Abstract
Cutaneous melanoma is a challenge to treat. Over the last 30 years, no drug or combination of drugs demonstrated significant impact to improve patient survival. From 1995 to 2000, the use of cytokines such as interferon and interleukin become treatment options. In 2011, new drugs were approved by the U.S. Food and Drug Administration, including peginterferon alfa-2b for patients with stage III disease, vemurafenib for patients with metastatic melanoma with the BRAF V600E mutation, and ipilimumab, a monoclonal antibody directed to the CTLA-4 T lymphocyte receptor, to combat metastatic melanoma in patients who do not have the BRAF V600E mutation. Both ipilimumab and vemurafenib showed results in terms of overall survival. Other trials with inhibitors of other genes, such as the KIT gene and MEK, are underway in the search for new discoveries. The discovery of new treatments for advanced or metastatic disease aims to relieve symptoms and improve patient quality of life.
Keywords: Antibodies, monoclonal; Antibody specificity; Interferons; Melanoma; Therapeutics
INTRODUCTION
Cutaneous melanoma is the more aggressive form of the disease, occurring when melanocytes undergo changes and become malignant.1 Melanoma is less common than basal cell and squamous cell carcinomas, but is the most dangerous skin cancer.2 According to the Brazilian National Cancer Institute (INCA), skin cancer is the most common cancer in Brazil and accounts for 25% of all malignant tumors registered in the country, with melanoma accounting for 4%. In 2009, 1392 deaths were recorded due to melanoma, and an estimated 6230 new cases are expected to occur in 2012.3 Treatment of this neoplasm has a major economic impact. Souza et al. (2009) demonstrated that the Brazilian state of São Paulo spent more than R$ 100,000.00 on diagnosis and treatment of melanoma between the years 2000 and 2007. Cases of stage III and IV disease accounted for 98% of this expenditure.4
Melanoma causes 50,000 deaths annually worldwide, and its incidence continues to increase. The incidence of malignant melanoma has increased fivefold from 1980 to 2009.5 If the tumor is detected early, before invading the dermis, surgical excision is curative in approximately 99% of patients.6 Therefore, early diagnosis is very important.5,7 The main treatments for melanoma currently are surgery, radiotherapy and chemotherapy. Surgery can provide efficient tumor resection, if there are no metastases. Radiation therapy is used in severe cases and in conjunction with surgery, to increase the efficiency of treatment.8 Antineoplastic therapy has been based on stimulation of the body's own defenses by immunotherapy with interferon alfα-2β (IFNα-2β) and interleukin-2 (IL-2).9
In 2011, new agents were approved by the U.S. Food and Drug Administration (FDA) for the treatment of advanced melanoma. These include peginterferon alfα-2β (IFNα-2β), which showed significant results in terms of progression-free survival (PFS, the time the patient remains alive with stable disease after treatment) and vemurafenib and ipilimumab, which, in randomized trials, demonstrated an improvement both in PFS and in overall survival (OS, the time between the start of treatment and the death of the patient).9-13
MATERIALS AND METHOD
This study aimed to conduct a bibliographic review on new advances in the treatment of cutaneous melanoma, mainly including articles published in Portuguese, Spanish, and English. The PubMed, LILACS, and SciELO databases were searched for articles published from 1995 to 2012, using strings such as "skin cancer and new treatments", "vemurafenib", "ipilimumab", "interleukin 2 skin cancer", "interferon alpha-2β skin cancer", and "pegylated interferon skin cancer". In addition, the reference lists of all articles considered relevant were consulted in search of new items for inclusion. Other sources, such as the National Cancer Institute (NCI), American Cancer Society, and FDA websites, were searched for additional information. Full-text availability was a criterion for selection.
RESULTS AND DISCUSSION
Our review of the literature related to new advances in the treatment of cutaneous melanoma revealed publications on new drugs that have been approved by the FDA, as well as some studies that show the expectations to researchers in the field, as the incidence of skin cancer has increased annually and melanoma is a leading cause of death by cutaneous diseases (Table 1). The search yielded a total of 60 articles: two published in Spanish, four published in Portuguese, and the remainder in English. A total of 27 articles were read and included; the others were read and found to have no relevance to the purpose of this study.
TABLE 1.
