Abstract
Aims/Introduction: The present study was designed to determine the effects of pioglitazone on glycemic control and atherosclerosis in patients with poorly controlled type 2 diabetes on insulin therapy.
Materials and Methods: The study was a prospective, randomized controlled trial involving 48 patients with inadequately controlled type 2 diabetes treated with insulin. We assigned patients to oral pioglitazone titrated from 15–30 mg (n = 22) or no pioglitazone (n = 26), to be taken in addition to their glucose‐lowering drugs and other medications. Daily insulin doses and numbers were recorded during the study period.
Results: The adjusted mean glycosylated hemoglobin (HbA1c) values decreased significantly by 1.13 ± 1.50% and 0.55 ± 0.76% in the pioglitazone and control groups, respectively. Significant decrease of HbA1c level was observed in the pioglitazone group compared with the control group (P < 0.05). The insulin dose lowered by 0.04 ± 0.10 units/kg/day in the pioglitazone group and increased by 0.03 ± 0.10 units/kg/day in the control group (P < 0.05). The number of insulin injections decreased by 0.1 ± 0.6 times/day in the pioglitazone group and increased by 0.2 ± 0.4 times/day in the control group (P < 0.05). The carotid intima‐media thickness estimated by B‐mode echography was carried out in both groups and decreased significantly at the end‐point only in the pioglitazone group, relative to the baseline.
Conclusions: These findings show that pioglitazone is useful in improving glycemic control and preventing the progression of atherosclerosis in poorly‐controlled type 2 diabetics on insulin therapy. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.00064.x, 2010)
Keywords: Pioglitazone, Insulin therapy, Atherosclerosis
Introduction
Patients with type 2 diabetes are at high risk of fatal and non‐fatal macro‐ and micro‐complications. Recent trials of intensive glycemic control in patients with type 2 diabetes and their impact on cardiovascular disease have resulted in confusion and speculation amongst physicians. The Action to Control Cardiovascular Risk in Diabetes Study was terminated early because of significant excess all‐cause mortality in the intensively‐treated group1. Furthermore, the ADVANCE and VADT trials did not show cardiovascular benefit with more intensive glycemic control2,3. However, there is indirect and direct evidence that agonists of peroxisome proliferator‐activated receptor gamma (PPARγ) can reduce macrovascular complications4–10.
Insulin therapy has been chosen as the final therapy for type 2 diabetes; but for some populations of patients, this therapy cannot yet achieve the desired goal. Combination therapy of pioglitazone with insulin improves glycemic control better than insulin or pioglitazone alone11–18. To our knowledge, there is no information on the effectiveness of the combination therapy of thiazolidinediones and insulin on the prevention of atherosclerotic complications in type 2 diabetes. The aim of the present study was to ascertain whether pioglitazone slows macrovascular changes in addition to improvement of glycemic control in patients with type 2 diabetes under inadequate glycemic control treated with insulin.
Materials and Methods
Patients
We screened type 2 diabetic patients who regularly attended Juntendo University Hospital and Funayama Clinic between July 2007 and December 2008. Among them, we selected those with the following criteria: (i) on insulin treatment >1 year; (ii) 40–75 years‐of‐age; (iii) HbA1c > 7.0%; (iv) stable glycemic control with HbA1c variation <1.0% during the preceding 6 months; and (v) negative history of thiazolidinediones therapy. Patients were also excluded if they had concomitant chronic diseases, such as anemia (hemoglobin ≤ 11.0 g/dL), kidney (plasma creatinine >1.50 mg/dL), liver (aspartate transaminase > 80 IU/L or alanine transferase > 80 IU/L) or cardiovascular disease; recent acute illness; history of glitazone therapy within 24 weeks; proliferative diabetic retinopathy, current steroid therapy, or were suspected or confirmed to be pregnant. The study protocol was carried out in accordance with the ethical principles stated in the Declaration of Helsinki and approved by the Ethic Review Committee of Juntendo University Hospital. All patients provided written informed consent and confirmed their willingness to inject insulin and carry out glucose self‐monitoring. The study protocol was registered under UMIN (ID: 000002333) termed ACTION‐J (Actos Combination Therapy for Insulin ON type 2 diabetes patients in Juntendo).
