Abstract
BACKGROUND
The cutaneous mycoses, mainly caused by dermatophyte fungi, are among the most common fungal infections worldwide. It is estimated that 10% to 15% of the population will be infected by a dermatophyte at some point in their lives, thus making this a group of diseases with great public health importance.
OBJECTIVE
To analyze the clinical, epidemiological, and therapeutic profile of dermatophytosis in patients enrolled at the Dermatology service of Universidade do Estado do Pará, Brazil, from July 2010 to September 2012.
METHOD
A total of 145 medical records of patients diagnosed with dermatophytosis were surveyed. Data were collected and subsequently recorded according to a protocol developed by the researchers. This protocol consisted of information regarding epidemiological and clinical aspects of the disease and the therapy employed.
RESULTS
The main clinical form of dermatophyte infection was onychomycosis, followed by tinea corporis, tinea pedis, and tinea capitis. Furthermore, the female population and the age group of 51 to 60 years were the most affected. Regarding therapy, there was a preference for treatments that combine topical and systemic drugs, and the most widely used drugs were fluconazole (systemic) and ciclopirox olamine (topical).
CONCLUSION
This study showed the importance of recurrent analysis of the epidemiological profile of dermatophytosis to enable correct therapeutic and preventive management of these conditions, which have significant clinical consequences, with chronic, difficult-totreat lesions that can decrease patient quality of life and cause disfigurement.
Keywords: Dermatomycoses, Fungi, Tinea
INTRODUCTION
The cutaneous mycoses, mainly caused by dermatophyte fungi, are among the most common fungal infections worldwide, affecting several age groups and adversely affecting the quality of life of infected patients.1,2
It is estimated that superficial fungal infections affect roughly 20-25% of the world population.3 In Brazil, surveys by Siqueira et al (2006) and Brilhante et al (2000)5 showed that the prevalence of dermatophytoses among cutaneous lesions ranges from 18.2 to 23.2%.4,5
In the Amazon region, the dermatophytoses have the highest incidence among the superficial mycotic infections.6 This is attributable to environmental factors characteristic of this region, such as the high temperature and relative humidity, which provide conditions favorable to fungal dispersion and development. Sociodemographic factors, such as precarious socioeconomic status, promiscuity, prolonged contact with animals, and poor hygiene conditions, are also conducive to the incidence and propagation of mycotic infections in the area.7
The dermatophytoses are dermatomycoses caused by a specific group of fungi known as ringworms or tineas, comprising the genera Microsporum, Trichophyton, and Epidermophyton.8 Transmission of dermatophytes may occur by direct contact with infected humans or animals or indirectly, by contact with contaminated fomites.9
Clinical manifestations vary depending on the causal agent and on the host immune response; they may last months or years, and may be asymptomatic or manifest only as pruritus.10 In the majority of cases, however, infection manifests itself as blistering, fissures, scales, or spots.11
Certain clinical signs predominate depending on the affected site. These manifestations include: scalp lesions, caused by tinea capitis (scalp ringworm); widespread lesions, caused by tinea corporis; lesions in the interdigital spaces and plantar regions of the foot, caused by tinea pedis (athlete's foot); and nail lesions, caused by tinea unguium (Figures 1-3).1,11,12,13
FIGURE 1.
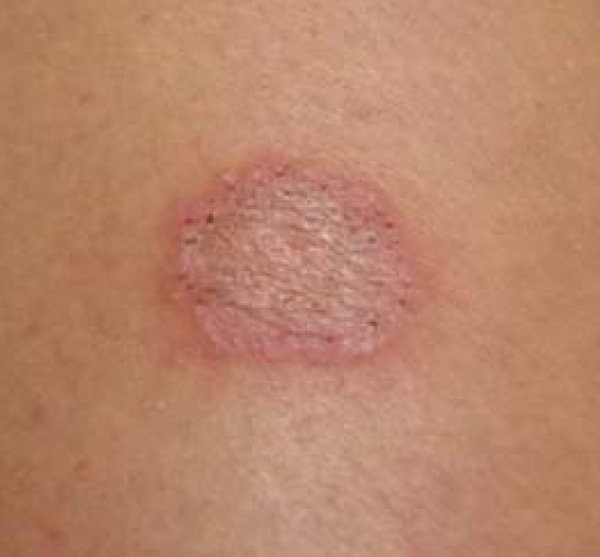
Tinea corporis
FIGURE 3.
