Abstract
A series of benzisothiazole- and indolizine-β-d-glucopyranoside inhibitors of human SGLT2 are described. The synthesis of the C-linked heterocyclic glucosides took advantage of a palladium-catalyzed cross-coupling reaction between a glucal boronate and the corresponding bromo heterocycle. The compounds have been evaluated for their human SGLT2 inhibition potential using cell-based functional transporter assays, and their structure−activity relationships have been described. Benzisothiazole-C-glucoside 16d was found to be an inhibitor of SGLT2 with an IC50 of 10 nM.
Keywords: Benzisothiazole- and indolizine-β-d-glucopyranoside inhibitors, SAR, SGLT2, benzisothiazole-C-glucoside
Inhibitors of sodium glucose cotransporter 2 (SGLT2) have received considerable attention lately for the treatment of type 2 diabetes.1,2 SGLT2 is a 14 trans-membrane protein located in the proximal tubule of the kidney and is responsible for the reuptake of glucose from the glomerular filtrate to the plasma. Inhibitors of SGLT2 prevent the reabsorption of glucose leading to the rapid elimination of glucose in the urine. In the clinic, such agents have proven to reduce blood glucose levels and cause modest weight loss in diabetic patients through a mechanism that does not depend on insulin release from β cells.3,4 It has also been demonstrated that obese animals treated with SGLT2 inhibitors undergo weight loss in proportion to the amount of glucose that is excreted.5 At the present time, several companies are conducting clinical evaluations of SGLT2 inhibitors for the treatment of metabolic disorders including diabetes and obesity.
Another closely related member of the sodium glucose cotransporter family, SGLT1, is located on enterocytes and transports dietary carbohydrates from the lumen of the small intestine to the systemic circulation. Inhibitors of SGLT1 delay glucose absorption and may also be useful in treating diabetes. Evidence from animal studies suggests that the profound improvements in glucose metabolism that result from Roux-en-Y gastric bypass surgery are directly related to altering SGLT1 expression and slowing glucose influx.6 We were interested in identifying compounds that would be selective for SGLT2 and that would be rapidly absorbed from the gastrointestinal (GI) tract with high oral bioavailability to act in the kidney. SGLT2 selectivity over SGLT1 may be important to avoid GI side effects that could result from carbohydrate malabsorption in the small intestine. In addition, because SGLT1 is highly expressed in cardiac myocytes7 and serves as a glucose transporter in the heart,8 we desired selectivity to avoid the potential disruption of glucose availability in cardiac muscle cells.
