97.0 ± 0.7% bound to human serum proteins and 96.3−97.9% bound to serum proteins in the animal species studied.
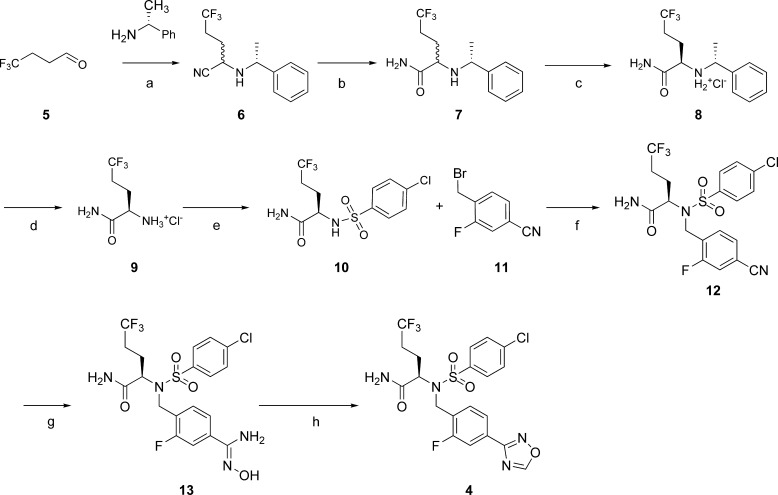
On the basis of favorable oral bioavailability and sufficient half-life for QD dosing, 4 was dosed orally in female rats and analyzed for its potential to reduce Aβ40 in plasma and brain over a time course of 24 h at doses of 1, 10, and 100 mg/kg. Compound 4 significantly reduced both plasma and brain Aβ40 levels relative to control at 10 and 100 mg/kg for the entire dosing interval (Figure 2A,B). The increase in plasma Aβ levels observed at the 1 mg/kg dose is related to an intrinsic characteristic of γ-secretase pharmacology and is observed with low levels of γ-secretase inhibition (Figure 2A).7 In brain, 4 demonstrated significant Aβ40 lowering for 8 h after an oral dose of 1 mg/kg (Figure 2B). In a separate study, when measured 5 h after single oral doses ranging from 3 to 100 mg/kg, 4 significantly lowered CSF Aβ40 levels in rats (data not shown).
Figure 2.
Plasma and brain Aβ40 following a single dose of 4 in female rats. The time course of changes in (A) plasma Aβ40 and (B) brain Aβ40. Mean ± SEM (n = 3 animals) for each time point shown.
In addition to the in vivo studies performed in rats, 4 was administered orally to male beagle dogs to measure Aβ40 lowering in brain and CSF. In this study, dogs were administered a single oral dose of 2 mg/kg of 4 and euthanized 2, 3, 5, 10, 16, and 24 h postdose (n = 1 or 2 per time point). Aβ40 measurements from the frontal cortex and CSF showed a rapid and sustained reduction in brain and CSF Aβ40 levels (Figure 3). In this experiment, CSF Aβ reductions measured in the dog correlated with Aβ reductions measured in the frontal cortex. Because it is not possible to directly measure human brain Aβ, a pharmacologic proof of concept of Aβ reductions in humans will rely primarily on measurement of a surrogate biomarker(s). On the basis of the correlation observed between brain and CSF Aβ40 lowering activity in dogs, our results suggest that CSF Aβ40 may serve as a surrogate biomarker for brain Aβ40 levels in humans.
Figure 3.
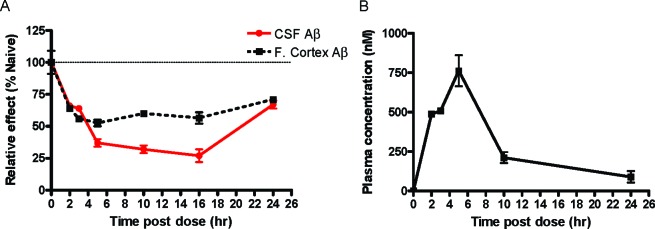
(A) Brain and CSF Aβ40 % reduction and (B) plasma concentration by 4 dosed PO 2 mg/kg in male dogs. Mean ± SEM (n = 1 or 2 animals) for each time point shown.
In summary, we designed and synthesized the novel oxadiazole-substituted sulfonamide 4 as a potent and selective γ-secretase inhibitor for the potential treatment of Alzheimer's disease. Replacement of the benzamide functionality in compound 3 with a bioisosteric oxadiazole as well as incorporation of a trifluoromethyl group24 in the side chain improved brain penetration and significantly increased the ability of this class of compounds to lower brain Aβ40. Compound 4 is currently undergoing clinical evaluation to determine its potential to act as a disease-modifying agent for the treatment of Alzheimer's disease. Results from these studies and a more detailed SAR summary surrounding the discovery of 4 will be presented in due course.