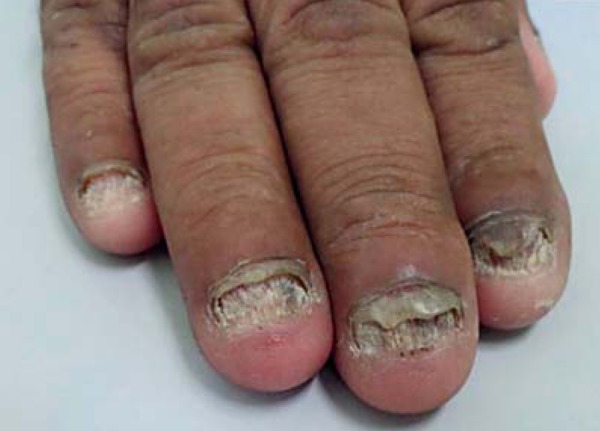
Onychomycosis
The diagnosis of dermatomycoses is primarily established by observation of clinical manifestations and by the characteristic distribution of lesions. When necessary, direct examination is performed for diagnostic confirmation.14
The choice of adequate treatment is determined by the site and extent of lesions, the fungal species involved, and the efficacy, safety profile, and pharmacokinetics of the available antifungal agents.15
First-line therapy is based on the use of topical agents, typically imidazole antifungals.15 When such therapy is ineffective, oral therapy with antifungal agents such as terbinafine, itraconazole, ketoconazole, and fluconazole usually follows.16 Combined therapy with topical and oral antifungals and anti-inflammatory agents has been employed in an attempt to increase the cure rate.8
Although these dermatoses are not serious in terms of mortality or psychological morbidity, they have substantial clinical consequences, producing chronic, difficult-to-treat cutaneous lesions.17 Furthermore, they lead to a decline in patient quality of life and cause disfigurement, with impacts on selfesteem and vanity, and can even result in social discrimination.18
Therefore, taking into account the high prevalence and great relevance of dermatophytoses, not only for the infection itself but also for its complications, the present study sought to determine the clinical, epidemiological, and therapeutic profile of dermatophyte infections in patients enrolled at the Dermatology service of Universidade do Estado do Pará, Brazil, from July 2010 to September 2012.
METHODS
This descriptive, retrospective, cross-sectional, observational study was carried out in Belém, state of Pará, Brazil, and consisted of a chart review of 145 patients seen at the Dermatology service of Centro de Ciências Biológicas e da Saúde da Universidade Estadual do Pará, a regional secondary referral center for cutaneous diseases.
The study sample comprised all patients treated at the service between July 2010 and September 2012 with a clinical diagnosis of dermatophytosis, regardless of causative species, site of infection, age, sex, or prior therapy. Cases with inconclusive clinical diagnosis were confirmed by fungal direct examination performed at the service's reference laboratory. Patients with incomplete or missing data in their charts and those with a clinical and/or mycological diagnosis inconsistent with dermatophytosis were excluded.
Data were collected by means of a chart review, and the parameters of interest were recorded in a protocol developed by the investigators. The questionnaire was designed to collect information on epidemiological data, clinical aspects, and therapy prescribed.
Microsoft Word 2010 and Microsoft Excel 2007 were used to process the manuscript, charts, and tables. Statistical analyses were carried out in the Bioestat 5.0 software environment. The chi-squared test of independence and Williams' corrected G-test were used for analysis.
This study was approved by the Universidade do Estado do Pará Research Ethics Committee with judgment number 180.297/2012.
RESULTS
Data analysis showed that, of the 145 patients included, 89 were female (61.4%) and 56 were male (38.6%). The most prevalent age group was 51-60 years.