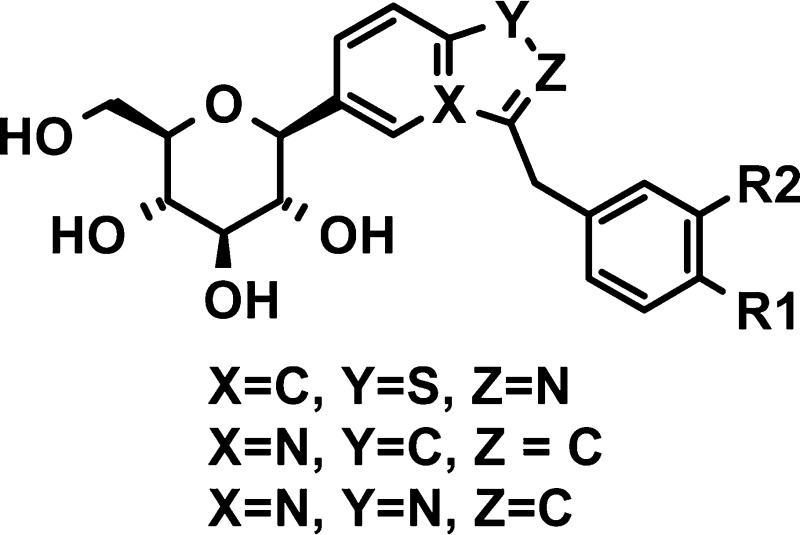
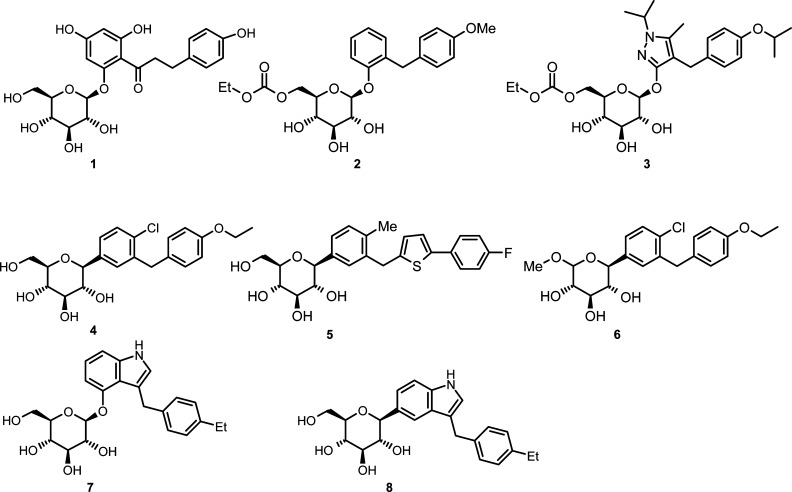
All known inhibitors of SGLT2 are carbohydrate-derived glycosides that trace their origins to the natural product phlorizin (1, Figure 1), which is a nonselective inhibitor of SGLT1 and SGLT2.9 Phlorizin is an O-glucoside and, as such, is subject to hydrolysis by O-glucosidases. As a result of the lability of glycosyl bonds, O-glucosides tend to have poor oral bioavailability unless the glucose moiety is masked as a prodrug, as is found in the 6′-O-ethyl carbonate derivatives, serglifozin etabonate (2) and remogliflozin etabonate (3). In diabetic rodent models, chronic treatment with 2 and 3 lowered fasting plasma glucose concentrations and improved glucose clearance following oral glucose treatment.10,11N-Glucosides and C-glucosides are more metabolically stable and tend to have higher oral bioavailability and plasma exposure without needing to be converted to a prodrug.2,12
Figure 1.
Scientists at Bristol-Myers Squibb have recently reported the discovery of a potent inhibitor of SGLT2, dapaglifozin (4), a C-glucoside with a long half-life and sustained duration of action.13 Johnson & Johnson in collaboration with Mitsubishi Tanabe have advanced canagliflozin (5), another C-aryl glucoside, to phase III clinical development. Most recently, scientists at Lexicon Pharmaceuticals have reported the discovery of metabolically stable O-xyloside inhibitors of SGLT2, their most potent analogue being 6, which possesses the same aglycone moiety as dapagliflozin.14 Beyond the monocyclic O- and C-glucosides, Zhang and co-workers at Johnson & Johnson have investigated O- and C-glucosides in which the aglycone is indole (7 and 8). They found that the C-glucosides had reduced potency as compared to the O-glucosides and proposed that the anomeric oxygen is important for full inhibition.15 In our program to discover novel and selective SGLT2 inhibitors, we set out to explore C-glucosides in which the aglycone is a benzo-fused heterocycle. Herein, we report our investigations into the synthesis and structure−activity relationships (SARs) of heterobicyclic β-d-glucopyranoside inhibitors of SGLT2.