Randomized Trial
Among 70 inadequately controlled insulin‐treated type 2 diabetic patients, 54 patients were assigned to participate in an open labeled randomized controlled trial carried out at Juntendo University Hospital and Funayama Clinic, Japan (ACTION‐J study).
Primary end‐points of the present study were: (i) effects of pioglitazone on glycemic control (HbA1c); and (ii) effects of pioglitazone on size of intima‐media thickness estimated with ultrasonography in type 2 diabetes with inadequate insulin therapy.
After the 4‐week screening period, eligible patients were randomized either to the pioglitazone group (oral pioglitazone titrated from 15–30 mg [n = 26]) or to the control group (no pioglitazone [n = 28]) based on a computer‐generated assignment using the minimization method employing age, sex, HbA1c, and systolic and diastolic pressure at baseline as adjustment factors.
Patients were provided with recommendations for diet therapy during the screening period and after randomization. No restriction was imposed on patients taking any oral diabetic agents, except thiazolidinediones for the control group, and other oral medications, such as blood pressure (BP)‐lowering and lipid‐lowering drugs, and the dosage of the insulin and drugs could be changed freely by the attending physician during the study.
At screening visit, baseline laboratory data including plasma adiponectin, BP, body mass index (BMI) and intima‐media thickness (IMT) were determined for each subject. Blood samples were obtained for the measurement of serum lipids (total cholesterol, high‐density lipoprotein (HDL)‐cholesterol and triglycerides) and HbA1c by standard laboratory techniques. BP was measured with a mercury sphygmomanometer. The baseline clinical characteristics of the subjects are shown in Table 1. The number of patients receiving antihypertensive drugs, lipid‐lowering drugs, oral hypoglycemic agents and antithrombotic drugs are shown in Table 2. Each patient was reviewed at least every 3 months, with their general health, compliance with medications, laboratory data, BP, and diet and exercise status being checked at each visit. At 48 months after the intervention, baseline laboratory data, BP, BMI and IMT were again determined for each patient. Average laboratory values for the observation period were calculated from the data obtained at each visit.
Table 1. Patients’ characteristics at baseline.
| Control group (n = 26) | Pioglitazone group (n = 22) | P‐value | |
|---|---|---|---|
| Age (years) | 57.2 ± 11.3 | 56.0 ± 9.7 | 0.70 |
| n, Sex (female/male) | 8/18 | 4/18 | 0.37 |
| Height (cm) | 164.0 ± 8.4 | 165.5 ± 9.0 | 0.53 |
| Weight (kg) | 73.4 ± 15.4 | 70.5 ± 11.0 | 0.47 |
| Body mass index (kg/m2) | 26.9 ± 4.4 | 25.5 ± 3.1 | 0.21 |
| Duration of diabetes (year) | 14.8 ± 7.2 | 13.4 ± 7.4 | 0.51 |
| Blood pressure | |||
| Systolic (mmHg) | 133.1 ± 13.7 | 123.2 ± 13.0 | 0.02 |
| Diastolic (mmHg) | 78.3 ± 11.1 | 75.4 ± 10.3 | 0.40 |
| HbA1c (%) | 8.64 ± 1.23 | 8.59 ± 1.28 | 0.58 |
| Total daily insulin dose (U) | 39.6 ± 14.5 | 37.2 ± 20.4 | 0.39 |
| Total daily insulin dose (U/day/kg) | 0.57 ± 0.26 | 0.54 ± 0.31 | 0.26 |
| Times of insulin injection (/day) | 3.4 ± 0.8 | 3.1 ± 0.6 | 0.24 |
Table 2. Comparison of ratios in concomitant drugs used at baseline and end‐point between non‐pioglitazone (control) and pioglitazone groups.
