Abstract
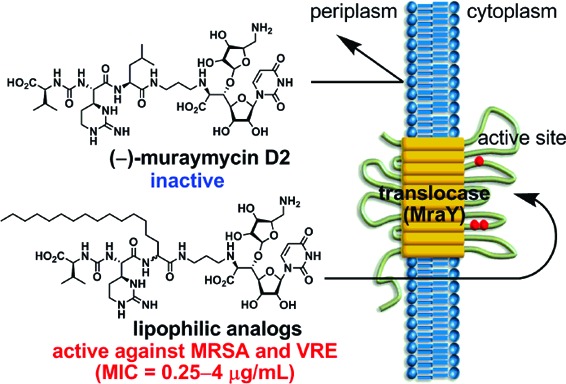
Muraymycin analogues with a lipophilic substituent were synthesized using an Ugi four-component assemblage. This approach provides ready access to a range of analogues simply by altering the aldehyde component. The impact of the lipophilic substituent on the antibacterial activity was very large, and analogues 7b−e and 8b−e exhibited good activity against a range of Gram-positive bacterial pathogens including methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. This study also showed that the accessory urea-dipeptide motif contributes to MraY inhibitory and antibacterial activity. The knowledge obtained from our structure−activity relationship study of muraymycins provides further direction toward the design of potent MraY inhibitors. This study has set the stage for the generation of novel antibacterial “lead” compounds based on muraymycins.
Keywords: Antibiotics, drug resistance, MraY, muraymycin, peptidoglycan, Ugi four-component reaction
The extensive use of antibiotics has raised a serious global public health problem. Because bacterial pathogens inevitably develop resistance to every new drug launched in the clinic, the need for new antibiotics to counteract drug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Staphylococcus aureus (VRSA) is critical.1 In choosing novel antibacterial agents to address this problem, several criteria need to be considered as follows: The target must be essential for growth, the agent must be different from existing drugs, and the initial “hit” scaffold must be amenable to structural changes that allow for optimization of the potency and efficacy to generate “lead” compounds.2−5
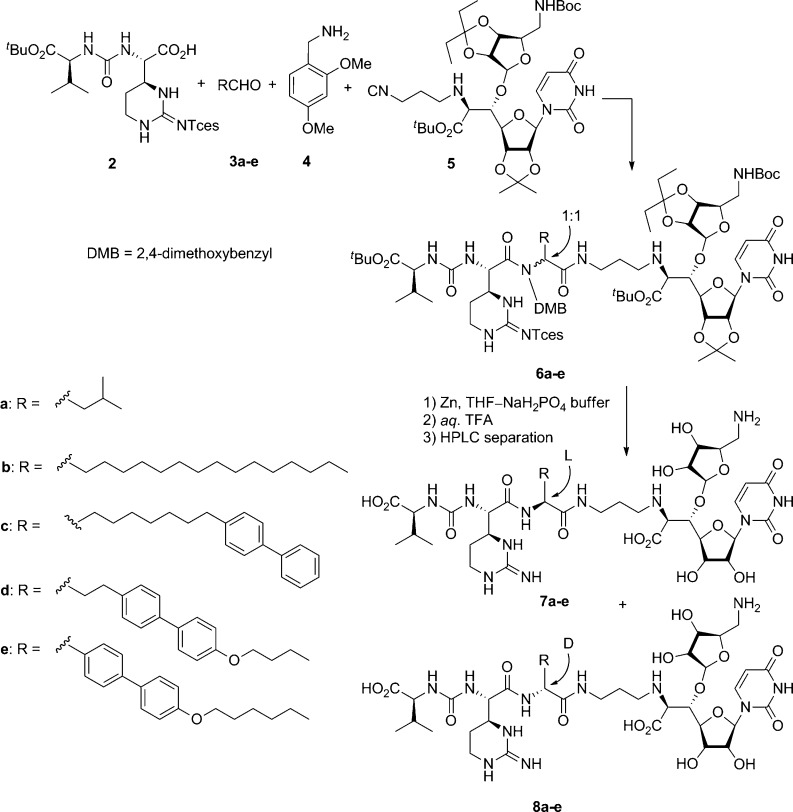
The muraymycins (MRYs) (Figure 1, 1), isolated from a culture broth of Streptomyces species,6,7 are members of a class of naturally occurring 6′-N-alkyl-5′-β-O-aminoribosyl-C-glycyluridine antibiotics.8,9 The MRYs that have a lipophilic side chain have been shown to exhibit excellent antimicrobial activity against Gram-positive bacteria. In particular, the efficacy of the MRYs in S. aureus-infected mice represents a promising lead for the development of new antibacterial agents. The MRYs inhibit the formation of lipid II and peptidoglycan and are believed to be inhibitors of the phospho-MurNAc-pentapeptide transferase (MraY), which is responsible for the formation of lipid I in the peptidoglycan biosynthesis pathway.10−14 Because MraY is an essential enzyme in bacteria,15 it is a potential target for the development of general antibacterial agents. Because of these promising biological properties, the MRYs have become intriguing, challenging synthetic targets.16−18 Recently, we have accomplished the first total synthesis of MRY D2 (7a) and its epimer (8a),19 featuring the convergent assemblage of the urea-dipeptide carboxylic acid 2, isovaleraldehyde (3a), 2,4-dimethoxybenzylamine (4), and the isonitrile derivative of the aminoribosyluridine 5 by an Ugi four-component reaction20 (U4CR) as shown in Scheme 1. Herein, we describe the synthesis and biological evaluation of MRY analogues associated with a lipophilic side chain as an initial structure−activity relationship (SAR) study of MRYs, and the MRY analogues that we discovered were effective against drug-resistant bacterial pathogens.