In our initial attempts to construct heterocyclic C-aryl glucosides, we took advantage of the attractive strategy outlined by Meng et al.,13 namely, lithium−halogen exchange of a bromoaromatic ring and addition of the resulting aryllithium to a gluconolactone followed by reduction of the resulting gluconolactol to give the aromatic C-aryl glucoside. Although this approach worked well when the aromatic system was substituted phenyl or benzothiophene, when the aromatic group was an aza heterocycle such as benzisothiazole 12a or 12p, we failed to achieve addition of the lithio species to the gluconolactone. Instead, we used an alternative approach involving the Pd-catalyzed cross-coupling of the bromoaromatic with a glucal boronate, a new glycosylation method that has been described in a patent application by scientists at Tanabe.16,17
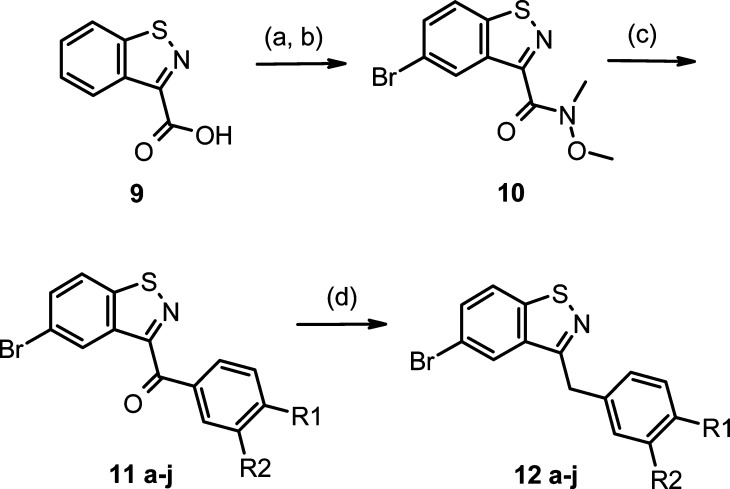
The synthesis of the benzisothiazole intermediates 12a−j is outlined in Scheme 1. Benzisothiazole 9 was readily prepared in three steps from thiophenol.18 Bromination of 9 under acidic conditions followed by condensation with N-methyl-O-methylhydroxylamine hydrochloride gave Weinreb amide 10, which was treated with various arylmagnesium bromides to give compounds 11a−j. The biaryl ketones were reduced with triethylsilane in TFA to provide compounds 12a−j.
Scheme 1.
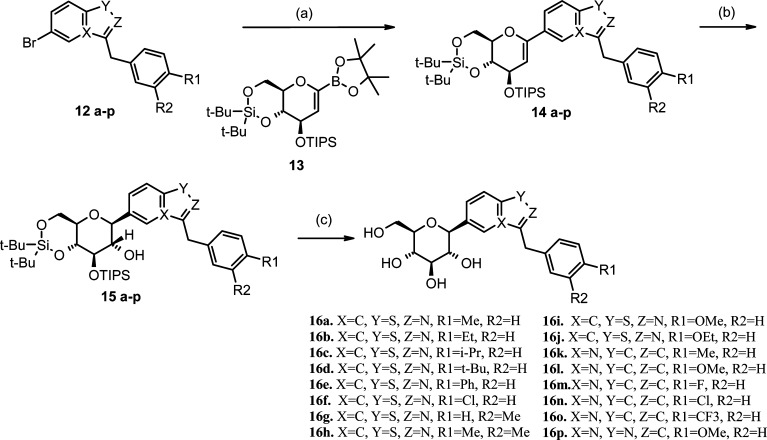
Heterocyclic C-aryl glucosides 16a−p were prepared from bromoheterocycles 12a−p as outlined in Scheme 2. Palladium-catalyzed cross-coupling of bromides 12a−p with pinacol boronate ester 13(16,17) provided compounds 14a−p. Hydroboration with borane-THF followed by oxidation with hydrogen peroxide afforded β-glucosides 15a−p as confirmed by the J coupling constant (∼9.5 Hz) between H1′ and H2′ in the 1H NMR spectrum. None of the α anomers were isolated. Finally, deprotection of 15a−p with HF/TEA or with TBAF gave target compounds 16a−p.
Scheme 2.