Comparative table between different studies for the treatment of melanoma
| Therapy | Disease Stage | Goal | Trial | Results | Reference |
|---|---|---|---|---|---|
| IL-2 (1998) | IV or recurrent melanoma | Determine the short- and long-term efficacy and toxicity of high-dose IL-2 | n = 70 → 600,000 or 720,000 IU/kg (IV). Cycle repeated after 6-9 days | n = 43 / PFS 8.9 months | (19) |
| n = 12 / PFS 12 months | |||||
| The results are more lasting for the patients with good pulmonary, heart and kidney function | |||||
| IV or recurrent melanoma, without previous treatment | Determine the antitumor efficacy of IL-2 | n = 24 → 30 mg/kg (IV) for 8 weeks | n = 9 / PFS 8 weeks | (21) | |
| n = 2 / PFS 16 weeks | |||||
| Response rate is lower and efficacy in terms of response rate is comparable to conventional therapies such as DTIC. | |||||
| IFNa-2b (1995) ESTG-1684 | IIB, IIC or III (with or without lymph node involvement) or recurrent melanoma | Determine the anti-tumor efficacy of high-dose IFNa-2b | n = 143 → 20 million IU/m2, 5 days a week for 4 weeks, followed by 10 million IU/m2, three times a week for 48 weeks | The analysis of 7 years of study showed that on average 1-1.7 years to PFS and 2.8-3.8 years to OS | (24) |
| First agent to show significant OS benefit (more pronounced benefit in patients with lymph node involvement). | |||||
| IFN-PEGa-2b (2011) EORTC 18991 | IIB, IIC or III (with or without lymph node involvement) or recurrent melanoma | Determine the anti-tumor efficacy of higher doses of PEG-IFNa-2b | n = 1256 / n = 627 → 6 Hg/kg weekly for 8 weeks, followed by 3 Hg/kg weekly for 5 years | PEG-IFNa-2b → the risk of recurrence and death in 7% of patients at 4 years and 13% for patients with nodal disease. | (27) |
| Determine the anti-tumor efficacy of low-dose PEG- IFNa-2b | n = 1388 → half received 10 million IU/m2, 5 days a week for 4 weeks, followed by 5 million IU/m2, three times a week for 2 years | During 2 years of analysis, the results of PFS were better with the use of high medication doses. | (28) | ||
| Biochemother apy | III, IV or recurrent melanoma | Determine whether the combination of chemotherapy and immunotherapy might have better efficacy | Patients were randomized to receive cisplatin, vin- blastine, and DTIC (CVD) alone or simultaneously with IL-2 and IFNa-2b | Those who received IL-2 and IFN-2b showed the slightly higher response rate and PFS than the group that received CVD alone. | (30) |
| There was no increase in OS or durable responses. | |||||
| Ipilimumab (2011) | III, IV or recurrent melanoma | Evaluate the median OS of patients who received an ipilimumab-containing regimen as compared with a group that received the gp100 vaccine alone | n = 403 → ipilimumab 3 mg/kg + gp100 peptide vaccine | Median OS in ipilimumab + gp100 group = 10 months | (33) |
| n = 137 → ipilimumab 3 mg/kg | Median OS with ipilimumab alone = 10.1 months | ||||
| n = 136 → gp100 vaccine peptide | Median OS with gp100 alone = 6.4 months | ||||
| Evaluate the average OS with different doses of ipilimumab | n = 73 → 10 mg/kg | OS was 11% at 10 mg/kg, 4.2% | (34) | ||
| n = 72 → 3 mg/kg | |||||
| n = 72 → 0.3 mg/kg | |||||
| every 3 weeks for 4 months by IV infusion | at 3 mg/kg and 0% at 0.3 mg/kg. | ||||
| Ipilimumab proved more effective at a dose of 10 mg/kg. | |||||
| Vemurafenib (2011) | III, IV or recurrent melanoma, without | Evaluate average OS with vemurafenib | n = 337, 960 mg vemurafenib orally twice daily | After 6 months, OS was 84% for vemurafenib and 64% for DTIC | (36) |
| treatment previous | n = 338 → 1,000 mg/nv* of DTIC, IV, once every 3 weeks | Vemurafenib showed a 63% relative reduction in the risk of death (OS) and 74% in the risk of progression (PFS) as compared with DTIC | |||
| Dabrafenib | III or IV, without previous treatment | Evaluate the antitumor efficacy of dabrafenib, another BRAF inhibitor | Patients were randomized to receive dabrafenib (n = 187) or DTIC (n = 63) | Average PFS was 5.1 months with dabrafenib versus 2.7 months with DTIC. | (39) |
| 70% → in risk of progression or death compared with DTIC | |||||
| Trametinib | III or IV | Cause a stronger response against the cancer and prevent resistance to treatment, as patients who use vemurafe-nib eventually develop resistance. | n = 125 → varying doses of dabrafenib / trameti-nib | Average PFS was 10.8 months in this group, and 15 of 24 patients (63%) achieved either CR or PR. This dosage (150/2 mg) is being assessed in a phase III randomized trial | (40) |
| Most PFS was achieved in 24 patients receiving 150 mg dabrafenib twice daily and 2 mg trametinib once daily | |||||
| Imatinib | III or IV | Ascertain the effectiveness of imatinib for melanoma with Kit gene mutation | n = 28 → 400 mg of imatinib twice daily | The durable overall response rate was 16%, with a median time to progression of 12 weeks, and mean OS 46.3 weeks. | (41) |
| n = 43 → 400 mg daily until disease progression or unacceptable toxicity to a mean of 12 months | 41.9% (n=18) CR | (42) | |||
| 30.2% (n=13) SD | |||||
| 23.3% (n=10) PR |
PFS, progression-free survival; OS, overall survival; PR, partial response; CR, complete response; SD, stable disease; ↓, decrease
DACARBAZINE
In 1970, dacarbazine (DTIC) became the first chemotherapeutic agent approved by the FDA for treatment of metastatic melanoma, based on overall response rates.14 DTIC is one of the triazene derivatives that acts through DNA alkylation, forming crosslinks within and between helices that lead to local denaturation of the DNA strand, interfering with its form and function and killing the cancer cell. DTIC is a prodrug and requires initial activation by cytochrome P450 through an N-demethylation reaction. In the target cell, spontaneous cleavage of the metabolite releases an alkylating compound, diazomethane.8 Recent data show that DTIC, alone or in combination with other drugs, are used to treat 40-80% of patients with any type of cancer.15 DTIC monotherapy produces an overall response rate of 15 to 25%, with an average response time of 5-6 months and complete response rates of 5%.16