Figure 1.

Structures of MRYs (1).
Scheme 1.
First, the inhibitory activity of 7a on the purified MraY enzyme (Bacillus subtilis) was examined by quantifying the incorporation of MurNAc-[14C]pentapeptide by MraY from UDP-MurNAc-[14C]pentapeptide into lipid I, the product of MraY (Table 1).21 It turned out that it was a strong MraY inhibitor with an IC50 value of 0.01 μM. Compound 8a, which is the epimer of 7a at the α-position of the Leu residue, exhibited good MraY inhibitory activity (IC50 = 0.09 μM) albeit reduced by a factor of 9 as compared to that of 7a. The antibacterial activity of 7a and 8a was then evaluated.22 However, no antibacterial activity was exhibited by these compounds when subjected to a range of Gram-positive bacteria up to 64 μg/mL (Table 2), despite the fact that they did exhibit potent inhibitory activity against their target enzyme MraY. Because the lipid bilayer of the cytoplasmic membrane is thought to be a common barrier in bacteria, this observed membrane permeability; these compounds lack the hydrophobic side chain found in MRY A and B classes (Figure 1), which have good antibacterial activity. Although not essential to MraY inhibition, the fatty acyl side chain attached to the peptide moiety of MRYs is necessary for antibacterial activity. Because the β-acyloxyleucine moiety found in the MRY A and B classes could be susceptible to β-elimination or hydrolysis by enzymes such as esterases, these shortcomings would have to be overcome. Therefore, we designed and synthesized analogues, which were linked to a hydrophobic substituent on the MRY core structure via a C−C bond, as chemically and biologically stable isosteres of the MRYs. Our synthetic route to 7a and 8a provides ready access to a range of analogues containing an unnatural amino acid simply by altering the aldehyde component. Thus, 2,194, 5,19 and hexadecanal (3b) gave, via the U4CR in EtOH, 6b containing a pentadecylglycine residue in its structure in 37% yield as a 1:1 mixture of diastereomers. Deprotection of 6b was achieved by a two-step sequence [Zn, 1 M aqueous NaH2PO4, tetrahydrofuran (THF), then 80% aqueous TFA] to afford the hydrophobic analogue 7b and its diastereomer 8b, which were easily separated by reverse-phase high-performance liquid chromatography (HPLC). The newly formed stereogenic center at the pentadecylglycine residue of each diastereomer was determined by conventional amino acid analysis23 by using l- or d-pentadecylglycine as the reference compound (see the Supporting Information, Scheme S1). The inhibitory effect of 7b and 8b on purified MraY activity was then evaluated (Table 1). The lipophilic analogues 7b and 8b were found to be weaker inhibitors of MraY than 7a but still potent (IC50 = 0.33 and 0.74 μM, respectively). Introducing the long lipophilic side chain was still acceptable for MraY inhibition. The nature of the lipophilic side chain could influence the antibacterial activity by modulating the membrane permeability or the affinity to the target enzyme MraY. Therefore, analogues incorporating a biphenyl