The main clinical form of dermatophytosis in the sample was onychomycosis (38.6%), followed by tinea corporis (24.1%), tinea capitis (22.1%), and tinea pedis (15.2%).
There was a significant proportion of patients with onychomycosis and tinea pedis in the adult age range (.<0.001), whereas tinea capitis was more common among children and adolescents (.=0.010). There were no significant differences in the age distribution of the other dermatophytoses.
Regarding treatment, there was a predominance of combination therapy with topical and systemic agents (62.8%), with fluconazole (33.1%) and ciclopirox olamine (49%) as the most commonly prescribed systemic and topical agents respectively.
Finally, we found that 31% of patients did not attend their follow-up visits.
DISCUSSION
Analysis of the characteristics of the research sample showed that the female population affected by dermatophytosis was approximately 1.5 times larger than the male population with these conditions (Table 1). Likewise, other studies have reported a higher prevalence in women; this difference may be explained by the fact that women are more likely to seek medical attention.6,19,20
TABLE 1.
Sociodemographic profile of the study sample
| Sociodemographic variables | n | % |
|---|---|---|
| Sex | ||
| Male | 56 | 38.6 |
| Female | 89 | 61.4 |
| Total | 145 | 100.0 |
| Age range | ||
| 0-10 years | 27 | 18.6 |
| 11-20 years | 15 | 10.3 |
| 21-30 years | 14 | 9.7 |
| 31-40 years | 9 | 6.2 |
| 41-50 years | 22 | 15.2 |
| 51-60 years | 35 | 24.1 |
| 61-80 years | 23 | 15.9 |
| Total | 145 | 100.0 |
| Occupation | ||
| Homemaker | 39 | 26.9 |
| Student | 34 | 23.4 |
| Not reported | 17 | 11.7 |
| Retired | 14 | 9.7 |
| Salesperson | 4 | 2.8 |
| Domestic servant | 3 | 2.1 |
| Driver | 3 | 2.1 |
| Builder | 3 | 2.1 |
| Other | 28 | 19.3 |
| Total | 145 | 100.0 |
The average age of patients with a diagnosis of dermatophytosis varies widely by study and region. In a 2010 study, Araújo et al. found that patients aged 0-20 years accounted for nearly half of all cases of dermatophytosis in the Brazilian state of Paraíba.21 In Natal, state of Rio Grande do Norte, Calado et al. (2006) found an average age of 47 years.22 Corroborating these findings, the highest prevalence of dermatophytosis in our sample was among the 51to-60 age range (Table 1).
Of the several clinical forms of dermatophytosis, onychomycosis predominated in the present study, followed by tinea corporis, tinea capitis, and tinea pedis (Table 2). Previous studies in Italy and Croatia reported this exact same order of prevalence of clinical forms.23,24
TABLE 2.
Clinical profile of the study sample
| Clinical variables | n | % |
|---|---|---|
| Type of dermatophytosis diagnoses* | ||
| Onychomycosis | 56 | 38.6 |
| Tinea corporis | 35 | 24.1 |
| Tinea capitis | 32 | 22.1 |
| Tinea pedis | 22 | 15.2 |
| Tinea cruris | 6 | 4.1 |
| Tinea interdigitalis | 2 | 1.4 |
| Tinea manuum | 2 | 1.4 |
| Body area affected* | ||
| Unknown | 36 | 24.8 |
| Head | 33 | 22.8 |
| Lower limbs | 26 | 17.9 |
| Fingernails | 20 | 13.8 |
| Toenails | 18 | 12.4 |
| Genitalia | 10 | 6.9 |
| Upper limbs | 10 | 6.9 |
| Trunk | 10 | 6,9 |
Note: The sum of the variables exceeds 100.0 due to cases of comorbid occurrence of more than one form of dermatophytosis in the same patient, as well as to extension of dermatophytosis to more than one body area.