Acknowledgments
We thank Rex Denton, and Gary Pilcher for evaluation of the in vivo toxicology studies of 4, Mark Thompson, Sam Varma, Carol Krause, Victoria Wong, and Harvey Ferguson for contributions to in vitro studies, and Nina Hoque, Jason Corsa, Tracey Fiedler, Maria Pierdomenico, Valerie Guss, Wendy Clarke and Sarah J. Taylor for contributions to in vivo studies.
Supporting Information Available
Experimental details for synthetic procedures and associated chemical data for compounds 3−13 as well as experimental details on the Aβ40 and mNotch1-ΔE assays. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Walsh D. M.; Selkoe D. J. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron 2004, 441181–193. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Alzheimer's disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 812741–766. [DOI] [PubMed] [Google Scholar]
- Barten D. M.; Guss V. L.; Corsa J. A.; Loo A.; Hansel S. B.; Zheng M.; Munoz B.; Srinivasan K.; Wang B.; Robertson B. J.; Polson C. T.; Wang J.; Roberts S. B.; Hendrick J. P.; Anderson J. J.; Loy J. K.; Denton R.; Verdoorn T. A.; Smith D. W.; Felsenstein K. M. Dynamics of β-amyloid reductions in brain, cerebrospinal fluid, and plasma of β-amyloid precursor protein transgenic mice treated with a γ-secretase inhibitor. J. Pharmacol. Exp. Ther. 2005, 3122635–643. [DOI] [PubMed] [Google Scholar]
- Zheng M.; Wang J.; Flint O. P.; Krishna R.; Yao M.; Pursley J. M.; Thakur A.; Boulton D. W.; Santone K. S.; Barten D. M.; Anderson J. J.; Felsenstein K. M.; Hansel S. B. Studies on the pharmacokinetics and metabolism of a γ-secretase inhibitor BMS-299897, and exploratory investigation of CYP enzyme induction. Xenobiotica 2009, 397544–555. [DOI] [PubMed] [Google Scholar]
- Mayer S. C.; Kreft A. F.; Harrison B.; Abou-Gharbia M.; Antane M.; Aschmies S.; Atchison K.; Chlenov M.; Cole D. C.; Comery T.; Diamantidis G.; Ellingboe J.; Fan K.; Galante R.; Gonzales C.; Ho D. M.; Hoke M. E.; Hu Y.; Huryn D.; Jain U.; Jin M.; Kremer K.; Kubrak D.; Lin M.; Lu P.; Magolda R.; Martone R.; Moore W.; Oganesian A.; Pangalos M. N.; Porte A.; Reinhart P.; Resnick L.; Riddell D. R.; Sonnenberg-Reines J.; Stock J. R.; Sun S.; Wagner E.; Wang T.; Woller K.; Xu Z.; Zaleska M. M.; Zeldis J.; Zhang M.; Zhou H.; Jacobsen J. S. Discovery of Begacestat, a Notch-1-Sparing γ-Secretase Inhibitor for the Treatment of Alzheimer's Disease. J. Med. Chem. 2008, 51, 7348–7351. [DOI] [PubMed] [Google Scholar]
- Siemers E. R.; Dean R. A.; Friedrich S.; Ferguson-Sells L.; Gonzales C.; Farlow M. R.; May P. C. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-β after inhibition of γ-secretase. Clin. Neuropharmacol. 2007, 30, 317–325. [DOI] [PubMed] [Google Scholar]
- Burton C. R.; Meredith J. E.; Barten D. M.; Goldstein M. E.; Krause C. M.; Kieras C. J.; Sisk L.; Iben L. G.; Polson C.; Thompson M. W.; Lin X.-A.; Corsa J.; Fiedler T.; Pierdomenico M.; Cao Y.; Roach A. H.; Cantone J. L.; Ford M. J.; Drexler D. M.; Olson R. E.; Yang M. G.; Bergstron C. P.; McElhone K. E.; Bronson J. J.; Macor J. E.; Blat Y.; Grafstrom R. H.; Stern A. M.; Seiffert D. A.; Zaczek R.; Albright C. F.; Toyn J. H. The amyloid-β rise and γ-secretase inhibitor potency depend on the level of substrate expression. J. Biol. Chem. 2008, 2833422992–23003. [DOI] [PubMed] [Google Scholar]
- Parker M. F.; Bronson J. J.; Barten D. M.; Corsa J. A.; Du W.; Felsenstein K. M.; Guss V. L.; Izzarelli D.; Loo A.; McElhone K. E.; Marcin L. R.; Padmanabha R.; Pak R.; Polson C. T.; Toyn J. H.; Varma S.; Wang J.; Wong V.; Zheng M.; Roberts S. B. Amino-caprolactam derivatives as γ-secretase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 5790–5795. [DOI] [PubMed] [Google Scholar]
- Aβ40 levels were determined as described in Anderson J. J.; Holtz G.; Baskin P. P.; Turner M.; Rowe B.; Wang B.; Kounnas M. Z.; Lamb B. T.; Barten D.; Felsenstein K.; McDonald I.; Srinivasan K.; Munoz B.; Wagner S. L. Reductions in β-amyloid concentrations in vivo by the γ-secretase inhibitors BMS-289948 and BMS-299897. Biochem. Pharmacol. 2005, 69, 689–698. [DOI] [PubMed] [Google Scholar]
- The stability in liver microsomes was determined by a high-throughput in-house assay using substrate concentrations of 3 μM and 1 mg/mL microsomal protein across species. Incubations were performed at 37 °C in sodium phosphate buffer (100 mM), pH 7.4, and quenched after 10 min. Samples were analyzed by LC/MS/MS, and the % remaining was reported.
