Abstract
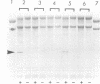
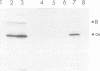
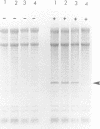
When recombinant ricin A chain transcripts are translated in a rabbit reticulocyte lysate the ribosomes are rapidly inactivated as shown by their inability to support translation of yeast preproalpha factor or chicken lysozyme transcripts added subsequently. In contrast, ribosomes which have translated transcripts encoding non-toxic polypeptides such as ricin B chain, readily translate the second transcript under identical conditions. Ribosome inactivation is accompanied by a highly specific modification of 28S rRNA which occurs at the same position as the N-glycosidic cleavage of an adenine residue and which is thought to cause inactivation of the ribosomes. Protein synthesis by wheat germ ribosomes was not inhibited under the conditions which inhibit reticulocyte ribosomes confirming earlier observations that plant cytoplasmic ribosomes are much less sensitive to inhibition by ricin A chain than are mammalian ribosomes. Using the same assay we have shown that deleting an internal hexapeptide, which shares homology with hamster elongation factor-2, completely abolishes catalytic activity. Deleting a second pentapeptide conserved between ricin A chain and the ribosome-inactivating plant toxin trichosanthin, had no effect. Deleting the first nine residues from the N-terminus of A chain did not affect toxicity whereas deleting a further three residues inactivated the polypeptide. Point mutations which individually converted arginine 48 and arginine 56 of ricin A chain to alanine residues or which deleted arginine 56 were also without effect on the catalytic activity of the toxin.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Straus J. W., Dudock B. S. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 1983;101:635–644. doi: 10.1016/0076-6879(83)01044-7. [DOI] [PubMed] [Google Scholar]
- Butterworth A. G., Lord J. M. Ricin and Ricinus communis agglutinin subunits are all derived from a single-size polypeptide precursor. Eur J Biochem. 1983 Dec 1;137(1-2):57–65. doi: 10.1111/j.1432-1033.1983.tb07795.x. [DOI] [PubMed] [Google Scholar]
- Cahn F., Schachter E. M., Rich A. Polypeptide synthesis with ribonuclease-digested ribosomes. Biochim Biophys Acta. 1970;209(2):512–520. doi: 10.1016/0005-2787(70)90748-3. [DOI] [PubMed] [Google Scholar]
- Calderwood S. B., Auclair F., Donohue-Rolfe A., Keusch G. T., Mekalanos J. J. Nucleotide sequence of the Shiga-like toxin genes of Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4364–4368. doi: 10.1073/pnas.84.13.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco L., Fernandez-Puentes C., Vazquez D. Effects of ricin on the ribosomal sites involved in the interaction of the elongation factors. Eur J Biochem. 1975 Jun;54(2):499–503. doi: 10.1111/j.1432-1033.1975.tb04162.x. [DOI] [PubMed] [Google Scholar]
- Cawley D. B., Hedblom M. L., Hoffman E. J., Houston L. L. Differential ricin sensitivity of rat liver and wheat germ ribosomes in polyuridylic acid translation. Arch Biochem Biophys. 1977 Aug;182(2):690–695. doi: 10.1016/0003-9861(77)90550-1. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Olvera J., Wool I. G. The structure of rat 28S ribosomal ribonucleic acid inferred from the sequence of nucleotides in a gene. Nucleic Acids Res. 1983 Nov 25;11(22):7819–7831. doi: 10.1093/nar/11.22.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grandis S., Ginsberg J., Toone M., Climie S., Friesen J., Brunton J. Nucleotide sequence and promoter mapping of the Escherichia coli Shiga-like toxin operon of bacteriophage H-19B. J Bacteriol. 1987 Sep;169(9):4313–4319. doi: 10.1128/jb.169.9.4313-4319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond D. R., Armstrong J., Colman A. The effect of capping and polyadenylation on the stability, movement and translation of synthetic messenger RNAs in Xenopus oocytes. Nucleic Acids Res. 1985 Oct 25;13(20):7375–7394. doi: 10.1093/nar/13.20.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Huber P. W., Wool I. G. The ribonuclease activity of the cytotoxin alpha-sarcin. The characteristics of the enzymatic activity of alpha-sarcin with ribosomes and ribonucleic acids as substrates. J Biol Chem. 1983 Feb 25;258(4):2662–2667. [PubMed] [Google Scholar]
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987 Apr 25;262(12):5908–5912. [PubMed] [Google Scholar]
- Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987 Jun 15;262(17):8128–8130. [PubMed] [Google Scholar]
- Endo Y., Tsurugi K., Yutsudo T., Takeda Y., Ogasawara T., Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988 Jan 15;171(1-2):45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Puentes C., Benson S., Olsnes S., Pihl A. Protective effect of elongation factor 2 on the inactivation of ribosomes by the toxic lectins abrin and ricin. Eur J Biochem. 1976 May 1;64(2):437–443. doi: 10.1111/j.1432-1033.1976.tb10320.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Puentes C., Vazquez D. Effects of some proteins that inactivate the eukaryotic ribosome. FEBS Lett. 1977;78(1):143–146. doi: 10.1016/0014-5793(77)80292-5. [DOI] [PubMed] [Google Scholar]