Other epidemiological studies confirm the high frequency of onychomycosis in relation to other forms of ringworm.25,26 This may be explained by increasing use of swimming pools, growing involvement in sports, wearing of occlusive footwear both in professional settings and during leisure time, and increasing incidence of diabetes and vascular disease.8,27,28
We found a relatively low incidence of onychomycosis in children, which may be attributable to faster nail growth, smaller nail surface area for invasion by infectious spores, and lower likelihood of trauma. Conversely, 98.2% of patients with onychomycosis were adult or elderly, which may be a result of the reduction in nail growth rate and the increased likelihood of trauma in these age ranges (Table 3).29
TABLE 3.
Association between type of dermatophytosis and age range
| Dermatophytosis | Age range | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Child/Adolescent | Adult | Elderly | |||||||
| n | (%) | n | (%) | n | (%) | n | (%) | P | |
| Onychomycosis | 1 | (1.8) | 37 | (66.1) | 18 | (32.1) | 56 | (100.0) | <0.001* |
| Tinea corporis | 12 | (34.3) | 19 | (54.3) | 4 | (11.4) | 35 | (100.0) | 0.352* |
| Tinea capitis | 29 | (90.6) | 2 | (6.3) | 1 | (3.1) | 32 | (100.0) | <0.001* |
| Tinea pedis | 1 | (4.5) | 14 | (63.6) | 7 | (31.8) | 22 | (100.0) | 0.010# |
| Tinea cruris | 1 | (16.7) | 5 | (83.3) | 0 | 6 | (100.0) | 0.204# | |
| Tinea interdigitalis | 0 | 2 | (100.0) | 0 | 2 | (100.0) | 0.402# | ||
| Tinea manuum | 0 | 2 | (100.0) | 0 | 2 | (100.0) | 0.402# | ||
Note: Chi-squared test of independence; #Williams’ corrected G-test.
Providing further evidence of the close relationship between age and topographic distribution of lesions, we found that the scalp was the predominant site of dermatophytosis in children (Table 3). Binder et al. (2011) and Cortez et al. (2012) reached the same conclusion, reporting that the highest prevalence of tinea capitis was found in the pediatric population.30,31
Tinea capitis is considered a disease of childhood; as seen in this study, adults are rarely affected. This prevalence is due to the absence of sebum secretion and colonization by Malassezia spp. characteristic of childhood, which reduce the ability of the scalp to protect itself from infection by these dermatophytes.32 Furthermore, children are more exposed to other risk factors, such as poor hygiene, crowded enclosed places (daycare facilities and schools), direct contact with household pets, and playing with sand.6
Treatment of dermatophytosis is generally a long and onerous process, typically involving the use of antifungal agents of the allylamine class (such as terbinafine) and the azoles (ketoconazole, miconazole, oxiconazole). Most infections can be managed with topical therapy alone; however, in an attempt to increase the cure rate, topical and systemic (oral) medications are often combined. In the present sample, 62.8% of patients received combination topical and systemic therapy (Table 4).33
TABLE 4.
Treatment profile of the study sample
| Treatment variables | n | % |
|---|---|---|
| Treatment modality | ||
| Topical | 25 | 17.2 |
| Systemic | 25 | 17.2 |
| Combined (topical + systemic) | 91 | 62.8 |
| Not reported | 4 | 2.8 |
| Total | 145 | 100.0 |
| Topical antifungals* | ||
| Ciclopirox olamine | 71 | 49.0 |
| Butenafine | 34 | 23.4 |
| Ketoconazole | 8 | 5.5 |
| Oxiconazole | 7 | 4.8 |
| Miconazole | 6 | 4.1 |
| Amorolfine | 3 | 2.1 |
| Clotrimazole | 2 | 1.4 |
| Fenticonazole | 2 | 1.4 |
| Betalfatrus® | 1 | 0.7 |
| Systemic antifungals* | ||
| Fluconazole | 48 | 33.1 |
| Griseofulvin | 45 | 31.0 |
| Terbinafine | 18 | 12.4 |
| Itraconazole | 9 | 6.2 |
| Ketoconazole | 1 | 0.7 |
Note: The sum of the variables exceeds 100.0 due to combined prescriptions of more than one agent or pharmaceutical form.
