Abstract
In the past two decades, we have acquired an enormous amount of knowledge regarding the epidemiology, diagnosis, pathophysiology and treatment of type 2 diabetes and its comorbidities. In addition to the earlier landmark blood lipid and blood pressure lowering trials, the latest blood glucose lowering megatrials represent the zenith of this global effort to prevent and control diabetes, and its devastating consequences. Although many of these latter trials have yielded negative results and have shown the narrow risk‐benefit ratio of intensive treatment in patients with advanced disease, the exceedingly low event rates in these high‐risk patients who were carefully monitored and intensively managed made possible in these clinical trial settings have not been emphasized enough. The heterogeneity of the clinical outcomes in these studies further highlight the complexity of diabetes, which is more than managing a disease, but the multiple needs of a patient with multisystem dysfunction. In the final analysis, what transpires from these megatrials is the need to translate the key components of these studies, namely, protocol, team, documentation and monitoring, into our daily clinical practice to enable the care team to stratify risk, define needs, individualize therapy, monitor progress and reinforce compliance in order to achieve positive outcomes. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.00063.x, 2010)
Keywords: Randomized clinical trials, Disease management, Diabetes
Diabetes and cardiovascular disease revisited
More than a decade ago, Haffner et al1. first reported the similar incidence of myocardial infarction (19% in 7 years) between type 2 diabetic patients without a history of myocardial infarction and non‐diabetic patients with a history of myocardial infarction. This landmark study also confirmed the fourfold higher risk of coronary heart disease (CHD) in diabetic subjects compared with non‐diabetic subjects (4% in 7 years) and their high risk of having recurrent events once they had CHD (45% in 7 years). Importantly, type 2 diabetic patients with myocardial infarction were more likely to die before hospitalization2 and during the post‐myocardial infarction period than non‐diabetic subjects3, thus emphasizing the importance of optimal control of CHD risk factors for both primary and secondary prevention in type 2 diabetes.
Residual cardiovascular risk in type 2 diabetes
Due to their high absolute risk for CHD, for the same intervention, whether blood pressure or lipid lowering or blockade of renin angiotensin system, more events were prevented in type 2 diabetic patients than their non‐diabetic counterparts in large randomized clinical trials4–6. However, despite control of these multiple risk factors, type 2 diabetic patients continue to have high residual risk with an annual CHD event rate of 3–5%. In the Steno 2 Study, although 30–80% of patients receiving multifaceted care attained lifestyle modification, blood pressure and lipid goals, less than 10% of patients achieved the predefined HbA1c goal of 6.5%7.
Intensive blood glucose lowering in type 2 diabetes
In the United Kingdom Prospective Diabetes Study (UKPDS), which recruited newly diagnosed type 2 diabetic patients, a difference of 0.9% in HbA1c (7% in the intensively‐treated group vs 7.9% in the standard treatment group) over a 10‐year period was translated to 13–24% reduction in all‐cause death, cardiovascular events and microvascular complication rates in the 10‐year post‐trial period8. In the epidemiological analysis of the UKPDS, there was a linear relationship between HbA1c and incidence of macrovascular complications beyond 7%, raising the possibility that the lower the HbA1c, the better the clinical outcomes9. Three subsequent large scale randomized clinical trials, ACCORD10, ADVANCE11 and VADT12 were carried out to address the question whether lowering HbA1c to <7% conferred additional cardiovascular benefits. However, these studies have yielded heterogeneous results with many controversies rather than consensus13.
Despite achieving a similar HbA1c level of 6.5%, intensively‐treated patients in the ACCORD study had a higher mortality rate than the standard treatment group10, but not in the ADVANCE study11. In the latter study, there was a 21% risk reduction in nephropathy, but not retinopathy11. In contrast, in the ACCORD study, intensive control of blood glucose and lipids was respectively associated with 33% and 44% risk reduction in retinopathy14. Apart from differences in baseline risk factors, patterns of drug use and rapidity in reaching target HbA1c15, it is noteworthy that 30% of patients in the ADVANCE study came from Asia, mainly from China, whereas participants in the ACCORD study were mainly Caucasians.
Importance of early intensive treatment to achieve long‐term benefits
In the Asia‐Pacific Collaborative Study Cohort, diabetes conferred 2–3‐fold increased risk of CHD in both Asian and Caucasian populations. However, the effect size was considerably higher with an odds ratio of 4 in subjects younger than 60 years compared with 2 in those aged 60 years or older16. These findings are particularly pertinent to Asia, where the major increase in diabetes prevalence will occur in the young to middle‐aged group17,18. In the subgroup analysis of the ACCORD study, intensive blood glucose lowering reduced CHD by 20% in patients with no previous history of CHD and HbA1c <8%10. Given that type 2 diabetes is the predominant form of disease in young Asian subjects18 and that disease duration is one of the most important determinants for CHD19, together with findings from the ACCORD10 and ADVANCE study11, it can be inferred that early intensive glycemic control in young patients is likely to bring major reductions in cardiorenal event and mortality rates.