moiety at the end (7c and 8c), in the middle (7d and 8d), and at the junction (7e and 8e) of the lipophilic moiety were also designed and synthesized. The corresponding aldehydes, precursors for the U4CR assemblage, were prepared as shown in Scheme 2. Cross-metathesis of 4-vinylbiphenyl (9) and 7-octen-1-ol in the presence of Grubbs second generation catalyst24 (77% yield) was followed by catalytic hydrogenation to give the saturated biphenylalcohol 10 (quant.). The primary alcohol was oxidized to give the aldehyde 3c. On the other hand, the aldehyde 11(25) was bis-homologated by the Horner−Wadsworth−Emmons reaction to give the corresponding α,β-unsaturated ester, which was reduced by catalytic hydrogenation to afford the saturated ester 12 in 82% yield over two steps. The ethyl ester was reduced with DIBAL to give the aldehyde 3d in quantitative yield. The last aldehyde 3e(26) was prepared from the carboxylic acid 13 by reduction of the acid with BH3·THF (95% yield) followed by oxidation with MnO2 (quant.). With these aldehyde units in hand, we have synthesized the analogues 7c−e and 8c−e via the U4CR followed by global deprotection in a manner similar to the preparation of 7b and 8b as shown in Scheme 1.27
Table 1. Inhibitory Activities of the Synthesized Compounds against the MraY Enzymea.
| 7a | 8a | 7b | 8b | 17 | |
|---|---|---|---|---|---|
| IC50 (μM) | 0.01 | 0.09 | 0.33 | 0.74 | 5 |
The activities of the compounds were tested against purified MraY from B. subtitis.21 The assay was performed in a reaction mixture of 10 mL containing, in final concentrations, 100 mM Tris-HCl, pH 7.5, 40 mM MgCl2, 1.1 mM C 55-P, 250 mM NaCl, 0.25 mM UDP-MurNAc-[14C]pentapeptide (337 Bq), and 8.4 mM N-lauroyl sarcosine. The mixture was incubated for 30 min at 37 °C. The radiolabeled substrate UDP-MurNAc-pentapeptide and reaction product (lipid I, product of MraY) were separated by TLC on silica gel plates. The radioactive spots were located and quantified with a radioactivity scanner. IC50 values were calculated with respect to a control assay without the inhibitor. Data represent the mean of independent triplicate determinations.
Table 2. Antibacterial Activity.
| MIC (ug/mL)a |
||||||
|---|---|---|---|---|---|---|
| compound | S. aureus ATCC 29213 (MSSA) | S. aureus SR3637 (MRSA) | E. faecalis ATCC 29212 | E. faecalis SR7914 (VRE) | E. faecium ATCC 19434 | E. faecium SR7917 (VRE) |
| 7a | >64 | >64 | >64 | >64 | >64 | >64 |
| 8a | >64 | >64 | >64 | >64 | >64 | >64 |
| 7b | 2 | 4 | 4 | 4 | 4 | 2 |
| 8b | 2 | 4 | 2 | 4 | 0.5 | 0.25 |
| 7c | 4 | 4 | 4 | 16 | 4 | 8 |
| 8c | 8 | 16 | 8 | 16 | 4 | 4 |
| 7d | 16 | 32 | 16 | 64 | 16 | 32 |
| 8e | 64 | 64 | 32 | 64 | 4 | 8 |
| 7e | 4 | 8 | 8 | 8 | 4 | 4 |
| 8e | 16 | 16 | 16 | 16 | 4 | 8 |
| 17 | >64 | >64 | 32 | 64 | 64 | 64 |
| vancomycin | 1 | 1 | 1 | >64 | 0.5 | >64 |
MICs were determined by a microdilution broth method as recommended by the NCCLS with cation-adjusted Mueller−Hinton broth (CA-MHB).22 Serial 2-fold dilutions of each compound were made in appropriate broth, and the plates were inoculated with 5 × 104 CFU of each strain in a volume of 0.1 mL. Plates were incubated at 35 °C for 20 h, and then, MICs were scored.