For many years, the first-line systemic therapy for patients with metastatic melanoma was DTIC. As in other malignancies, several attempts were made to obtain better results with DTIC by adding other agents.17 Temozolomide (a DTIC analog), cisplatin, carmustine, fotemustine, vinblastine, and tamoxifen were added to DTIC in a variety of regimens that induced responses in metastatic lesions in the liver, bones, and brain, but were unable to produce any survival benefit for patients.12,18 DTIC is still regarded as the default choice of chemotherapy for most patients with melanoma, despite its lack of superiority in comparison with other treatments or best supportive care in phase III studies.17
Toxicities include nausea and vomiting, in over 90% of patients, which usually develop 1 to 3 hours after treatment, myelosuppression (both leucopenia and thrombocytopenia), which is usually mild to moderate; and flu-like illness, consisting of chills, fever, malaise, and myalgia. Hepatotoxicity, alopecia, facial flushing, neurotoxicity, and skin reactions have also been reported.16
INTERLEUKIN-2
The use of high-dose IL-2 was approved by the FDA in 1998 on the basis of a phase II study showing long-term, durable complete responses in previously treated patients with metastatic melanoma (Stage IV).8,19 Through a mechanism of immunomodulation, IL-2 stimulates the growth of T cells and NK (natural killer) cells and thus facilitates its cytolytic effect, causing malignant cells to be destroyed. The exclusive use of high-dose IL-2 is an alternative therapy in cases of patients with metastatic melanoma and good performance status, because the drug is associated with frequent and very severe acute toxicity.20
Atkins et al. (1999) aimed to determine the toxicity and efficacy of high-dose IL-2 in the short and long term. The study evaluated 70 patients in eight clinical trials conducted between 1985 and 1993, with dosages ranging from 600,000 to 720,000 IU/kg, administered by intravenous infusion (IV) as clinically tolerated. After 6-9 days, a second course of treatment identical to the first was administered.19
The overall response rate was 16% (95% CI 12-21%), including 17 complete responses (CR) (6%) and 26 partial responses (PR) (10%). The median duration of response for all respondents (43 patients) was 8.9 months. Ten patients who had CR and two who had PR (59%) had a PFS of 12 months, and their responses were durable during this period. Therefore, high doses of IL-2 appear to benefit some patients with metastatic melanoma, producing partial responses or durable complete responses.16
Davis et al. (2009), with the goal of discovering an effective antimelanoma agent, conducted a phase I study in 14 patients with previously untreated advanced melanoma, who received IL-2 at a dose of 30 mcg/kg IV for 8 weeks. CR was observed. In phase II, according to the protocol, 10 patients were added for a total of 24, and the overall response rate was 8.3%. In the 8th week, 9 patients had PFS; in the 16th week, 2 patients had PFS, and of these 2, one had CR and one PR (both with lung metastases). Although the overall response rate was low in this study, the antitumor efficacy in terms of response rate was comparable to conventional therapies such as DTIC.21
Although the promise of cure with IL-2 is attractive, its use is still limited due to high toxicity (20). This treatment can be offered only to patients who have adequate cardiac, pulmonary, and kidney function. It also requires that the physician have adequate training and resources in terms of trained personnel to perform cardiac monitoring and administer IL-2 safely.9,21 The main toxic effects associated with high doses of IL-2 include hypotension, vascular leak syndrome, cardiac arrhythmias, hepatic dysfunction, fever, nausea, diarrhea, catheter-related sepsis, and death.16,22
An important observation is that patients who have not responded to chemotherapy have the same chance of responding to IL-2 than patients without prior treatment. This discovery has led some physicians and patients to consider less toxic treatment options initially, with the understanding that IL-2 therapy may be considered as second-line treatment.9
INTERFERON ALFA-2β
The mechanism of action of IFNa-2b is unclear. It is known that it inhibits DNA/RNA replication, having an antiproliferative effect on cell growth and a cytostatic effect on malignant cells, as well immunomodulating actions on various elements of the immune system, such as stimulation of lytic activity of NK cells, cytotoxic T lymphocytes, and macrophages of infected tumor cells; modification of antibody production by B lymphocytes; regulation of antigen expression by major histocompatibility complex (MHC) on the cell membrane; and stimulation of IFNa-2b production.23
IFNα-2β was approved in 1995 for adjunctive treatment of patients with advanced melanoma at high risk of recurrence after surgery, stages IIB (between 2 and 4 mm thick, with ulceration, or > 4mm without ulceration), IIC (> 4mm thick with ulceration), or stage III (melanoma of any thickness, with or without ulceration).24 According to a study by Kirkwood, IFNα-2β at high doses may increase PFS as well as OS.20 In a randomized clinical trial conducted by the Eastern Cooperative Oncology Group, ESTG-1684, 287 patients (stage IIB, IIC, or III) received high doses of IFNα-2β (20 million international units [IU]/m2, IV, 5 days a week for 4 weeks, followed by 10 million IU/m2 subcutaneously three times a week for 48 weeks). At a median of 7 years (6.9-7.6 years), the study demonstrated a significant prolongation of PFS and OS for patients receiving high doses of IFNα-2β. The mean PFS (1.0-1.7 years, P = 0.023) and OS (2.8-3.8 years, P = 0.237). The benefit of therapy with IFNα-2β was more pronounced in patients with lymph node involvement. IFNα-2β is the first agent to show a significant OS benefit in patients at high risk of recurrence.24