Importance of detecting renal disease to prevent CHD
The predilection of Asian type 2 diabetic patients to renal disease was first reported in the World Health Organization Multicenter Study for Vascular Disease in Diabetes (WHO‐MSVDD)20. This was subsequently confirmed by the reported 60% prevalence of nephropathy in Asian type 2 diabetic patients21 compared with 40% in their Caucasian counterparts22. In a subanalysis of the RENAAL study, Asian type 2 diabetic patients with moderate renal insufficiency had a higher rate of end‐stage renal disease (35%) than Caucasian patients (30%) in the placebo group after receiving comparable treatments for 3.5 years23,24. Similarly, in the ADVANCE study, Asian patients (5%) had the highest incidence of new onset or progression of nephropathy compared with Caucasian (3%) and eastern European populations (4%) after 5 years of follow up25.
Importance in stratifying risk and personalizing therapy
Albuminuria, a marker of endothelial damage, predicts cardiovascular and renal disease in both diabetic and non‐diabetic subjects26. In the Hong Kong Diabetes Registry, albuminuria and estimated glomerular filtration rate (eGFR) were the most consistent predictors for cardiorenal events and all‐cause death27,28. These findings emphasize the importance of periodic monitoring of renal parameters to stratify risks and assess treatment effectiveness.
In the UKPDS29 and Hong Kong Diabetes Registry30, HbA1c was a major determinant for progression of albuminuria and deterioration of renal function. With the onset of diabetic kidney disease (DKD), arbitrarily defined as eGFR <60 mL/min/1.72 m2, the risk of CHD is increased by 4–5‐fold compared with those without. This is mainly due to further changes in internal milieu associated with DKD, which include anemia, vascular calcification, oxidative stress and inflammation24.
Of note, the onset of DKD increases the risk of hypoglycemia as a result of impaired pharmacokinetics and pharmacodynamics with drug–drug interactions, prolonged effects of blood glucose lowering drugs and reduced counterregulation31. In these high‐risk subjects who often have autonomic neuropathy32 and silent cardiac ischemia33, hypoglycemia might precipitate cardiac events. Given the multiplicative effects of these risk factors and complications of clinical course, the need to phenotype and individualize treatment represents the first step to good clinical practice34.
Using structured care and team approach to improve clinical outcomes
In the ACCORD study, one or more hypoglycemic episodes requiring assistance was associated with an increased risk of death, although the effect size was considerably lower in the intensively‐treated group with a hazard ratio of 1.4 compared with 2.3 in the standard treatment group35. Counter‐intuitively, the risk of death in intensively‐treated patients who had severe hypoglycemia requiring medical assistance had a lower hazard ratio of 0.55 for mortality compared with the standard treatment group. These seemingly paradoxical findings suggest that with intensive monitoring, the adverse effects of intensive treatment might be mitigated, resulting in clinical benefits.
Changing our clinical practice and health care system
These observations led to a growing consensus on the need to use a team of trained health‐care personnel to stratify risk and deliver care protocols in order to get these patients to treatment goals safely and effectively36. Indeed, using these disease management protocols with predefined procedures, targets and decision support, major event rates can be reduced by 50–70% compared with usual care, which often lacks integration, coordination, monitoring and feedback37–40.
Despite the endorsement of the International Diabetes Federation on these principles41, there is a general lack of resources or incentives to develop care systems that incorporate these components, except in a few areas or centers. Without these changes in practice environment to facilitate integrated care and self management42, doctors managing patients with chronic diseases will not be able to fully utilize their expertise and knowledge to benefit their patients, just like a surgeon working without an operating theatre or a cardiologist working without a catheterization laboratory.
In contrast to real‐life practice, where fewer than 5% of patients attained three treatment goals (HbA1c <7%, blood pressure <130/80 mmHg and LDL‐cholesterol <2.6 mmol/L)43, nearly all patients in these megatrials attained treatment targets and were put on life‐saving drugs15. These changes in practice made possible by enrolment into a trial might explain the often negative results in these megatrials as a result of underestimation of these ‘trial effects’. Thus, although there is a need to learn from these negative findings, we must not lose sight of the extremely low annual event rate in these megatrials.