Topical therapy with the fungicidal allylamine antifungals is associated with slightly higher cure rates and shorter courses of treatment than therapy with the fungistatic azoles.34,35,36 However, this therapeutic advantage is offset by their significantly higher cost.37 Therefore, as the present study was conducted in a public health system setting, we may infer that therapeutic options had to be adapted to meet the patients' economic status. This may explain why there were no prescriptions of topical terbinafine therapy.
Followed by topical terbinafine, ciclopirox olamine is the most effective agent against dermatophytosis.38 This helps explain the findings of the present study, in which ciclopirox olamine accounted for 49% of topical prescriptions (Table 4).
The high efficacy of ciclopirox notwithstanding, this finding is also attributable to the fact that onychomycosis was the most prevalent clinical form of dermatophytosis in the sample; ciclopirox enamel is the first-line drug of choice for this condition, as it can even be used in patients who are unable or unwilling to undergo systemic therapy.39
Butenafine, an allylamine-like antifungal, was the second most commonly prescribed topical agent in the sample. This corroborates the findings of previous studies, which have shown butenafine to be an excellent option, particularly for tinea corporis, pedis, and cruris, due to its anti-inflammatory action and prolonged skin retention after topical application (Table 4).40,41
Systemic therapy is indicated when lesions are generalized, recurrent, chronic, or unresponsive to topical therapy. Conventional oral treatment regimens are associated with long treatment duration and poor adherence.42
The most widely used systemic antifungals for this purpose are terbinafine, itraconazole, ketoconazole, and fluconazole. The last three, however, require elevated concentrations to achieve a fungistatic effect, which is consistent with the mechanism of action of azole antifungals.43
Several comparative studies found that fluconazole was the least active of the antifungal agents assessed, with its effect varying depending on the causative species.38,44 Nevertheless, in the present sample, it was the most widely used agent for systemic treatment of dermatophytosis (Table 4).
Griseofulvin was the second leading systemic antifungal in our sample. This agent is considered the standard of care, particularly for tinea capitis (Table 4).35 As compared with ketoconazole, griseofulvin produces excellent outcomes, with a faster onset of action and no side effects.32,45
Due to the difference in cost between the azole and allylamine antifungals, the incorporation of economic outcomes (such as cost-effectiveness analyses) to existing systematic reviews should help define which therapeutic option produces the best clinical outcomes per currency unit invested, and is thus the most efficient alternative for each condition.
Finally, we found that 31% of patients did not attend follow-up appointments. This high rate may be explained by improvement of lesions before the date scheduled for follow-up, as the average course of treatment of dermatophytosis (excluding onychomycosis) lasts 20 to 45 days, which corresponds to the average time elapsed between the initial visit and first followup visit at the facility where the study was conducted.1
CONCLUSION
This study provides evidence of the importance of recurrent analysis of the epidemiological profile of dermatophytoses to enable correct therapeutic and preventive management of these conditions, which have significant clinical consequences, producing chronic, difficult-to-treat lesions that can decrease patient quality of life and cause disfigurement.
FIGURE 2.
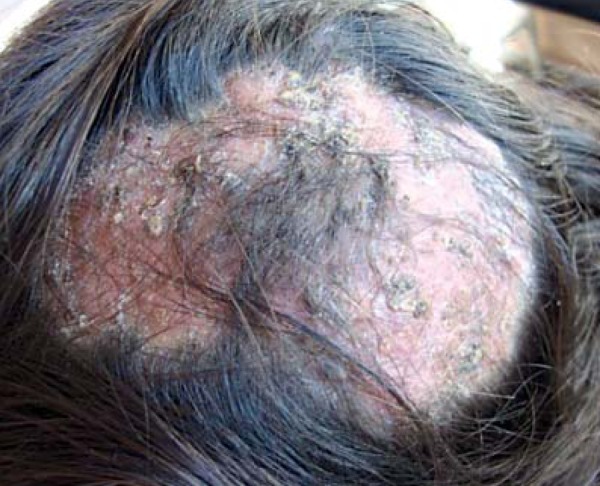
Tinea capitis
Footnotes
Conflict of Interest: None
Financial Support: None
How to cite this article: Pires CAA, Cruz NFS, Lobato AM, Sousa PO, Carneiro FRO, Mendes AMD. Clinical, epidemiological, and therapeutic profile of dermatophytosis. An Bras Dermatol. 2013;88(2):259-64.