This benefit must be balanced against the toxicities associated with the use of IFN to decide whether adjuvant treatment with IFNα-2β is appropriate.9 Within 12 hours after administration, 80% to 90% of patients develop flu-like illness, which is the main adverse reaction, characterized by fever, chills, headache, myalgia, arthralgia, and malaise. Skin reactions such as rashes, or, in some cases, alopecia, can occur. Fatigue and hepatotoxicity have been reported, especially in the elderly.22 Treatment with IFNa-2b can also have effects on the central nervous system, such as irritability, depression, memory impairment, and drowsiness. Antidepressants and other mood stabilizers may be needed. These symptoms can be severe in some patients, and can result in discontinuation of treatment. However, they usually occur only at the beginning of therapy.25
PEGINTERFERON ALFA-2β
IFN-PEG-2b is characterized by the incorporation of a polyethylene glycol molecule (pegylation) to IFNα-2β, which makes it larger and decreases its metabolism, with the benefit of prolonging plasma concentrations and thus allowing the administration of only one dose weekly, unlike the non-pegylated formulation, which is administered three times a week.16
IFN-PEGa-2b was approved by the FDA in 2011 as adjunctive therapy in the treatment of patients with advanced melanoma at high risk of recurrence after surgery, disease stage IIB, IIC, or III. This approval was based on a randomized phase III clinical trial conducted under the auspices of the European Organization for Research and Treatment of Cancer -EORTC 18991.26 The study involved 1,256 patients with advanced melanoma in the postoperative period. Half of the patients (n = 627) were randomized to receive high-dose IFN-PEGa-2b (6 mg/kg per week for 8 weeks as induction followed by 3 mg/kg per week for 5 years) and the remaining 629 patients received standard therapy.27
Patients were followed on average for 3.8 years (3.2-4.2 years). There was a statistically significant improvement in PFS (P = 0.011) in patients treated with IFN-PEGa-2b compared with the observation group (45.6% versus 38.9%), but no such improvement in OS. In all patients, IFN-PEGa-2b therapy resulted in a 7% reduction in the risk of recurrence or death at 4 years of treatment (13% for patients with microscopic nodal disease [N1] or other lymph node involvement). There were no unexpected toxicities with IFN-PEGa-2b and toxicity did not increase with treatment duration. However, 355 patients in the IFN-PEGa-2b group (58%) had dose reductions due to toxicity. Adverse events of any grade occurred in 30% of patients, the most common being fatigue, hepatotoxicity, and depression.27
A 2005 study conducted by Eggermont et al. (EORTC 18952) examined the effect of intermediate doses of IFN-PEGa-2b (10 million IU/m2, 5 days a week for 4 weeks, followed by 5 million IU/m2 3 times a week for 2 years), alone or in combination with IL-2, in 1,388 patients to determine whether similar efficacy could be achieved with less toxicity. At 2-year follow-up, this trial demonstrated less benefit than that observed with high doses of IFNα-2β in terms of PFS.28
Although these studies have demonstrated a benefit in PFS compared to the placebo group, this benefit was lost as soon as the treatment was stopped, raising the possibility that prolonged treatment may be required. Eggermont et al., in a combined analysis of these studies, demonstrated a significant effect in patients whose tumor reached the lymph nodes.27
BIOCHEMOTHERAPY
Biochemotherapy is the combination of chemotherapy and immunotherapy. This combination has been presented in various ways over the years, but the regimen best known for its results and longer development time is the combination of DTIC, cisplatin, vinblastine, IFNα-2β, and IL-2.29
In one phase III clinical trial, 395 patients were randomized to receive cisplatin, vinblastine, and DTIC (CVD) alone (n = 195) or simultaneously with IL-2 and IFNα-2β (n = 200). Those who received IL-2 and IFN-2b had a slightly higher response rate (19.5% versus 13.8%, P = 0.140) and longer time to progression (4.8 months versus 2.9 months, P = 0,015) than patients given only CVD. Although IL-2 and IFN-2b were able to produce slightly higher response rates and mean PFS, these were not associated with an increase in mean OS (9.0 months versus 8.7 months) or durable responses corresponding to the percentage of patients alive at one year (41% versus 36.9%). Considering its high toxicity and complexity, this combined IL-2 and IFN regimen has limited use for melanoma.30
IPILIMUMAB
The FDA recently approved ipilimumab for treatment of metastatic melanoma (stage III and IV) and recurrent melanoma in patients who had previously been treated with other chemotherapeutic agents. It has been clearly demonstrated that patients with metastatic melanoma live longer if they receive ipilimumab.11,13
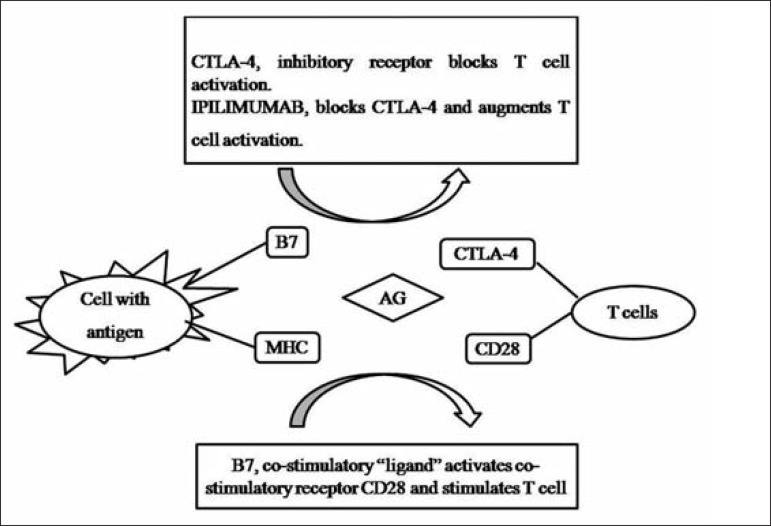
The drug in question is a monoclonal antibody directed to the CTLA-4 lymphocyte receptor. Its mechanism of action is to fundamentally interfere with the process of antigen presentation (Figure 1). In this process, the antigen-presenting cell (APC) presents the antigen via the MHC.31 T lymphocytes then receive this signal through a specific receptor. However, T-cell activation is dependent on co-stimulatory factors. An accessory stimulus is provided by the interaction of a cell surface protein called host B7, which binds to the CD28 protein. Along with this process, it triggers a counterbalance system of immune activation, a kind of "brake", which is exerted by CTLA-4.32 Ipilimumab interacts with CTLA-4 to block this inhibitory activity, "releasing the brake" on activation. The immediate result is increased immune activation, allowing the immune system to recognize, home to, and attack malignant cells.33
FIGURE 1.