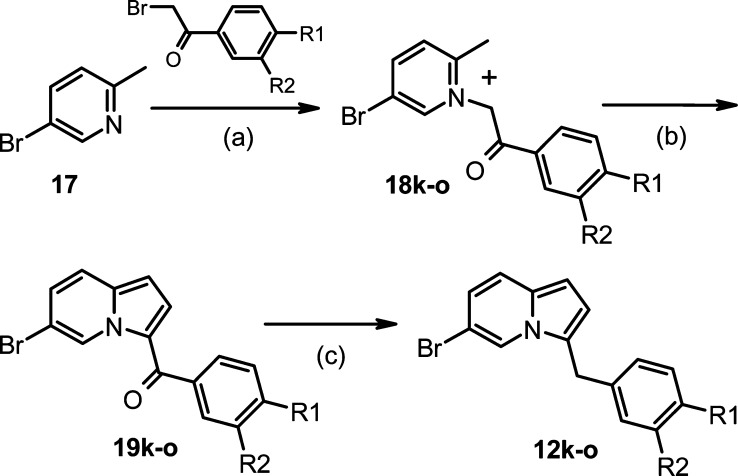
As shown in Scheme 3, the indolizine intermediates 19k−o were prepared in two steps from 3-bromopicoline 17. Compound 17 was treated with commercially available bromoacetophenones to afford picolinium salts 18k−o, which were treated with dimethylsulfate-DMF to provide ketones 19k−o.19,20 The ketones 19k−o were reduced to diarylmethanes 12k−o using sodium borohydride with aluminum chloride.
Scheme 3.
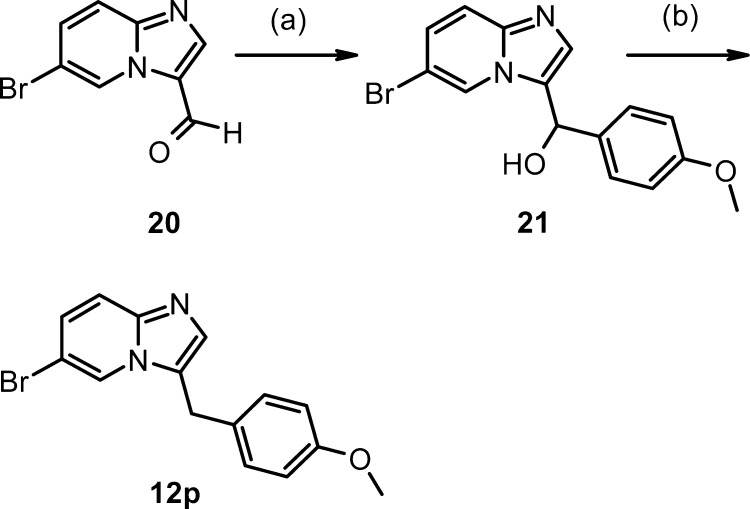
The imidazo[1,2-a]pyridine 12p was prepared in two steps from commercially available aldehyde 20 (Scheme 4). Treatment of 20 with 4-methoxyphenylmagnesium bromide followed by reduction of the resulting alcohol 21 with TFA/NaBH4 gave 12p.
Scheme 4.
The methodology that we have used to construct C-aryl glucosides, namely, the Pd-catalyzed cross-coupling of glucal boronates, will be a useful addition to the array of techniques that are available to carbohydrate chemists. The method allowed the construction of benzo-fused C-glucosides, which were not accessible using the conventional method.
The SGLT1 and SGLT2 inhibitory activity (IC50) of the heterocyclic C-glucosides was determined by monitoring the inhibition of uptake of 14C-labeled α-methyl-d-glucopyranoside by COS-7 cells, which transiently expressed human SGLT2 or SGLT1 using BacMam technology.21 The results are summarized in Table 1. Focusing initially on the benzisothiazole series, we found that the 4-methyl benzyl- (16a) and the 4-ethyl benzyl- (16b) substituted derivatives were potent inhibitors with pIC50 values of 80 and 50 nM, respectively. Increasing the size of the 4-substituent appeared to increase the inhibition potential. Following this trend, we synthesized the 4-isopropyl (16c) and the 4-tert-butyl (16d) analogues and found that 16d achieved inhibition with an IC50 of 10 nM. Exploring the size limits of the 4-substituent, we found that the 4-phenyl benzyl analogue (16e) lost potency. Likewise, the 4-chlorobenzyl analogue (16f) displayed less inhibition potential than the 4-alkyl analogues. We observed that the 4-methoxy and 4-ethoxy derivatives (16i and 16j) were less potent than the corresponding alkyl analogues. The 3-methylbenzyl and the 3,4-dimethylbenzyl analogues 16g and 16h were significantly less potent than 16a, indicating that a methyl group in the meta position was not tolerated.