| Drug treatment | Baseline | P‐value | End‐point | P‐value | ||
|---|---|---|---|---|---|---|
| Control (%) | Pioglitazone (%) | Control (%) | Piolglitazone (%) | |||
| Insulin | 26/26 (100) | 22/22 (100) | 1 | 26/26 (100) | 22/22 (100) | 1 |
| Metformin | 16/26 (62) | 14/22 (64) | 0.88 | 16/26 (62) | 12/22 (55) | 0.62 |
| α‐Glucosidase inhibitor | 8/26 (31) | 6/22 (27) | 0.79 | 12/26 (46) | 6/22 (27) | 0.18 |
| Sulfonylureas | 0/26 (0) | 2/22 (9) | 0.12 | 3/26 (12) | 4/22 (18) | 0.52 |
| Calcium‐channel blockers | 10/26 (38) | 4/22 (18) | 0.12 | 14/26 (54) | 6/22 (27) | 0.06 |
| ACE‐I/ARB | 12/26 (46) | 7/22 (32) | 0.31 | 18/26 (69) | 11/22 (50) | 0.18 |
| Statins | 7/26 (27) | 10/22 (45) | 0.18 | 12/26 (46) | 15/22 (68) | 0.66 |
| Fibrates | 2/26 (8) | 1/22 (5) | 0.65 | 3/26 (12) | 2/22 (10) | 0.78 |
| Antiplatelet medications | 8/26 (31) | 2/22 (9) | 0.65 | 13/26 (50) | 4/22 (18) | 0.02 |
The patients were provided with blood glucose meters (Glutest Neo or Pro; Sanwa Kagaku Kenkyu‐syo, Nagoya, Japan) and diaries, and were instructed to self‐monitor blood glucose at least before breakfast and dinner. Safety was also assessed by general physical examination, assessment of vital signs, ECG, clinical hematology and chemistry, urinalysis and reporting of adverse events.
Assessment of Intima‐media Thickness of the Common Carotid Artery
Ultrasonography of the carotid arteries was carried out using an echotomographic system (EUB‐555; Hitachi Medico, Chiyoda Tokyo, Japan) with a linear transducer (midfrequency range of 7.5–10 MHz). Scanning of the extracranial carotid arteries in the neck was carried out in three different longitudinal projections (anterior oblique, lateral, and posterior oblique) and in the transverse projection, as reported previously19–21. This allowed the common carotid artery to be scanned bilaterally. All obtained images were photographed. The carotid intima‐media wall was defined as the distance between lumen/intima borderline and media/adventitia borderline on the far wall. In each longitudinal projection, the site of the greatest intima‐media thickness (IMT) was detected by scanning along the vessel from the common carotid artery, which defined the area from 10–20 mm below the flow divider. Three measurements of the IMT were made; one at the site of the greatest thickness and two at other points (1 cm proximal and 1 cm distal to the site) on the anterior, lateral and posterior projections of the far wall for each patient and always in plaque‐free segments. These measurements were carried out on both sides. The average value of the six highest intima‐media wall measurements (three from the left side and three from the right side) was used as the mean common carotid artery IMT for each patient. All scans were carried out by a single physician, whereas all IMT measurements were carried out by another physician, and both were blinded to the clinical information. We have previously shown a good intraday and interday reproducibility of our examination19–21. The annual change in IMT was calculated by using the following equation, which was used as the primary endpoint:
 |
Statistical analysis
All data are expressed as mean ± SD unless otherwise indicated. The Fisher’s exact test was carried out to compare the difference between the two groups in changes made to the concomitantly used medications during the course of the study. To compare the differences between baseline and 48‐week, paired t‐test was carried out. To compare between‐group differences, non‐paired t‐test was carried out. A P‐value < 0.05 was considered statistically significant.
Results
Figure 1 shows a flow chart of the trial profile of the present study. A total of 70 patients were recruited and 16 of them dropped out as a result of improvement of glycemic control or liver dysfunction during the run‐in period. The remaining patients were randomly assigned to either the pioglitazone group (n = 26) or the non‐pioglitazone group (n = 28).
Figure 1.
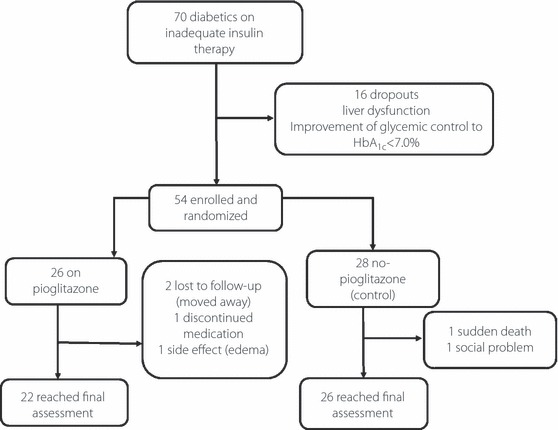
Schematic diagram of the study protocol.