Work performed at the Dermatology service, Universidade do Estado do Pará (UEPA), and Centro de Ciências Biológicas e da Saúde (CCBS) - Belém (PA), Brazil.
REFERENCES
- 1.Azulay RD, Azulay DR, Azulay-Abulafia L. Dermatologia. 5. ed. Rio de Janeiro: Guanabara Koogan; 2008. [Google Scholar]
- 2.Peres NTA, Maranhão FCA, Rossi A, Martinez-Rossi NM. Dermatophytes: host-pathogen interaction and antifungal resistance. An Bras Dermatol. 2010;85:657–667. doi: 10.1590/s0365-05962010000500009. [DOI] [PubMed] [Google Scholar]
- 3.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51:2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 4.Siqueira ER, Ferreira JC, Maffei CML, Candido RC. Occurrence of dermatophyte, in nails, feet and hands of university students. Rev Soc Bras Med Trop. 2006;39:269–271. doi: 10.1590/s0037-86822006000300008. [DOI] [PubMed] [Google Scholar]
- 5.Brilhante RSN, Paixão GC, Salvino LK, Diógenes MJN, Bandeira SP, Rocha MFG, et al. Epidemiology and ecology of dermatophytoses in the City of Fortaleza: Trichophyton tonsurans as important emerging pathogen of Tinea capitis. Rev Soc Bras Med Trop. 2000;33:417–425. doi: 10.1590/s0037-86822000000500002. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira JAA, Barros JA, Cortez ACA, Oliveira JRSL. Superficial mycoses in the City of Manaus/AM between March and November/2003. An Bras Dermatol. 2006;81:238–243. [Google Scholar]
- 7.Lacaz CS, Porto E, Melo NT. Guia para identificação: fungos, actimomicetos e algas de interesse médico. São Paulo: Savier; 1998. [Google Scholar]
- 8.Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335–352. doi: 10.1007/s11046-008-9100-9. [DOI] [PubMed] [Google Scholar]
- 9.Degreef H. Clinical Forms of Dermatophytosis (Ringworm Infection) Mycopathologia. 2008;166:257–265. doi: 10.1007/s11046-008-9101-8. [DOI] [PubMed] [Google Scholar]
- 10.Turchin I, Barankin B, Alanen KW, Saxinger L. Dermatophyte infection (tinea) Can Fam Physician. 2005;51:499–501. [PMC free article] [PubMed] [Google Scholar]
- 11.Somenzi CC, Ribeiro TS, Menezes A. Características particulares da micologia clínica e o diagnóstico laboratorial de micoses superficiais. Newslab. 2006;77:106–118. [Google Scholar]
- 12.Sampaio SAP, Rivitti EA. Dermatologia. 3. ed. São Paulo: Artes Médicas; 2007. [Google Scholar]
- 13.Fitzpatrick TB. Tratado de Dermatologia. 7. ed. Rio de Janeiro: Editora Revinter; 2010. [Google Scholar]
- 14.Campanha AM, Tasca RS, Svidzinski TIE. Dermatomicoses: freqüência, diagnóstico laboratorial e adesão de pacientes ao tratamento em um sistema público de saúde, Maringá-PR, Brasil. Lat Am J Pharm. 2007;26:442–448. [Google Scholar]
- 15.Kassem MA, Esmat S, Bendas ER, El-Komy MH. Efficacy of topical griseofulvin in treatment of tinea corporis. Mycoses. 2006;49:232–235. doi: 10.1111/j.1439-0507.2006.01221.x. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Torres B, Cabañes FJ, Carrillo-Muñoz AJ, Esteban A, Inza I, Abarca L, et al. Collaborative evaluation of optimal antifungal susceptibility testing condition for dermatophytes. J Clin Microbiol. 2002;40:3999–4003. doi: 10.1128/JCM.40.11.3999-4003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wille MP, Arantes TD, Silva JLM. Epidemiologia das dermatomicoses em população da periferia de Araraquara - SP. Rev Bras Clin Med. 2009;7:295–298. [Google Scholar]