Scheme 2.
The antibacterial activity of this series of compounds was evaluated, and the results are summarized in Table 2. The impact of the lipophilic substituent on the antibacterial activity was very good, and both 7b and 8b exhibited good activity against a range of Gram-positive bacterial pathogens including S. aureus SR3637 (MRSA) and Enterococcusfaecium SR7917 (VRE) with minimum inhibitory concentration (MIC) values of 0.25−4 μg/mL. The activity was comparable to that reported for MRY A and B classes.6,7 Thus, the membrane permeability plays an important role in terms of the antibacterial activity among this class of natural products. Of significance is the discovery of 8b with the “unnatural” d-pentadecylglycine residue, which exhibited eight times more potent antibacterial activity against E. faecium SR7917 than that of 7b with the “natural” stereochemistry. Overall, analogues with the “natural” stereochemistry were slightly more potent than those with “unnatural” stereochemistry against Staphylococci. Introducing the rigid biphenyl group into the lipophilic side chain did not improve antibacterial activity but rather reduced potency in the case of 7d and 8d, which contain the biphenyl group in the middle of the side chain. Further optimization of the lipophilic side chain therefore will be necessary. All of the compounds prepared in this study exhibited no cytotoxicity against human hepatoceller liver carcinoma (HepG2) cells (IC50 > 100 μg/mL).
MRYs share the accessory urea-dipeptide motif in addition to the 5′-O-aminoribosyl-5′-C-glycyluridine moiety. To rapidly see the impact of the accessory motif, a truncated analogue 17, where the accessory motif was completely removed from 7b or 8b, was prepared from 15(28) as shown in Scheme 3, and the biological properties were compared. The truncated analogue 17 was found to be a much weaker MraY inhibitor with an IC50 value of 5 μM, which was a 6−12-fold reduction of the inhibitory activity as compared to 7b and 8b (Table 1). The antibacterial activity of 17 was greatly decreased with MICs ranging from 32−64 μg/mL, although 17 possessed a hydrophobic substituent (Table 2). These results clearly show that the urea-dipeptide accessory motif is also a contributing factor in the interaction with MraY to result in strong antibacterial inhibitory activity. In addition to and apart from the binding pocket interacting with the 5′-O-aminoribosyl-5′-C-glycyluridine moiety, it is noteworthy that there would be an additional binding site in MraY, which recognizes the accessory urea-dipeptide motif.
Scheme 3.
In summary, MRY analogues with a lipophilic substituent were synthesized by U4CR, which enabled us easily to prepare MRY analogues containing unnatural amino acids. The impact of the lipophilic substituent on the antibacterial activity was very large, and analogues 7b−e and 8b−e exhibited good activity against a range of Gram-positive bacterial pathogens, including MRSA and VRE. This study also indicated that the accessory urea-dipeptide motif contributes to MraY inhibitory and antibacterial activity. The knowledge obtained from our SAR study of MRYs would provide further direction toward the design of potent MraY inhibitors. Because MRYs possess relatively high molecular weight and polarity, these structural features are in good agreement with the property space characteristics of antibacterial agents.29−32 This initial study has set the stage for the generation of novel antibacterial “lead” compounds based on MRYs and is currently being expanded.
Acknowledgments
We thank Kouichi Uotani (Discovery Research Laboratories, Shionogi & Co., Ltd.) for evaluating the antibacterial activities of the synthesized analogues. We thank S. Oka and A. Tokumitsu (Center for Instrumental Analysis, Hokkaido University) for measurement of the mass spectra.
Supporting Information Available
Full experimental procedures, compound purities by HPLC, and NMR data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Department of Internal Medicine, Faculty of Medicine and Health Sciences, United Arab Emirates University, P.O. Box 17666, Al Ain, United Arab Emirates.
T.T. contributed to the synthesis of muraymycin analogues. B.A.-D. contributed to the MraY inhibitory assay. S.I. was the PI of T.T., made significant writing and editing contributions, and provided significant intellectual input. A.B. was the PI of B.A.-D. and a collaborator of the Matsuda lab and provided significant intellectual input. H.O. contributed to the cytotoxicity assay. A.M. was the main PI of the entire project, was the overseeing PI of T.T., S.I., and A.B., made significant writing and editing contributions, and provided significant intellectual input.