- Gillman K. W.; et al. Unpublished results.
- Saunders J.; MacLeod A. M.; Merchant K.; Showell G. A.; Snow R. J.; Street L. J.; Baker R. Ester Bio-isosteres: Synthesis of Oxadiazolyl-1-azabicyclo[2.2.1]heptanes as Muscarinc Agonists. J. Chem. Soc., Chem. Commun. 1988, 1618–1619. [Google Scholar]
- Watjen F.; Baker R.; Englestoff M.; Herbert R.; Macleod A.; Knight A.; Merchant K.; Moseley J.; Saunders J.; Swain C. J.; Wong E.; Springer J. P. Novel Benzodiazepine Receptor Partial Agonists: Oxadiazolylimidazobenzodiazepines. J. Med. Chem. 1989, 32, 2282–2291. [DOI] [PubMed] [Google Scholar]
- Street L. J.; Baker R.; Book T.; Kneen C. O.; MacLeod A. M.; Merchant K. J.; Showell G. A.; Saunders J.; Herbert R. H.; Freedman S. B.; Harley E. A. Synthesis and Biological Activity of 1,2,4-Oxadiazole Derivatives: Highly Potent and Efficacious Agonists for Cortical Muscarinic Receptors. J. Med. Chem. 1990, 33, 2690–2697. [DOI] [PubMed] [Google Scholar]
- Starrett J. E. Jr.; Gillman K. W.; Olson R. E.. Novel alpha-(N-sulfonamido)acetamide compound as an inhibitor of beta amyloid peptide production. U.S. Patent Application 2009/0111858 A1, 2009.
- Bayston D. J.; Griffin J. L. W.; Gruman A.; Polywka M. E. C.; Scott R. M.. Process for the preparation of cyclopropylglycine. U.S. Patent 6,191,306, 2001.
- Kitamura S.; Fukushi H.; Miyawaki T.; Kawamura M.; Terashita Z.; Naka T. Orally active GPIIb/IIIa antagonists: synthesis and biological activities of masked amidines as prodrugs of 2-[(3S)-4-[(2S)-2-(4-amidinobenzoylamino)-3-(4-methoxyphenyl) propanoyl]-3-(2-methoxy-2-oxoethyl)-2-oxopiperazinyl]acetic acid. Chem. Pharm. Bull. 2001, 493268–277. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S; Rand M. D.; Lake R. J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 2845415770–776. [DOI] [PubMed] [Google Scholar]
- Kadesch T. Notch signaling: A dance of proteins changing partners. Exp. Cell Res. 2000, 26011–8. [DOI] [PubMed] [Google Scholar]
- Searfoss G. H.; Jordan W. H.; Calligaro D. O.; Galbreath E. J.; Schirtzinger L. M.; Berridge B. R.; Gao H.; Higgins M. A.; May P. C.; Ryan T. P. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. J. Biol. Chem. 2003, 278, 46107–46116. [DOI] [PubMed] [Google Scholar]
- Wong G. T.; Manfra D.; Poulet F. M.; Zhang Q.; Josien H.; Bara T.; Engstrom L.; Pinzon-Ortiz M.; Fine J. S.; Lee H.-J. J.; Zhang L.; Higgins G. A.; Parker E. M. Chronic treatment with the gamma-Secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J. Biol. Chem. 2004, 279, 12876–12882. [DOI] [PubMed] [Google Scholar]
- Milano J.; McKay J.; Dagenais C.; Foster-Brown L.; Pognan F.; Gadient R.; Jacobs R. T.; Zacco A.; Greenberg B.; Ciaccio P. J.; et al. Modulation of Notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 2004, 82, 341–358. [DOI] [PubMed] [Google Scholar]
- Female rats were employed due to differences in the pharmacokinetic profile of 4 following administration to male and females rats. A discussion of this phenomenon will be the subject of a future publication.
- Tam T. F.; Leung-Toung R.; Wang Y.; Zhao Y.. Preparation of fluorinated derivatives of deferiprone as iron chelators for treating iron-overload diseases including neurodegenerative disorders. WO 2008/116301 A1,2009.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.