- 18.Lopes JO, Alves SH, Mari CR, Oliveira LT, Brum LM, Westphalen JB, et al. A ten-year survey of onychomycosis in the central region of the Rio Grande do Sul, Brazil. Rev Inst Med Trop São Paulo. 1999;41:147–149. doi: 10.1590/s0036-46651999000300002. [DOI] [PubMed] [Google Scholar]
- 19.Schoeler AP, Sguissardi CH, Bernardi E, Cembranel LR, Fuentefria AM. Prevalência de dermatófitos na rotina de micologia em hospital particular de médio porte na cidade de Chapecó, estado de Santa Catarina, Brasil. Rev Ciênc Farm Básica Apl. 2010;31:103–106. [Google Scholar]
- 20.Couto MT, Pinheiro TF, Valença O, Machin R, Silva GSN, Gomes R, et al. O homem na atenção primária à saúde: discutindo (in)visibilidade a partir da perspectiva de gênero. Interface Comunic Saude Educ. 2010;14:257–270. [Google Scholar]
- 21.Araújo Gde M, Araújo ND, Farias RP, Cavalcanti FC, Lima Mdo L, Braz RA. Superficial mycoses in Paraíba: a comparative analysis and bibliographical revision. An Bras Dermatol. 2010;85:943–946. doi: 10.1590/s0365-05962010000600031. [DOI] [PubMed] [Google Scholar]
- 22.Calado NB, Sousa F, Jr, Gomes NO, Cardoso FR, Zaror LC, Milan EP. Fusarium nail and skin infection: a report of eight cases from Natal, Brazil. Mycopathologia. 2006;161:27–31. doi: 10.1007/s11046-005-0136-9. [DOI] [PubMed] [Google Scholar]
- 23.Vena GA, Chieco P, Posa F, Garofalo A, Bosco A, Cassano N. Epidemiology of dermatophytoses: retrospective analysis from 2005 to 2010 and comparison with previous data from 1975. New Microbiol. 2012;35:207–213. [PubMed] [Google Scholar]
- 24.Miklić P, Skerlev M, Budimcić D, Lipozencić J. The frequency of superficial mycoses according to agents isolated during a ten-year period (1999-2008) in Zagreb area, Croatia. Acta Dermatovenerol Croat. 2010;18:92–98. [PubMed] [Google Scholar]
- 25.Maraki S. Epidemiology of dermatophytoses in Crete, Greece between 2004 and 2010. G Ital Dermatol Venereol. 2012;147:315–319. [PubMed] [Google Scholar]
- 26.Drakensjö IT, Chryssanthou E. Epidemiology of dermatophyte infections in Stockholm, Sweden: a retrospective study from 2005-2009. Med Mycol. 2011;49:484–488. doi: 10.3109/13693786.2010.540045. [DOI] [PubMed] [Google Scholar]
- 27.Kaur R, Kashyap B, Bhalla P. Onychomycosis - epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26:108–116. doi: 10.4103/0255-0857.40522. [DOI] [PubMed] [Google Scholar]
- 28.Nenoff P, Hanselmayer GG, Tietz HJ. Fungal nail infections--an update: Part 1:Prevalence, epidemiology, predisposing conditions, and differential diagnosis. Hautarzt. 2012;63:30–38. doi: 10.1007/s00105-011-2251-5. [DOI] [PubMed] [Google Scholar]
- 29.Araújo AJG, Bastos OMP, Souza MAJ, Oliveira JC. Onicomicoses por fungos emergentes: análise clínica, diagnóstico laboratorial e revisão. An Bras Dermatol. 2003;78:445–455. [Google Scholar]
- 30.Binder B, Lackner HK, Poessl BD, Propst E, Weger W, Smolle J, et al. Prevalence of tinea capitis in Southeastern Austria between 1985 and 2008: up-to-date picture of the current situation. Mycoses. 2011;54:243–247. doi: 10.1111/j.1439-0507.2009.01804.x. [DOI] [PubMed] [Google Scholar]