Importance of protocol, team, documentation and monitoring
Using the ACCORD Study as an example, despite the old age, long disease duration and high percentage of patients with prior history of CHD, the annual rate of cardiovascular event was only 1–1.5%10. This is in stark contrast to 2% per year in the younger and newly diagnosed patients in the UKPDS44, 3–5% per year in patients with multiple risk factors, but no prior history of CHD in the Steno 2 study, and 3–8% per year in the East‐West Study where treatment was less intensive in the early 1970–90s1 (Figure 1). Thus, what really transpires from these megatrials is the need to incorporate the key components of clinical trials (protocols, team, monitoring and feedback) into our daily clinical practice with cost‐effective analysis in order to persuade policy makers and payors to make these care systems accessible, affordable and sustainable (Figure 2).
Figure 1.
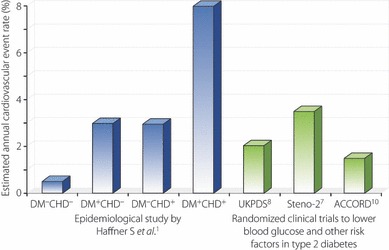
Estimated annual cardiovascular event rates in large scale epidemiological studies and randomized clinical trials since 1990. Despite the high‐risk nature of type 2 diabetic (DM) patients in the ACCORD study, more than 30% of whom had a history of coronary heart disease (CHD), intensive treatment and monitoring in a trial setting has given rise to event rates lower than the younger and newly diagnosed patients in the UKPDS, and patients with multiple risk factors without prior history of CHD in the Steno‐2 study, who were managed less intensively.
Figure 2.
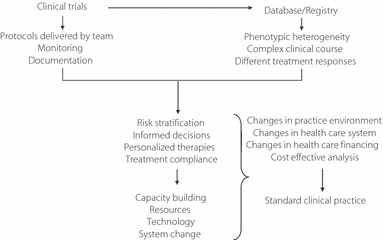
Learning from recent megatrials. The key components of a clinical trial include baseline assessments and delivery of protocol by a team with frequent monitoring and documentation of processes and responses. This team‐based approach enables risk stratification, informed decisions, individualized regimens, regular monitoring, improved compliance and better outcomes. In order to increase the accessibility of these care models, changes in clinical practice and health care system is needed to ensure its accessibility, affordability and sustainability.
Conclusion
During the past two decades, we have gained enormous insights into the epidemiology, pathophysiology and treatment of type 2 diabetes and its comorbidities. The heterogeneity of age, sex, ethnicity, disease duration, risk factors and complications interact in a multiplicative manner to increase the complexity of the clinical course and treatment responses. Thus, treating diabetes is more than treating a disease(s), but managing the multiple needs of an individual with multisystem dysfunction. By using a systematic approach to document these risk factors, complications, processes and outcomes, care providers will be in a better position to define their needs, make informed decisions and individualize treatment in order to maximize benefits and minimize harm. Although our ultimate goal is to discover a cure for diabetes and change our environment and lifestyle to prevent diabetes, given the compelling evidence from these megatrials, there is an urgent need to reform our health care system to ensure those who already have the disease receive proper education, care, monitoring and support using a team approach to reduce the societal and personal impacts of this devastating condition.
Acknowledgements
The author has received research grants, speakers’ fees and consultancy fee from Astra‐Zeneca, Bayer, Bristol‐Myers‐Squibbs, Daiichi‐Sankyo, Lilly, MSD, Pfizer and sanofi‐aventis, all of which have been donated to the Chinese University of Hong Kong to support ongoing research and education in the field of diabetes.
References
- 1.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234 [DOI] [PubMed] [Google Scholar]
- 2.Miettinen H, Lehto S, Salomaa V, et al. Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study Group. Diabetes Care 1998; 21: 69–75 [DOI] [PubMed] [Google Scholar]
- 3.Sprafka JM, Burke GL, Folsom AR, et al. Trends in prevalence of diabetes mellitus in patients with myocardial infarction and effect of diabetes on survival. The Minnesota Heart Survey. Diabetes Care 1991; 14: 537–543 [DOI] [PubMed] [Google Scholar]
- 4.Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the losartan intervention for endpoint reduction on hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359: 1004–1010 [DOI] [PubMed] [Google Scholar]
- 5.Heart Outcomes Prevention Evaluation (HOPE) Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet 2000; 355: 235–259 [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 7.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393 [DOI] [PubMed] [Google Scholar]
- 8.Holman RR, Paul SK, Bethel MA, et al. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 9.Stratton IM, Aler AI, Neil HA, et al. Association of glycemia with microvascular and macrovascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 12.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139 [DOI] [PubMed] [Google Scholar]