CTLA-4 mechanism of action. B7: cell surface protein; CD28: T lymphocyte surface protein; CTLA-4: T lymphocyte receptor that acts by inhibiting T cell activation; MHC: major histocompatibility complex. MHC proteins encoded are expressed on the cell surface and exposed to antigens through the MHC
Hodi et al. (2010) tested the safety and efficacy of ipilimumab in a study with 676 patients, where 403 patients received a combination of ipilimumab 3 mg/kg and gp100 peptide vaccine, 137 patients received ipilimumab 3 mg/kg as monotherapy, and 136 patients received only gp100. Patients received four doses of ipilimumab as tolerated (induction therapy) for 3 weeks. The aim of the study was to evaluate the median OS of patients receiving a regimen containing ipilimumab as compared with the group receiving only gp100.33
The median OS was 10 months among patients receiving ipilimumab plus gp100 vaccine versus 6.4 months among patients receiving gp100 vaccine alone (hazard ratio for death, 0.68; P < 0.001). The median OS of patients who received only ipilimumab was 10.1 months (hazard ratio for death compared to gp100 alone, 0.66; P = 0.003). Hence, ipilimumab with or without gp100 vaccine improved median OS as compared with gp100 vaccine alone.33
Wolchok et al. (2010) conducted a phase II study of 217 melanoma patients with stages III or IV disease, refractory to antitumor treatment, who received ipilimumab at a fixed dose (induction phase) of 10 mg/kg (n = 73), 3 mg/kg (n = 72), or 0.3 mg/kg (n = 72) by IV infusion every 3 weeks for 4 months. The best survival rates were 11.1% (95%CI 4.9-20.7%) in the 10 mg/kg group, 4.2% (0.9-11.7%) in the 3 mg/kg group, and 0% (0.0-4.9%) in the 0.3 mg/kg group (P = 0.0015). Therefore, ipilimumab was most effective at a dose of 10 mg/kg.34
Common side effects that may result from autoimmune reactions associated with the use of ipilimumab include fatigue, diarrhea, and skin rashes, which are often pruritic.11 Diarrhea is very frequent; in severe cases, which are fortunately rare, it may even lead to intestinal perforation. The use of steroids since the early stages is the best way to control bowel symptoms. There may also be hormonal changes such as hypothyroidism and hypopituitarism, both of which can be controlled with hormone replacement therapy.33
Due to these unusual and severe side effects, health professionals should conduct a complete description of these risks through the Risk Evaluation and Mitigation Strategies (REMS), which consists of a Communication Plan to inform health professionals about the serious risks of ipilimumab, to facilitate early identification of these risks, and an overview of management of patients with moderate or severe immune-mediated adverse reactions.13
VEMURAFENIB
Vemurafenib is another new discovery and represents a major step forward for melanoma research. It is a BRAF V600 inhibitor, and when used for the treatment of metastatic melanoma (stage III and IV) and recurrent melanoma, can slow the progression of lesions.35 Vemurafenib is indicated as monotherapy for the treatment of adult patients with metastatic melanoma positive for the BRAF V600 mutation.36 Before administering vemurafenib, patients must have confirmation that their tumor is positive for the BRAF V600 mutation using a validated test (Test of 4800 BRAF V600 Mutation COBAS).37 The test is based on a real-time polymerase chain (PCR) that detects BRAF V600 mutations in tumor samples of human melanoma. Patients whose tumors carry BRAF V600 mutations can benefit from vemurafenib treatment. The test is performed from DNA extracted from formalin-fixed, paraffin-embedded malignant melanoma tissue samples (resected tumors or biopsy specimens).36
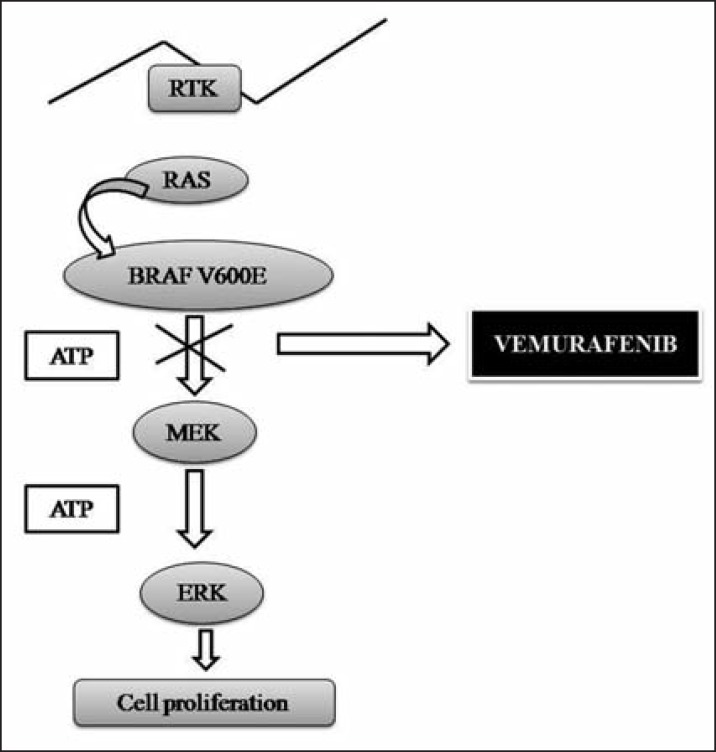
The BRAF protein is normally involved in regulating cell growth, but is mutated in about half of patients with end-stage melanoma.36 Vemurafenib is designed to selectively inhibit BRAF and is capable of blocking the function of the V600E mutant BRAF protein (Figure 2).16 Vemurafenib is a low-molecularweight, orally available, serine/threonine-protein kinase BRAF inhibitor. Mutations in the BRAF gene replacing the valine at amino acid position 600 result in constitutively activated BRAF proteins, which can cause cell proliferation in the absence of growth factors that would normally be required for proliferation.10,16,36,38
FIGURE 2.