We acknowledge the CNRS UMR 8619 (support of B.A.-D. and A.B.).
Supplementary Material
References
- Rice L. B. Unmet medical needs in antibacterial therapy. Biochem. Pharmacol. 2006, 71, 991–995. [DOI] [PubMed] [Google Scholar]
- Payne D. J.; Gwynn M. N.; Holms D. J.; Pompliano. D. L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nature Rev. Drug Discovery 2007, 6, 29–40. [DOI] [PubMed] [Google Scholar]
- Talbot G. H.; Bradley J.; Edwards J. E. Jr.; Gilbert D.; Scheld M.; Bartlett J. G. Bad bugs need drugs: An update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 42, 657–668. [DOI] [PubMed] [Google Scholar]
- Overbye K. M.; Barrett. J. F. Antibiotics: Where did we go wrong?. Drug Discovery Today 2005, 10, 45–52. [DOI] [PubMed] [Google Scholar]
- Monagham R. L.; Barrett J. F. Antibacterial drug discovery—Then, now and the genomics future. Biochem. Pharmacol. 2006, 71, 901–909. [DOI] [PubMed] [Google Scholar]
- Walsh C. Where will new antibiotics come from?. Nature Rev. Microbiol. 2003, 1, 65–70. [DOI] [PubMed] [Google Scholar]
- McDonald L. A.; Barbieri L. R.; Carter G. T.; Lenoy E.; Lotvin J.; Petersen P. J.; Siegel M. M.; Singh G.; Williamson R. T. Structures of the muraymycins, novel peptidoglycan biosynthesis inhibitors. J. Am. Chem. Soc. 2002, 124, 10260–10261. [DOI] [PubMed] [Google Scholar]
- Carter G. T.; Lotvin J. A.; McDonald L. A.. WO 2002085310 A2, 2002.
- Ochi K.; Ezaki M.; Iwani M.; Komori T.; Kohsaka M.. EP-333177, 1989.
- Yoshida Y.; Yamanaka H.; Sakane K.. JP H05-78385, 1993.
- Bugg T. D. H.; Lloyd A. J.; Roper D. I. Phospho-MurNAc-pentapeptide translocase (MraY) as a target for antibacterial agents and antibacterial proteins. Infect. Dis. Drug Targets 2006, 6, 85–106. [DOI] [PubMed] [Google Scholar]
- Kimura K.; Bugg T. D. H. Recent advances in antimicrobial nucleoside antibiotics targeting cell wall biosynthesis. Nat. Prod. Rep. 2003, 20, 252–273. [DOI] [PubMed] [Google Scholar]
- Bouhss A.; Mengin-Lecreulx D.; Le Beller D.; Van Heijenoort J. Topological analysis of the MraY protein catalyzing the first membrane step of peptidoglycan synthesis. Mol. Microbiol. 1999, 34, 576–585. [DOI] [PubMed] [Google Scholar]
- Bouhss A.; Trunkfield A. E.; Bugg T. D.; Mengin-Lecreulx D. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol. Rev. 2008, 32, 208–33. [DOI] [PubMed] [Google Scholar]
- Al-Dabbagh B.; Henry X.; El Ghachi M.; Auger G.; Blanot D.; Parquet C.; Mengin-Lecreulx D.; Bouhss A. Active site mapping of MraY, a member of the polyprenyl-phosphate N-acetylhexosamine 1-phosphate transferase superfamily, catalyzing the first membrane step of peptidoglycan biosynthesis. Biochemistry 2008, 47, 8919–8928. [DOI] [PubMed] [Google Scholar]
- Winn M.; Goss R. J. M.; Kimura K.; Bugg T. D. H. Antimicrobial nucleoside antibiotics targeting cell wall assembly: Recent advances in structure−function studies and nucleoside biosynthesis. Nat. Prod. Rep. 2010, 27, 279–304. [DOI] [PubMed] [Google Scholar]
- Yamashita A.; Norton E.; Petersen P. J.; Rasmussen B. A.; Singh G.; Yang Y.; Mansour T. S.; Ho D. M. Muraymycins, novel peptidoglycan biosynthesis inhibitors: synthesis and SAR of their analogues. Bioorg. Med. Chem. Lett. 2003, 13, 3345–3350. [DOI] [PubMed] [Google Scholar]