- Glass J. R., MacKrell M., Duffy J. J., Gerner E. W. Ornithine decarboxylase production in vitro by using mouse cDNA. Biochem J. 1987 Jul 1;245(1):127–132. doi: 10.1042/bj2450127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Halling K. C., Halling A. C., Murray E. E., Ladin B. F., Houston L. L., Weaver R. F. Genomic cloning and characterization of a ricin gene from Ricinus communis. Nucleic Acids Res. 1985 Nov 25;13(22):8019–8033. doi: 10.1093/nar/13.22.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley M. R., Wheeler A., Ellis R. J. Protein synthesis in chloroplasts. V. Translation of messenger RNA for the large subunit of fraction I protein in a heterologous cell-free system. J Mol Biol. 1975 Jan 5;91(1):67–77. doi: 10.1016/0022-2836(75)90372-1. [DOI] [PubMed] [Google Scholar]
- Hovde C. J., Calderwood S. B., Mekalanos J. J., Collier R. J. Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2568–2572. doi: 10.1073/pnas.85.8.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K., Uchida T., Ohkubo H., Nakanishi S., Nakanishi T., Fukui T., Ohtsuka E., Ikehara M., Okada Y. Amino acid sequence of mammalian elongation factor 2 deduced from the cDNA sequence: homology with GTP-binding proteins. Proc Natl Acad Sci U S A. 1986 Jul;83(14):4978–4982. doi: 10.1073/pnas.83.14.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Fritz H. J. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984 Oct;38(3):879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb F. I., Roberts L. M., Lord J. M. Nucleotide sequence of cloned cDNA coding for preproricin. Eur J Biochem. 1985 Apr 15;148(2):265–270. doi: 10.1111/j.1432-1033.1985.tb08834.x. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Hays T. R., Eyring H. Effect of molecular rotation on the vibration-vibration energy transfer in condensed media. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3571–3575. doi: 10.1073/pnas.76.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Montfort W., Villafranca J. E., Monzingo A. F., Ernst S. R., Katzin B., Rutenber E., Xuong N. H., Hamlin R., Robertus J. D. The three-dimensional structure of ricin at 2.8 A. J Biol Chem. 1987 Apr 15;262(11):5398–5403. [PubMed] [Google Scholar]
- Murphy G., Kavanagh T. Speeding-up the sequencing of double-stranded DNA. Nucleic Acids Res. 1988 Jun 10;16(11):5198–5198. doi: 10.1093/nar/16.11.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare M., Roberts L. M., Thorpe P. E., Watson G. J., Prior B., Lord J. M. Expression of ricin A chain in Escherichia coli. FEBS Lett. 1987 May 25;216(1):73–78. doi: 10.1016/0014-5793(87)80759-7. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Fernandez-Puentes C., Carrasco L., Vazquez D. Ribosome inactivation by the toxic lectins abrin and ricin. Kinetics of the enzymic activity of the toxin A-chains. Eur J Biochem. 1975 Dec 1;60(1):281–288. doi: 10.1111/j.1432-1033.1975.tb21001.x. [DOI] [PubMed] [Google Scholar]
- Ready M., Wilson K., Piatak M., Robertus J. D. Ricin-like plant toxins are evolutionarily related to single-chain ribosome-inhibiting proteins from Phytolacca. J Biol Chem. 1984 Dec 25;259(24):15252–15256. [PubMed] [Google Scholar]
- Roberts L. M., Lord J. M. The synthesis of Ricinus communis agglutinin, cotranslational and posttranslational modification of agglutinin polypeptides. Eur J Biochem. 1981 Sep;119(1):31–41. doi: 10.1111/j.1432-1033.1981.tb05573.x. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Stewart T. S. Purification and properties of a translation inhibitor from wheat germ. Biochemistry. 1979 Jun 12;18(12):2615–2621. doi: 10.1021/bi00579a028. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Webb J. R., Ammerer G., Meyer D. I. Secretion in yeast: structural features influencing the post-translational translocation of prepro-alpha-factor in vitro. EMBO J. 1987 Nov;6(11):3455–3463. doi: 10.1002/j.1460-2075.1987.tb02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Bailey S., Miller S. P., Bodley J. W. Modification of ribosomal RNA by ribosome-inactivating proteins from plants. Nucleic Acids Res. 1988 Feb 25;16(4):1349–1357. doi: 10.1093/nar/16.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Barbieri L. Ribosome-inactivating proteins up to date. FEBS Lett. 1986 Jan 20;195(1-2):1–8. doi: 10.1016/0014-5793(86)80118-1. [DOI] [PubMed] [Google Scholar]
- Veldman G. M., Klootwijk J., de Regt V. C., Planta R. J., Branlant C., Krol A., Ebel J. P. The primary and secondary structure of yeast 26S rRNA. Nucleic Acids Res. 1981 Dec 21;9(24):6935–6952. doi: 10.1093/nar/9.24.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S. Synergy between immunotoxins prepared with native ricin A chains and chemically-modified ricin B chains. J Immunol. 1986 Mar 1;136(5):1880–1887. [PubMed] [Google Scholar]
- Watanabe K., Funatsu G. Involvement of arginine residues in inhibition of protein synthesis by ricin A-chain. FEBS Lett. 1986 Aug 18;204(2):219–222. doi: 10.1016/0014-5793(86)80815-8. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Murray G. J., Neville D. M., Jr Ricin linked to monophosphopentamannose binds to fibroblast lysosomal hydrolase receptors, resulting in a cell-type-specific toxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5559–5562. doi: 10.1073/pnas.76.11.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]