Table 1. SGLT2 and SGLT1 Inhibition of Compounds 16a−p and 4a.

| pIC50 |
|||||||
|---|---|---|---|---|---|---|---|
| X | Y | Z | R1 | R2 | SGLT2 | SGLT1 | |
| 16a | C | S | N | Me | H | 7.09 ± 0.21 | <5 |
| 16b | C | S | N | Et | H | 7.30 ± 0.07 | <5 |
| 16c | C | S | N | i-Pr | H | 7.29 ± 0.23 | <5 |
| 16d | C | S | N | t-Bu | H | 8.00 ± 0.18 | <5 |
| 16e | C | S | N | Ph | H | 6.41 ± 0.15 | ND |
| 16f | C | S | N | Cl | H | 6.85 ± 0.04 | ND |
| 16 g | C | S | N | H | Me | 5.27 ± 0.01 | ND |
| 16 h | C | S | N | Me | Me | 6.21 ± 0.04 | ND |
| 16i | C | S | N | OMe | H | 6.83 ± 0.08 | <5 |
| 16j | C | S | N | OEt | H | 6.43 ± 0.18 | ND |
| 16k | N | C | C | Me | H | 7.21 ± 0.13 | <5 |
| 16l | N | C | C | OMe | H | 6.87 ± 0.02 | ND |
| 16m | N | C | C | F | H | 6.03 ± 0.21 | ND |
| 16n | N | C | C | Cl | H | 6.81 ± 0.20 | ND |
| 16o | N | C | C | CF3 | H | 6.95 ± 0.06 | <5 |
| 16p | N | N | C | OMe | H | <5 | ND |
| 4 | 9.84 ± 0.14 | <5 | |||||
Data represent −log (IC50) ± standard error.
Similar SAR trends were observed in the indolizine series. The 4-methylbenzyl (16k) and the 4-trifluoromethylbenzyl (16o) analogues had high SGLT2 inhibition (IC50 = 60 and 110 nM, respectively). The 4-methoxybenzyl (16l) and the 4-halobenzyl analogues (16m and 16n) were less potent than the 4-alkyl derivatives. Probing alternative aza heterocycle isosteres, we found that imidazo pyridine 16p, although having a similar overall shape and size as the benzisothiazole 16i (IC50 = 155 nM) and indazole 16l (IC50 = 135 nM), was devoid of SGLT2 inhibition activity. The most potent SGLT2 inhibitors were tested for SGLT1 inhibition and were found to be inactive against SGLT1 in all cases. Compound 4 was tested as a comparator and was found to be a highly selective inhibitor of SGLT2 and more potent than the benzisothiazoles.
The pharmacokinetic profiles of two of the benzisothiazole analogues, 16b and 16c, were determined in male CD rats. The results are summarized in Table 2. Both compounds were rapidly absorbed after oral dosing. Although the plasma clearance is high for these molecules, the bioavailability (% F, ratio of plasma drug concentrations after oral dosing versus IV dosing) is high, indicating that the compounds were stable enough in the GI tract to be absorbed well. C-Glucosides 16b and 16c did not require conversion to prodrug to achieve oral absorption. Although the prodrug approach has proven viable for SGLT2 inhibitors such as 2 and 3, which have clear antidiabetic effects in rodent models of diabetes,10,11 it would be preferable to avoid the extra chemical step of appending the prodrug moiety. A prodrug approach also introduces the additional development risk that the conversion of prodrug to parent may not translate from animals to humans. Furthermore, there can be large intrapatient variability in the pharmacokinetics of prodrug to parent conversion.