Vemurafenib mechanism of action. RAS: protein encoded by genes; TKR: tyrosine kinase receptor; MEK: mitogen-activated by protein kinase; ERK: protein kinase regulated by extracellular signals
Flaherty et al. (2010) conducted a phase I dose-escalation study in 49 patients diagnosed with melanoma, which established a maximum tolerated dose of 960 mg twice daily. An additional phase II extension involved 32 patients who had metastatic melanoma with BRAF V600E mutations, to whom the maximum dose established without adverse effects such as skin rash, fatigue, and arthralgia was administered twice daily until disease progression. In selected patients, tumor biopsy was performed before and during treatment to validate BRAF inhibition. In the phase I dose-escalation trial, 16 of the 49 patients had a BRAF V600E mutation. These 16 patients received 240 mg or more of vemurafenib twice daily. At the end of the analysis, 10 had achieved CR and one had a PR. Among the 32 patients in phase II, 24 had a PR and two achieved CR. The estimated median PFS among all patients was over 7 months.37
Later, Chapman et al. (2010) conducted a phase III study comparing vemurafenib with DTIC. A total of 2,107 patients were screened in 104 centers across 12 countries worldwide. Absence of the BRAF V600 mutation was the main reason for exclusion; patients with life expectancy < 3 months, aged < 18 years, liver failure and/or renal impairment, or CNS metastases were also not included in the study. The final sample comprised 675 patients with metastatic melanoma and BRAF V600E mutation who had not been previously treated. Patients were randomized to receive either vemurafenib (n = 337, 960 mg orally two times a day) or DTIC (n = 338, 1,000 mg/m2, IV, once every 3 weeks). After 6 months, the OS was 84% (95%CI 78-89%) versus 64% (95%CI 56-73%) in the vemurafenib and DTIC groups respectively. On interim analysis for OS and final analysis for PFS, vemurafenib showed a 63% relative reduction in the risk of death (OS) and a 74% reduction in the risk of death or progression (PFS) as compared with DTIC (P < 0.001 for both comparisons).36 Average survival appeared to be significantly better at around 2.5 months, and the response rate was 48% for vemurafenib versus 5% for DTIC.32
The results of phase I, II, and III clinical trials show that treatment with vemurafenib led to complete or partial tumor regression in most patients with the BRAF V600E mutation, as well as improvements in PFS and OS.38 According to Data and Safety Monitoring Board (DSMB) recommendations, these results were released in January 2011 and the study was modified to allow patients in the DTIC arm to cross over to vemurafenib, which was more effective.36
The most frequent adverse reactions with the use of vemurafenib include alopecia, fatigue, rash, and keratosis of the extremities, but two events stand out: photosensitivity and the emergence of new skin cancers. Grade 2 or 3 skin photosensitivity reactions were observed in 12% of patients; grade 3 reactions, characterized by blistering, might be avoided with the use of the sunscreen in some patients.16,36,37 Small tumors, such as squamous cell carcinomas, may arise in the skin of patients receiving vemurafenib, and must be treated by local excision.36,37 The median time to onset of a squamous cell carcinoma was 8 weeks after initiation of treatment, but with low invasive potential and practically no metastases.37 Ipilimumab is being approved with a Medication Guide to inform health care professionals and patients of the potential risks of the drug.36
DABRAFENIB
Dabrafenib is another BRAF inhibitor and was recently compared with DTIC in a phase III study. The results were very similar to DTIC. A total of 250 patients with stage III or IV melanoma, previously untreated, were randomized to receive dabrafenib (n = 187), 150 mg orally or DTIC (n = 63), 1000 mg/m2 IV. The median PFS was 5.1 months for dabrafenib versus 2.7 months for DTIC. The overall response rate of 93% for dabrafenib included 3% CR, 50% PR, and 40% with stable disease (SD); these rates were 0%, 19%, and 30%, respectively, with chemotherapy. The treatments were well tolerated overall. Previous studies have suggested that patients taking vemurafenib are at increased risk of sun sensitivity and secondary skin tumors, such as squamous cell carcinoma; however, patients using dabrafenib in this study reported a low incidence of adverse events.39
Dabrafenib showed an impressive 70% reduction in risk of progression or death in patients with melanoma and BRAF mutation as compared with DTIC treatment. Further studies are underway to test the efficacy of combining dabrafenib with trametinib, an inhibitor of another gene (MEK).39
TRAMETINIB
The combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib showed promising activity in patients with stages III or IV melanoma and fewer side effects compared with vemurafenib. The study objective was to create a stronger response to fight cancer and to prevent or delay resistance to treatment, since patients who use vemurafenib eventually develop resistance. A total of 125 patients received dabrafenib/trametinib at a range of doses. A subgroup analysis included 77 patients who had not previously been treated with BRAF inhibitors. Among these 77 patients, the overall response rate was 56%; 6 patients achieved CR, 38 had PR, and 29 had SD. The average PFS was 7.4 months, which is comparable to the results observed with vemurafenib.40
However, dabrafenib/trametinib were given at four different dosage levels. Most PFS was achieved in the 24 patients receiving 150 mg dabrafenib, twice daily and 2 mg trametinib once daily. In this group, the average PFS was 10.8 months, and 15 of 24 patients (63%) achieved either CR or PR. This dosage (150/2 mg) has been evaluated in a Phase III clinical trial. Although the use of BRAF inhibitors is associated with serious adverse effects, particularly skin lesions, which develop in approximately 25% of patients using vemurafenib, these side effects appeared less frequently in patients receiving combined dabrafenib/trametinib therapy; grade ≥ 2 skin toxicities occurred in 14% of patients, while only 2% developed squamous cell carcinoma and 2% developed premalignant actinic keratosis.40
IMATINIB
A better understanding of the process of melanoma development and mutation profile of different human genes provided knowledge that is the basis for targeted drug development [9]. The first clinical evidence of the potential of this concept was observed by blocking the KIT gene, long known for its role in gastrointestinal tumors and chronic myeloid leukemia. A drug capable of inhibiting its activity has been developed: imatinib.41
Reports from two Phase II open-label studies using imatinib for melanoma with KIT mutation have recently been published.42 The study conducted by Carvajal et al. (2011) analyzed 28 patients who received imatinib at a dose of 400 mg orally, two times per day.41 Guo et al. (2011) assessed 43 patients who also received 400 mg of imatinib daily until disease progression or unacceptable toxicity, with a mean follow-up duration of 12 months. In this study, 10 patients (23.3%, 95%CI 10.2-36.4%) had a PR and 13 patients (30.2%, 95%CI 16.0-44.0) had SD, with 18 of 43 patients (41.9%) demonstrating tumor regression.42
Imatinib was well tolerated and showed no very serious adverse effects at a dose of 400 mg. On the basis of these data, imatinib has shown promising results as a therapeutic agent in patients with metastatic melanoma and mutations in the KIT gene.16 A multicenter phase III trial comparing the use of nilotinib, another KIT inhibitor, with DTIC is underway. The major limitation of this study has been the rarity of mutations in this gene, which occur in less than 5% of patients with melanoma.32 Sunitinib, dasatinib, and nilotinib, among other tyrosine kinase inhibitors, have been cited in the literature in search of new drugs for melanoma, but showed no significant effects.12
FINAL THOUGHTS
A better understanding of the process of melanoma development and of the mutation profile of various genes has yielded knowledge that became the basis for development of new treatments.
Among the drugs recently approved by the FDA, vemurafenib has a high response rate and prolonged time to disease progression, while ipilimumab brings the possibility of durable responses. Deciding which medicine to use depends on the clinical picture and the patient. Patients who present with BRAF mutation are more suited to treatment with vemurafenib.
Drugs used as immunotherapy, such as IFN and IL-2, which stimulate the immune system to fight the cancer, can be effective in combination with chemotherapy and surgery. Patients in good physical condition and with a low burden of disease may be candidates for treatment with high-dose IL-2, which is characterized by higher curative potential as compared with IFN, but has many side effects that can be difficult to tolerate. If the cancer has reached nearby lymph nodes, these lymph nodes may also need to be removed, and treatment with IFN after surgery may be effective for these patients.
Although new drugs and expectations have arisen for patients with melanoma in recent years, it is generally still incurable. The new discovered treatments prolong patient life and can shrink the tumor and relieve symptoms, but their cost is high. New treatments are not as aggressive as conventional chemotherapy and do not cause hair loss, although they are associated with a wide range of toxicities. In Brazil, as in other countries, these new therapies have not yet been approved, However, not only doctors, but also patients await the launch of new medicines to treat melanoma cancer.
Footnotes
Conflict of interest: None
Financial Support: None
How to cite this article: Foletto MC, Haas SE. Cutaneous melanoma: new advances in treatment. An Bras Dermatol. 2014;89(2):301-10.
Work performed at the School of Pharmacy, Universidade Federal do Pampa (UNIPAMPA) - Uruguaiana (RS), Brazil.