All patients were given the minimum diet education program, including restriction of sodium intake to prevent any increase in bodyweight or edema induced by pioglitazone. A total of 22 of the 26 patients on pioglitazone (two moved elsewhere, one dropped out with moderate edema and one interrupted the treatment for unknown reasons) and 26 of the 28 non‐pioglitazone patients (one dropped out as a result of sudden death and one with social problems) completed the study. As shown in Table 1, the two groups were well matched with respect to the baseline characteristics, except for systolic blood pressure. At the time of randomization, just two patients from the pioglitazone group were being treated with a sulfonylurea, and ∼60% of the patients were on metformin (Table 2). Table 2 lists the other related medications taken concomitantly by the groups. No significant differences in these medications at baseline were noted between the two groups.
Table 2 also describes the changes in the concomitant medications during the course of the study. The use of antiplatelet medications increased significantly in patients of the control group in comparison with those in the pioglitazone group only after 48‐weeks of treatment.
As shown in Figure 2 and Table 3, significant differences were evident at the end of the study (48 weeks); HbA1c values as the first primary end‐point improved significantly from 8.59 ± 1.28% at baseline to 7.33 ± 0.86% at the end of the study in the pioglitazone group and from 8.64 ± 1.23% to 8.09 ± 1.39% in the control group, respectively. Furthermore, significant improvement in HbA1c was also observed in the pioglitazone group compared with the control group after 48‐week of treatment with pioglitazone (P < 0.05; Table 4).
Figure 2.
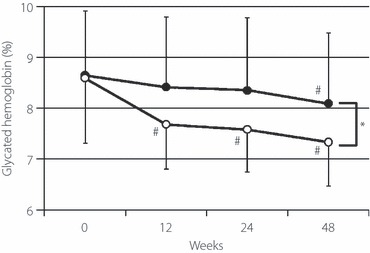
Comparison of changes in mean (±SD) HbA1c of patients with pioglitazone (open circles) and non‐pioglitazone‐treated control group (closed circles). #P < 0.01 vs 0 week, *P < 0.05, between the pioglitazone and control groups.
Table 3. Baseline and end‐point values of various parameters in the non‐pioglitazone (control) and pioglitazone groups.
| Control (n = 26) | Pioglitazone (n = 22) | |||
|---|---|---|---|---|
| Baseline | End‐point | Baseline | End‐point | |
| Weight (kg) | 73.4 ± 15.4 | 73.5 ± 15.5 | 70.5 ± 11.0 | 72.5 ± 12.4* |
| Blood pressure | ||||
| Systolic (mmHg) | 133.1 ± 13.7 | 136.5 ± 13.3 | 123.2 ± 13.0 | 132.4 ± 13.3 |
| Diastolic (mmHg) | 78.3 ± 11.1 | 77.8 ± 12.1 | 75.4 ± 10.3 | 76.6 ± 11.7 |
| Total daily insulin dose (U/day/kg) | 0.57 ± 0.23 | 0.59 ± 0.20 | 0.54 ± 0.31 | 0.50 ± 0.32‡ |
| Number of insulin injection (times/day) | 3.4 ± 0.8 | 3.5 ± 0.9 | 3.1 ± 0.6 | 2.9 ± 0.9‡ |
| HbA1c (%) | 8.64 ± 1.23 | 8.09 ± 1.39* | 8.59 ± 1.28 | 7.33 ± 0.86*‡ |
| Fasting serum glucose (mg/dL) | 185.2 ± 67.8 | 165.3 ± 51.5 | 184.1 ± 44.6 | 148.9 ± 44.0† |
| Fasting serum C‐peptide (ng/mL) | 1.64 ± 1.25 | 1.65 ± 1.03 | 1.61 ± 0.72 | 1.63 ± 0.66 |
| LDL cholesterol (mg/dL) | 129.6 ± 31.9 | 117.8 ± 26.5† | 125.0 ± 23.6 | 120.6 ± 19.5 |
| HDL cholesterol (mg/dL) | 50.3 ± 14.1 | 49.8 ± 14.2 | 51.8 ± 14.1 | 54.6 ± 14.1† |
| Triglycerides (mg/dL) | 162.4 ± 94.1 | 154.8 ± 95.9 | 157.9 ± 96.8 | 125.5 ± 50.9 |
| High sensitive‐CRP (mg/dL) | 0.160 ± 0.200 | 0.162 ± 0.169 | 0.098 ± 0.069 | 0.069 ± 0.045 |
| High‐molecular adiponectin (μg/mL) | 2.31 ± 2.20 | 2.11 ± 1.69 | 1.89 ± 1.71 | 4.44 ± 2.38*‡ |
| Intima‐media thickness (mm) | 0.923 ± 0.139 | 0.884 ± 0.140 | 0.927 ± 0.143 | 0.877 ± 0.127* |
*P < 0.01 vs baseline, †P < 0.05 vs baseline, ‡P < 0.05 vs control.