- Lin Y.-I.; Li Z.; Francisco G. D.; McDonald L. A.; Davis R. A.; Singh G.; Yang Y.; Mansour T. S. Muraymycins, novel peptidoglycan biosynthesis inhibitors: Semisynthesis and SAR of their derivatives. Bioorg. Med. Chem. Lett. 2002, 12, 2341–2344. [DOI] [PubMed] [Google Scholar]
- Spork A. P.; Koppermann S.; Ducho C. Improved convergent synthesis of 5′-epi-analogues of muraymycin nucleoside antibiotics. Synlett 2009, 2503–2507. [Google Scholar]
- Tanino T.; Ichikawa S.; Shiro M.; Matsuda A. Total synthesis of (−)-muraymycin D2 and its epimer. J. Org. Chem. 2010, 75, 1366–1377. [DOI] [PubMed] [Google Scholar]
- For a review, see Dömling A.; Ugi I. Multicomponent reactions with isocyanides. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. [DOI] [PubMed] [Google Scholar]
- Bouhss A.; Crouvoisier M.; Blanot D.; Mengin-Lecreulx D. Purification and characterization of the bacterial MraY translocase catalyzing the first membrane step of peptidoglycan biosynthesis. J. Biol. Chem. 2004, 279, 29974–29980. [DOI] [PubMed] [Google Scholar]
- Maki H.; Miura K.; Yamano Y. Katanosin B and plusbacin A 3, inhibitors of peptidoglycan synthesis in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1823–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a review, see Bhushan R.; Brückner H. Marfey's reagent for chiral amino acid analysis: A review. Amino Acids 2004, 27, 231–247. [DOI] [PubMed] [Google Scholar]
- Scholl M.; Ding S.; Lee C. W.; Grubbs R. H. Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands. Org. Lett. 1999, 1, 953–956. [DOI] [PubMed] [Google Scholar]
- Kovganko V. N.; Kovganko N. N. 4,5-Dihydroisoxazoles in the synthesis of new metallomesogens. Russ. J. Org. Chem. 2006, 42, 430–434. [Google Scholar]
- Doherty G. A.; Li Z.; Hale J. J.; Mills S. G.. WO 2003062248 A2, 2003.
- In HPLC analysis, a similar behavior was observed between compounds with the R-configuration (7a,b, longer retention times) and those with the S-configuration (8a,b, shorter retention times) at the newly formed stereogenic center in the U4CR. On the basis of the relative retention times in HPLC analysis, the newly formed stereogenic centers of 7c−e and 8c−e were tentatively assigned according to the retention time.
- Hirano S.; Ichikawa S.; Matsuda A. Total synthesis of (+)-FR-900493 and establishment of its absolute stereochemistry. Tetrahedron 2007, 63, 2798–2804. [Google Scholar]
- Mishra R. K.; Garcia-Domenech R.; Galvez J. Getting discriminant functions of antibacterial activity from physicochemical and topological parameters. J. Chem. Inf. Comput. Sci. 2001, 41, 387–393. [DOI] [PubMed] [Google Scholar]
- Cronin M. T.; Aptula A. O.; Dearden J. C.; Duffy J. C.; Netzeva T. I.; Patel H.; Rowe P. H.; Schultz T. W.; Worth A. P.; Voutzoulidis K.; Schuurman G. Structure-based classification of antibacterial activity. J. Chem. Inf. Comput. Sci. 2002, 42, 869–878. [DOI] [PubMed] [Google Scholar]
- Murcia-Soler M.; Perez-Gimenez F.; Garcia-March F. J.; Salabert-Salvador M. T.; az-Villanueva W.; Castro-Blea M. J.; Villanueva-Pareja A. Artificial neural networks and linear discriminant analysis: A valuable combination in the selection of new antibacterial compounds. J. Chem. Inf. Comput. Sci. 2004, 44, 1031–1041. [DOI] [PubMed] [Google Scholar]
- O'Shea R.; Moser H. E. Physicochemical properties of antibacterial compounds: Implications for drug discovery. J. Med. Chem. 2008, 51, 2871–2878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.