- 13.Del Prato S. Megatrials in type 2 diabetes. From excitement to frustration? Diabetologia 2009; 52: 1219–1226 [DOI] [PubMed] [Google Scholar]
- 14.Chew EY, Ambrosius WT, Davis MD, et al Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010; 233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32: 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodward M, Zhang X, Barzi F, et al. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia‐Pacific region. Diabetes Care 2003; 26: 360–366 [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101 [DOI] [PubMed] [Google Scholar]
- 18.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301: 2129–2140 [DOI] [PubMed] [Google Scholar]
- 19.Yang X, So WY, Kong AP, et al. Development and validation of a total coronary heart disease risk score in type 2 diabetes mellitus. Am J Cardiol 2008; 101: 596–601 [DOI] [PubMed] [Google Scholar]
- 20.Morrish NJ, Wang S, Stevens LK, et al. Mortality and causes of death in the WHO Multinational Survey of Vascular Diseases in Diabetes. Diabetologia 2001; 44: S14–S21 [DOI] [PubMed] [Google Scholar]
- 21.Wu AY, Kong NC, de Leon FA, et al. An alarmingly high prevalence of diabetic nephropathy in Asian type 2 diabetic patients: the MicroAlbuminuria Prevalence (MAP) Study. Diabetologia 2005; 48: 1674–1675 [DOI] [PubMed] [Google Scholar]
- 22.Parving HH, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006; 69: 2057–2063 [DOI] [PubMed] [Google Scholar]
- 23.Chan JC, Wat NM, So WY, et al. Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes. An Asian perspective from the RENAAL study. Diabetes Care 2004; 27: 874–879 [DOI] [PubMed] [Google Scholar]
- 24.Luk A, Chan JC. Diabetic nephropathy‐‐what are the unmet needs? Diabetes Res Clin Pract 2008; 82(Suppl 1): S15–S20 [DOI] [PubMed] [Google Scholar]
- 25.Clarke PM, Glasziou P, Patel A, et al. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med 2010; 7: e1000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286: 421–426 [DOI] [PubMed] [Google Scholar]
- 27.Yang X, So WY, Tong PC, et al. Development and validation of an all‐cause mortality risk score in type 2 diabetes. Arch Intern Med 2008; 168: 451–457 [DOI] [PubMed] [Google Scholar]
- 28.So WY, Kong AP, Ma RC, et al. Glomerular filtration rate, cardiorenal end points, and all‐cause mortality in type 2 diabetic patients. Diabetes Care 2006; 29: 2046–2052 [DOI] [PubMed] [Google Scholar]
- 29.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232 [DOI] [PubMed] [Google Scholar]
- 30.Luk AO, So WY, Ma RC, et al. Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: A 5‐year prospective analysis of the Hong Kong diabetes registry. Diabetes Care 2008; 31: 2357–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol 2009; 4: 1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev 2008; 24: 353–363 [DOI] [PubMed] [Google Scholar]
- 33.Desouza C, Salazar H, Cheong B, et al. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 2003; 26: 1485–1489 [DOI] [PubMed] [Google Scholar]
- 34.Pozzilli P, Leslie RD, Chan J, et al. The A1C and ABCD of glycaemia management in type 2 diabetes: a physician’s personalized approach. Diabetes Metab Res Rev 2010; 26: 239–244 [DOI] [PubMed] [Google Scholar]
- 35.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340: b4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall SM. Intensive diabetes management for high‐risk patients: how best to deliver? Diabetes Care 2009; 32: 1132–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan JCN, So WY, Yeung CY, et al. The SURE study: effects of structured versus usual care on renal endpoint in type 2 diabetes: a randomized multi‐centre translational study. Diabetes Care 2009; 32: 977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grant RW, Hamrick HE, Sullivan CM, et al. Impact of population management with direct physician feedback on care of patients with type 2 diabetes. Diabetes Care 2003; 26: 2275–2280 [DOI] [PubMed] [Google Scholar]
- 39.So WY, Chan JC. The role of the multidisciplinary team In: Goldstein DB, Cockram CS (eds). Textbook of Diabetes. Wiley‐Blackwell, 2010; pp. 969–983 [Google Scholar]
- 40.Ko GT, So WY, Tong PC, et al. From design to implementation‐‐the Joint Asia Diabetes Evaluation (JADE) program: a descriptive report of an electronic web‐based diabetes management program. BMC Med Inform Decis Mak 2010; 10: 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.IDF Clinical Guidelines Task Force . Global guideline for type 2 diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med 2006; 23: 579–593 [DOI] [PubMed] [Google Scholar]
- 42.Funnell MM, Anderson RM. Changing office practice and health care systems to facilitate diabetes self‐management. Curr Diab Rep 2003; 3: 127–133 [DOI] [PubMed] [Google Scholar]
- 43.Chan JCN, Gagliardino JJ, Baik SH, et al. Multi‐faceted determinants for achieving glycaemic control: The International Diabetes Management Practice Study (IDMPS). Diabetes Care 2009; 32: 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.UKPDS . Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853 [PubMed] [Google Scholar]