Table 4. Delta changes in various parameters at end of 48 weeks in the non‐pioglitazone (control) and pioglitazone groups.
| Control | Pioglitazone | P‐value | |
|---|---|---|---|
| HbA1c (%) | −0.55 ± 0.76 | −1.13 ± 1.50 | 0.048 |
| Bodyweight (kg) | 0.18 ± 1.95 | 1.96 ± 3.67 | 0.087 |
| Total daily insulin dose (U/day/kg) | 0.03 ± 0.10 | −0.04 ± 0.10 | 0.030 |
| No. insulin injections (times/day) | 0.2 ± 0.4 | −0.1 ± 0.6 | 0.048 |
| Triglycerides (mg/dL) | −8.4 ± 66.2 | −31.6 ± 74.3 | 0.23 |
| LDL cholesterol (mg/dL) | −11.8 ± 21.3 | −4.4 ± 24.9 | 0.37 |
| HDL cholesterol (mg/dL) | −0.5 ± 9.0 | 2.5 ± 6.9 | 0.20 |
| Fasting serum glucose (mg/dL) | −19.8 ± 67.9 | −35.2 ± 53.5 | 0.40 |
| High sensitive‐CRP (mg/dL) | 0.002 ± 0.136 | −0.032 ± 0.083 | 0.32 |
| High‐molecular adiponectin (μg/mL) | −0.14 ± 0.89 | 2.68 ± 2.16 | <0.00001 |
The mean plasma glucose level before breakfast improved significantly in the pioglitazone group (Table 3), but no significant differences were observed between the two groups at the end of the study (Table 4). The mean change in bodyweight was greater in the pioglitazone group than the control group, but the difference was not significant (P = 0.087).
Although the daily total dose of insulin was similar between the two groups at baseline (Table 2), the change in the daily dose from the study commencement to 48 weeks in the pioglitazone group (−0.04 ± 0.10 units/kg/day) was significantly smaller than that of the control group (0.03 ± 0.10 units/kg/day, P < 0.05, Figure 3a). The frequency of insulin injections used by patients of the pioglitazone group decreased significantly from 0–48 weeks, whereas that of the control group tended to increase significantly (Figure 3b).
Figure 3.
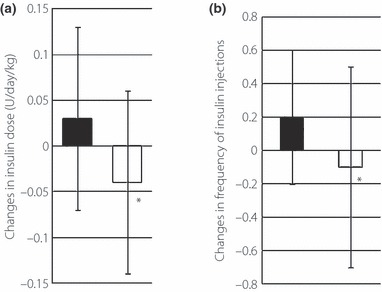
Changes in (a) total daily dose of insulin and (b) number of daily insulin injections in the pioglitazone (open squares) and control (closed squares) groups. Data are mean ± SD. *P < 0.05, between the pioglitazone and control groups.
We also compared the changes in serum lipid profile between the two groups. At the end of the study, low‐density lipoprotein (LDL)‐cholesterol was lower than the baseline in the control group. Serum HDL‐cholesterol level increased significantly in the pioglitazone group (Table 3). The increase in serum adiponectin level from baseline was significantly larger in the pioglitazone group than the control group (Table 4).
With regard to the carotid IMT as the second primary end‐point, a modest but significant decrease in this parameter, relative to the baseline, was observed in the pioglitazone group (0.927 ± 0.143 mm to 0.877 ± 0.127 mm, P < 0.05), whereas such a decrease in the control group was not significant (0.923 ± 0.139 mm to 0.884 ± 0.140 mm, not significant) at the end of the follow‐up period (Table 3). The annual change in the IMT was not significantly different between the two groups.
In relation to adverse effects of pioglitazone, edema is the most frequent and physicians sometimes have to stop this drug. In the present study, the number of patients who had edema was one of 26 in the pioglitazone group and none in the control group.