Table 2. Pharmacokinetic Profiles of Compounds 16b,c in Male CD Rats Following Intravenous and Oral Dosing.
| dose | 16b | 16c |
|---|---|---|
| IV 2 mg/kg | ||
| t1/2 (h) | 1.2 | 1.7 |
| AUCinf (h ng/mL) | 424 | 393 |
| Vss (L/kg) | 4.3 | 5.3 |
| CL (mL/min/kg) | 79.1 | 85.5 |
| PO 10 mg/kg | ||
| Cmax (ng/mL) | 422 | 321 |
| tmax (h) | 0.5 | 0.5 |
| AUCinf (h ng/mL) | 1642 | 1483 |
| F (%) | 77 | 74 |
In summary, we have taken advantage of an underutilized Pd-catalyzed cross-coupling reaction to synthesize a series of benzisothiazole and indolizine C-glucosides, which have extended our understanding of SGLT2 inhibitor SARs. We have established that potent SGLT2 inhibitors can be found in C-glucosides in which the aglycone is a benzo-fused heterocycle. We have also seen that the weakly basic imidazo pyridines, although possessing the same overall shape as the benzisothiazoles and indolizines, have no SGLT2 inhibition at all. Further work concerning the optimization of SGLT2 inhibitors suitable for clinical development will be reported in subsequent publications.
Acknowledgments
We thank William Clay for generating the SGLT2 and SGLT1 BacMam viruses and Sandy Kerner for the BacMam constructs.
Supporting Information Available
Description of the inhibition assay, pharmacokinetic study methods, and the synthesis of compounds 10−21. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Bays H. From victim to ally: The kidney as an emerging target for the treatment of diabetes mellitus. Curr. Med. Res. Opin. 2009, 25, 671–681. [DOI] [PubMed] [Google Scholar]
- Washburn W. Evolution of sodium glucose co-transporter 2 inhibitors as anti-diabetic agents. Expert Opin. Ther. Pat. 2009, 19, 1485–1499. [DOI] [PubMed] [Google Scholar]
- List J. F.; Woo V.; Morales E.; Tang W.; Fiedorek F. T. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009, 32, 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding J. P. H.; Norwood P.; T'joen C.; Bastien A.; List J. F.; Fiedorek F. T. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: Applicability of a novel insulin-independent treatment. Diabetes Care 2009, 32, 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenny J.; Harvey S.; Rooney S.; Godonis H.; Washburn W.; Whaley J.; Taylor S.; Pelleymounter M. A.. The Effect of Dapagliflozin, a Highly Selective SGLT2 Inhibitor, on Body Weight in Diet-induced Obese Rats. Annual Scientific Meeting of the North American Association for the Study of Obesity (NAASO), New Orleans, LA, October 20−24, 2007. [Google Scholar]
- Stearns A. T.; Balakrishnan A.; Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G950–G957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L.; Cryan E. V.; D'Andrea M. R.; Belkowski S.; Conway B. R.; Demarest K. T. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J. Cell. Biochem. 2003, 90, 339–346. [DOI] [PubMed] [Google Scholar]
- Banerjee S. K.; McGaffin K. R.; Pastor-Soler N. M.; Ahmad F. SGLT1 is a novel glucose transporter that is perturbed in disease states. Cardiovasc. Res. 2009, 84, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenkranz J. R. L.; Lewis N. G.; Kahn C. R.; Roth J. Phlorizin: A review. Diabetes/Metab. Res. Rev. 2005, 21, 31–38. [DOI] [PubMed] [Google Scholar]