REFERENCES
- 1.Koch CM. Melanoma. Oncol. 2004;27:99–101. [Google Scholar]
- 2.Bárzaga HOV. Caracterización clínica e histopatológica del cáncer cutáneo no melanoma. Arch Méd Camaguey. 2010;14:0. [Google Scholar]
- 3.Inca.gov.br. Ministério da Saúde. Instituto Nacional do Câncer . Câncer de Pele Melanoma 2012. [acesso 22 Mar 2012]. [página na internet] Disponível em: http://www2.inca.gov.br/wps/wcm/connect/tiposdecancer/site/home/pele_melanoma/definicao. [Google Scholar]
- 4.Souza RJ, Mattedi AP, Rezende ML, Corrêa Mde P, Duarte EM. An estimate of the cost of treating melanoma disease in the state of Sao Paulo - Brazil. An Bras Dermatol. 2009;84:237–243. doi: 10.1590/s0365-05962009000300004. [DOI] [PubMed] [Google Scholar]
- 5.Naser N. Cutaneous melanoma - a 30-year-long epidemiological study conducted in a city in southern Brazil, from 1980-2009. An Bras Dermatol. 2011;86:932–941. doi: 10.1590/s0365-05962011000500011. [DOI] [PubMed] [Google Scholar]
- 6.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 7.Bonfá R, Bonamigo RR, Bonfá R, Duro KM, Furian RD, Zelmanowicz Ade M. Early diagnosis of cutaneous melanoma: an observation in southern Brazil. An Bras Dermatol. 2011;86:215–221. doi: 10.1590/s0365-05962011000200003. [DOI] [PubMed] [Google Scholar]
- 8.Almeida VL, Leitão A, Reina Barrett LC, Montanari CA, Donnici CL, Lopes MTP. Câncer e agentes antineoplásicos ciclo-celular específicos e ciclo-celular não específicos que interagem com o DNA: uma introdução. Quim Nova. 2005;28:118–129. [Google Scholar]
- 9.Rietschel P, Chapman PB. Immunotherapy of melanoma. Hematol Oncol Clin North Am. 2006;20:751–766. doi: 10.1016/j.hoc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Weeraratna AT. RAF around the edges--the paradox of BRAF inhibitors. N Engl J Med. 2012;366:271–273. doi: 10.1056/NEJMe1111636. [DOI] [PubMed] [Google Scholar]
- 11.Graziani G, Tentori L, Navarra P. Ipilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol Res. 2012;65:9–22. doi: 10.1016/j.phrs.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27–35. doi: 10.1016/j.molmed.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traynor K. Ipilimumab approved for metastatic melanoma. Am J Health Syst Pharm. 2011;68:768. doi: 10.2146/news110025. [DOI] [PubMed] [Google Scholar]
- 14.Cheng S, Koch WH, Wu L. Co-development of a companion diagnostic for targeted cancer therapy. N Biotechnol. 2012;29:682–688. doi: 10.1016/j.nbt.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Neves AP, Vargas M.D. Complexos de Platina(II) na Terapia do Câncer. Rev Virtual Quim. 2011;3:196–209. [Google Scholar]
- 16.Lee B, Mukhi N, Liu D. Current management and novel agents for malignant melanoma. J Hematol Oncol. 2012;5:3. doi: 10.1186/1756-8722-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Day S, Boasberg P. Management of metastatic melanoma 2005. Surg Oncol Clin N Am. 2006;15:419–437. doi: 10.1016/j.soc.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Eggermont AM. Advances in systemic treatment of melanoma. Ann Oncol. 2010;21:vii339–vii344. doi: 10.1093/annonc/mdq364. [DOI] [PubMed] [Google Scholar]
- 19.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 20.Wainstein AJA, Belfort F.A. Conduta para o melanoma cutâneo. Rev Col Bras Cir. 2004;31:204–214. [Google Scholar]
- 21.Davis ID, Brady B, Kefford RF, Millward M, Cebon J, Skrumsager BK, et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. 2009;15:2123–2129. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- 22.Norris BL, Beam S. New Therapeutic Approaches to Metastatic Melanoma. Oncol Nurs. 2008;1:1–12. [Google Scholar]
- 23.Santana H, Martínez E, Sánchez JC, Moya G, Sosa R, Hardy E, et al. Molecular Characterization of Recombinant Human Interferon Alpha-2b Produced in Cuba. Biotecnol Apl. 1999;16:154–159. [Google Scholar]
- 24.Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 25.Silva CM, Viana MB, Gontijo B, Fernandes RA, Pereira LB. Giant hemangioma treated with interferon alpha-2a. J Pediatr (Rio J) 1997;73:277–280. doi: 10.2223/jped.559. [DOI] [PubMed] [Google Scholar]
- 26.Agarwala SS, O'Day SJ. Current and future adjuvant immunotherapies for melanoma: blockade of cytotoxic T-lymphocyte antigen-4 as a novel approach. Cancer Treat Rev. 2011;37:133–142. doi: 10.1016/j.ctrv.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–126. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 28.Eggermont AM, Suciu S, MacKie R, Ruka W, Testori A, Kruit W, et al. Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet. 2005;366:1189–1196. doi: 10.1016/S0140-6736(05)67482-X. [DOI] [PubMed] [Google Scholar]
- 29.Khayat D, Bernard-Marty C, Meric JB, Rixe O. Biochemotherapy for advanced melanoma: maybe it is real. J Clin Oncol. 2002;20:2411–2414. doi: 10.1200/JCO.2002.20.10.2411. [DOI] [PubMed] [Google Scholar]
- 30.Atkins MB, Hsu J, Lee S, Cohen GI, Flaherty LE, Sosman JA, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748–5754. doi: 10.1200/JCO.2008.17.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danielli R, Ridolfi R, Chiarion-Sileni V, Queirolo P, Testori A, Plummer R, et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol Immunother. 2012;61:41–48. doi: 10.1007/s00262-011-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eggermont AM, Robert C. New drugs in melanoma: it's a whole new world. Eur J Cancer. 2011;47:2150–2157. doi: 10.1016/j.ejca.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 33.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 35.Robert C, Arnault JP, Mateus C. RAF inhibition and induction of cutaneous squamous cell carcinoma. Curr Opin Oncol. 2011;23:177–182. doi: 10.1097/CCO.0b013e3283436e8c. [DOI] [PubMed] [Google Scholar]
- 36.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slipicevic A, Herlyn M. Narrowing the knowledge gaps for melanoma. Ups J Med Sci. 2012;117:237–243. doi: 10.3109/03009734.2012.658977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 40.Weber JSF, Flaherty KT, Infante JR, Falchook GS, Kefford R, Daud A, et al., editors. Updated safety and efficacy results from a phase I/II study of the oral BRAF inhibitor dabrafenib (GSK2118436) combined with the oral MEK 1/2 inhibitor trametinib (GSK1120212) in patients with BRAFi-naive metastatic melanoma. ASCO Annual Meeting; 2012. [Google Scholar]
- 41.Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305:2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29:2904–2909. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]