- 31.Cortez AC, de Souza JV, Sadahiro A, de Oliveira JA. Frequency and aetiology of dermatophytosis in children age 12 and under in the state of Amazonas, Brazil. Rev Iberoam Micol. 2012;29:223–226. doi: 10.1016/j.riam.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Rebollo N, López-barcenas AP, Arenas R. Tiña de la cabeza. Actas Dermosifiliogr. 2008;99:91–100. [PubMed] [Google Scholar]
- 33.Gupta AK, Cooper EA. Update in antifungal therapy of dermatophytosis. Mycopathologia. 2008;166:353–367. doi: 10.1007/s11046-008-9109-0. [DOI] [PubMed] [Google Scholar]
- 34.Andrews MD, Burns M. Common tinea infections in children. Am Fam Physician. 2008;77:1415–1420. [PubMed] [Google Scholar]
- 35.Bell-Syer SE, Khan SM, Torgerson DJ. Oral treatments for fungal infections of the skin of the foot. Cochrane Database Syst Rev. 2012;10: doi: 10.1002/14651858.CD003584.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotta I, Otuki MF, Sanches AC, Correr CJ. Eficácia de antifúngicos tópicos em diferentes dermatomicoses: uma revisão sistemática com metanálise. Rev Assoc Med Bras. 2012;58:308–318. [PubMed] [Google Scholar]
- 37.Martins GG. Terapêutica de onicomicoses humanas: epidemiologia, manifestações clínicas e uso clínico de antifúngicos [tese] Belo Horizonte, MG: Universidade Federal de Minas Gerais; 2009. [Google Scholar]
- 38.Magagnin CM, Stopiglia CD, Vieira FJ, Heidrich D, Machado M, Vetoratto G, et al. Antifungal susceptibility of dermatophytes isolated from patients with chronic renal failure. An Bras Dermatol. 2011;86:694–701. doi: 10.1590/s0365-05962011000400011. [DOI] [PubMed] [Google Scholar]
- 39.Shemer A, Nathansohn N, Trau H, Amichai B, Grunwald MH. Ciclopirox nail lacquer for the treatment of onychomycosis: an open non-comparative study. J Dermatol. 2010;37:137–139. doi: 10.1111/j.1346-8138.2009.00773.x. [DOI] [PubMed] [Google Scholar]
- 40.Singal A. Butenafine and superficial mycoses: current status. Expert Opin Drug Metab Toxicol. 2008;4:999–1005. doi: 10.1517/17425255.4.7.999. [DOI] [PubMed] [Google Scholar]
- 41.Das S, Barbhuniya JN, Biswas I, Bhattacharya S, Kundu PK. Studies on comparison of the efficacy of terbinafine 1%cream and butenafine 1% cream for the treatment of Tinea cruris. Indian Dermatol Online J. 2010;1:8–9. doi: 10.4103/2229-5178.73249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shivakumar V, Okade R, Rajkumar V, Sajitha K, Prasad SR. Intermittent pulse-dosed terbinafine in the treatment of tinea corporis and/or tinea cruris. Indian J Dermatol. 2011;56:121–122. doi: 10.4103/0019-5154.77579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrillo-Muñoz AJ, Tur-Tur C, Hernández-Molina JM, Santos P, Cárdenes D, Giusiano G. Antifungal agents for onychomycoses. Rev Iberoam Micol. 2010;27:49–56. doi: 10.1016/j.riam.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Manzano-Gayosso P, Mendez-Tovar LJ, Hernandez-Hernandez F, Lopez-Martinez R. La resistencia a los antifúngicos: un problema emergente en México. Gac Méd Méx. 2008;144:23–26. [PubMed] [Google Scholar]
- 45.Zaraa I, Hawilo A, Trojjet S, El Euch D, Mokni M, Ben Osman A. Letter: tinea capitis in infants in their first 2 years of life: a 12-year study and a review of the literature. Dermatol Online J. 2012 Jul 15;18:16. [PubMed] [Google Scholar]