- Fujimori Y.; Katsuno K.; Nakashima I.; Ishikawa-Takemura Y.; Fujikura H.; Isaji M. Remogliflozin etabonate, in a novel category of selective low-affinity sodium glucose cotransporter (SGLT2) inhibitors, exhibits antidiabetic efficacy in rodent models. J. Pharmacol. Exp. Ther. 2008, 327, 268–276. [DOI] [PubMed] [Google Scholar]
- Fujimori Y.; Katsuno K.; Ojima K.; Nakashima I.; Nakano S.; Ishikawa-Takemura Y.; Kusama H.; Isaji M. Sergliflozin etabonate, a selective SGLT2 inhibitor, improves glycemic control in streptozotocin-induced diabetic rats and Zucker fatty rats. Eur. J. Pharmacol. 2009, 609, 148–154. [DOI] [PubMed] [Google Scholar]
- Isaji M. Sodium-glucose cotransporter inhibitors for diabetes. Curr. Opin. Invest. Drugs (Thomson Sci.) 2007, 8, 285–292. [PubMed] [Google Scholar]
- Meng W.; Ellsworth B. A.; Nirschl A. A.; McCann P. J.; Patel M.; Girotra R. N.; Wu G.; Sher P. M.; Morrison E. P.; Biller S. A.; Zahler R.; Deshpande P. P.; Pullockaran A.; Hagan D. L.; Morgan N.; Taylor J. R.; Obermeier M. T.; Humphreys W. G.; Khanna A.; Discenza L.; Robertson J. G.; Wang A.; Han S.; Wetterau J. R.; Janovitz E. B.; Flint O. P.; Whaley J. M.; Washburn W. N. Discovery of Dapagliflozin: A Potent, Selective Renal Sodium-Dependent Glucose Cotransporter 2 (SGLT2) Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2008, 51, 1145–1149. [DOI] [PubMed] [Google Scholar]
- Goodwin N. C.; Mabon R.; Harrison B. A.; Shadoan M. K.; Almstead Z. Y.; Xie Y.; Healy J.; Buhring L. M.; DaCosta C. M.; Bardenhagen J.; Mseeh F.; Liu Q.; Nouraldeen A.; Wilson A. G. E.; Kimball S. D.; Powell D. R.; Rawlins D. B. Novel l-Xylose Derivatives as Selective Sodium-Dependent Glucose Cotransporter 2 (SGLT2) Inhibitors for the Treatment of Type 2 Diabetes. J. Med. Chem. 2009, 52, 6201–6204. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Urbanski M.; Patel M.; Cox G. G.; Zeck R. E.; Bian H.; Conway B. R.; Beavers M. P.; Rybczynski P. J.; Demarest K. T. Indole-glucosides as novel sodium glucose co-transporter 2 (SGLT2) inhibitors. Part 2. Bioorg. Med. Chem. Lett. 2006, 16, 1696–1701. [DOI] [PubMed] [Google Scholar]
- Nomura S.; Kawanishi E.; Ueta K. U.S. Patent 5,233,988. Chem. Abstr. 2005, 143, 387313.
- Nomura S.; Kawanishi E.; Ueta K.. WO 2005/012326. Chem. Abstr. 2005, 142, 219491.
- Xie W.; Herbert B.; Ma J.; Nguyen T. M.; Schumacher R.. WO2005/063767. Chem. Abstr. 2005, 143, 133366.
- Li H.; Xia Z.; Chen S.; Koya K.; Ono M.; Sun L. Development of a Practical Synthesis of STA-5312, a Novel Indolizine Oxalylamide Microtubule Inhibitor. Org. Process Res. Dev. 2007, 11, 246–250. [Google Scholar]
- Przewloka T.; Chen S.; Xia Z.; Li H.; Zhang S.; Chimmanamada D.; Kostik E.; James D.; Koya K.; Sun L. Application of DMF-methyl sulfate adduct in the regioselective synthesis of 3-acylated indolizines. Tetrahedron Lett. 2007, 48, 5739–5742. [Google Scholar]
- Condreay J. P.; Witherspoon S. M.; Clay W. C.; Kost T. A. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.