Discussion
The present findings showed that the addition of pioglitazone to insulin therapy significantly improved glycemic control, despite the decrease in daily insulin dose and frequency of injections. To our knowledge, this is the first report of the benefits of the combined use of pioglitazone and insulin in patients of Asian ethnicity with type 2 diabetes. Pioglitazone also increased serum adiponectin levels in these patients, with associated reduction in the carotid IMT as a surrogate of lessening of atherosclerogenesis. The novel finding of the present study is that the addition of pioglitazone has an anti‐atherosclerotic effect, even in patients with type 2 diabetes under inadequate glycemic control treated with insulin. How pioglitazone reduces the IMT in these patients is unclear at this moment. The significant increase in adiponectin, which improves insulin sensitivity and has anti‐atherogenic effects, might contribute to the decreased IMT in the pioglitazone group22,23. We should have evaluated visceral fat and considered the relationship between amount of visceral fat and effectiveness of pioglitazone on glycemic control and atherosclerosis. It will need to be evaluated in a future study.
In addition to the metabolic improvement, the significant increase in the mean HDL‐cholesterol in the pioglitazone group might have contributed to the decrease in IMT. Particle number or density of LDL‐cholesterol, which also influence the decrease in IMT, might be changed with pioglitazone, but neither was accessed in the present study. Pioglitazone treatment resulted in a significant decrease in the mean total daily insulin dose and the frequency of insulin injections, suggesting a remarkable improvement in insulin sensitivity with pioglitazone in patients under poor glycemic control by insulin therapy.
Unfortunately, the difference in changes in the IMT between the two groups was not significant. The main reason for this finding might be the difference in the use of other medications during the course of the study. The use of antiplatelet medications was significantly more common in the control group at the end‐point. These differences and the small, but significant, improvement in HbA1c in the control group might minimize the difference in the change in IMT.
In the present study, only a few patients of the pioglitazone group developed peripheral edema. A previous study reported that the incidence of edema with pioglitazone was higher in patients treated with pioglitazone combined with insulin (15.3%) compared with those receiving pioglitazone monotherapy (4.8%)24,25. Based on this background, we provided all patients the minimum diet education program, including limitation of sodium intake, to prevent any increase in bodyweight or edema by pioglitazone. This probably explains the low rate of pioglitazone‐related edema. Even though frequency of edema was low, the mean change in bodyweight was greater in the pioglitazone group than the control group. Because mean BMI at baseline was originally large in both groups, we do not expect the participants followed a proper diet, despite being offered several opportunities for nutrition consultations. More optimum education before the use of pioglitazone might further reduce the frequency or severity of edema or heart failure and increase of bodyweight in patients treated with pioglitazone, including those on insulin or sulfonylurea.
Certain study limitations of the present study should be recognized. First, the study was composed of a comparatively small number of the patients and was carried out in a few institutions. Second, no study group achieved <7.0% of HbA1c as the glycemic target. We expect that factors other than insulin therapy itself exist, such as inappropriate diet or exercise. In general, further studies must be needed to evaluate the long‐term effects of pioglitazone on insulin therapy, inviting a larger number of patients and after more intensive education.
In conclusion, the beneficial effects of pioglitazone, including both glycemic control and anti‐atherosclerosis, were observed in diabetic patients inadequately treated with insulin.
Acknowledgements
We thank Noriko Iijima and Emi Miyazawa for their excellent technical assistance. TH and RK have received grant support from Takeda and Nippon Ili Lilly. TH has also acted as a spokesperson for Takeda, Nippon Ili Lilly and Sanofi Aventis. YF has received grant support from Takeda. All other authors declare no conflict of interest.
References
- 1.The Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 3.Pftzner A, Marx N, Lbben G, et al. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the Pioneer study. J Am Coll Cardiol 2005; 45: 1925–1931 [DOI] [PubMed] [Google Scholar]
- 4.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139 [DOI] [PubMed] [Google Scholar]
- 5.Langenfeld MR, Forst T, Hohberg C, et al. Pioglitazone decreases carotid intima‐media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized study. Circulation 2005; 111: 2525–2531 [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Matsuda T, Kawagoe Y, et al. Effect of pioglitazone on carotid intima‐media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism 2004; 53: 1382–1386 [DOI] [PubMed] [Google Scholar]
- 7.Koshiyama H, Shimono D, Kuwamura N, et al. Inhibitory effect of pioglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab 2001; 86: 3452–3456 [DOI] [PubMed] [Google Scholar]
- 8.Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima‐media thickness in type 2 diabetes: a randomized trial. JAMA 2006; 296: 2572–2581 [DOI] [PubMed] [Google Scholar]
- 9.Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008; 299: 1561–1573 [DOI] [PubMed] [Google Scholar]
- 10.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366: 1279–1289 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz S, Raskin P, Fonseca V, et al. Effect of troglitazone in insulin‐treated patients with type II diabetes mellitus. N Engl J Med 1998; 338: 861–866 [DOI] [PubMed] [Google Scholar]
- 12.Buse JB, Gumbiner B, Mathias NP, et al. Troglitzzone use in insulin‐treated type 2 diabetic patients. Diabetes Care 1998; 21: 1455–1461 [DOI] [PubMed] [Google Scholar]
- 13.Fonseca V, Foyt HL, Shen K, et al. Long‐term effects of troglitazone. Open‐label extension studies in type 2 diabetic patients. Diabetes Care 2000; 23: 354–359 [DOI] [PubMed] [Google Scholar]
- 14.Raskin P, Rendell M, Riddle MC, et al. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin‐treated type 2 diabetes. Diabetes Care 2001; 24: 1226–1232 [DOI] [PubMed] [Google Scholar]
- 15.Mattoo V, Eckland D, Widel M, et al. Metabolic effects of pioglitazone in combination with insulin in patients with type 2 diabetes mellitus whose disease is not adequately controlled with insulin therapy: results of a six‐month, randomized, double‐blind, prospective, multicenter, parallel‐group study. Clin Ther 2005; 27: 554–567 [DOI] [PubMed] [Google Scholar]
- 16.Davidson JA, Perez A, Zhang J, The Pioglitazone 343 Study Group . Addition of pioglitazone to stable insulin therapy in patients with poorly controlled type 2 diabetes: results of a double‐blind, multicentre, randomized study. Diabetes Obes Metab 2006; 8: 164–174, [DOI] [PubMed] [Google Scholar]
- 17.Berhanu P, Perez A, Yu S. Effect of pioglitazone in combination with insulin therapy on glycaemic control, insulin dose requirement and lipid profile in patients with type 2 diabetes previously poorly controlled with combination therapy. Diabetes Obes Metab 2007; 9: 512–520 [DOI] [PubMed] [Google Scholar]
- 18.Strowig SM, Raskin P. Combination therapy using metformin or thiazolidinediones and insulin in the treatment of diabetes mellitus. Diabetes Obes Metab 2005; 7: 633–641 [DOI] [PubMed] [Google Scholar]
- 19.Mitsuhashi N, Tanaka Y, Kubo S, et al. Effect of cilostazol, a phosphodiesterase inhibitor, on carotid IMT in Japanese type 2 diabetic patients. Endocr J 2004; 51: 545–550 [DOI] [PubMed] [Google Scholar]
- 20.Mita T, Watada H, Uchino H, et al. Association of C‐reactive protein with early‐stage carotid atherosclerosis in Japanese patients with early‐state type 2 diabetes mellitus. Endocr J 2006; 53: 693–698 [DOI] [PubMed] [Google Scholar]
- 21.Mita T, Watada H, Shimizu T, et al. Nateglinide reduces carotid intima‐media thickening in type 2 diabetic patients under good glycemic control. Atheroscler Thromb Vasc Biol 2007; 27: 2456–2462 [DOI] [PubMed] [Google Scholar]
- 22.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 2002; 277: 25863–25866 [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, Shimomura I, Sata M, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo‐vascular axis. J Biol Chem 2002; 277: 37487–37491 [DOI] [PubMed] [Google Scholar]
- 24.Rosenstock J, Emhorn D, Hershon K, et al. Efficacy and safety of pioglitazone in type 2 diabetes a randomized, placebo controlled study in patients receiving stable insulin therapy. Int J Clin Pract 2002; 56: 251–257 [PubMed] [Google Scholar]
- 25.Mudliar S, Chang AR, Henry RR. Thiazolinediones, peripheral edema, and type 2 diabetes incidence, pathophysiology and clinical implications. Endocr Pract 2003; 9: 406–416 [DOI] [PubMed] [Google Scholar]


