Abstract
Thrombopoietin-receptor agonists increase platelet counts by stimulating the thrombopoietin receptor. Bone marrow fibrosis has been reported in patients receiving thrombopoietin-receptor agonists. This study determined the extent of myelofibrosis, its clinical relevance, and incidence of phenotypic or karyotypic abnormalities in patients with immune thrombocytopenia treated with thrombopoietin-receptor agonists. The grade of myelofibrosis was assessed before (n=15), during (n=117) and after (n=9) treatment in bone marrow biopsies from 66 patients. The proportion of bone marrow biopsies showing no fibrosis (myelofibrosis grade 0) decreased from 67% pre-treatment to 22% at last biopsy, of which 59% had grade 1 myelofibrosis and 18% had grade 2 myelofibrosis. The median duration of treatment with thrombopoietin-receptor agonists to last bone marrow biopsies was 29 months; patients who had two or more biopsies significantly more frequently had myelofibrosis grades 2/3 in the last bone marrow biopsies as compared to the first. Older age was associated with higher grades of fibrosis. No differences in blood counts or lactate dehydrogenase levels were found between patients with myelofibrosis grades 0/1 and those with grade 2. No clonal karyotypic or immunophenotypic abnormalities emerged. This study found that thrombopoietin-receptor agonists induce myelofibrosis grades 2/3 in approximately one-fifth of patients with immume thrombocytopenia, increasingly with >2 years of treatment with thrombopoietin-receptor agonists. Annual/biannual follow-up with bone marrow biopsies is, therefore, recommended in patients being treated with thrombopoietin-receptor agonists in order to enable prompt discontinuation of these drugs should grades 2/3 myelofibrosis develop. Discontinuation of thrombopoietin-receptor agonists may prevent development of clinical manifestations by stopping progression of fibrosis in grade 2/3.
Introduction
Thrombopoietin-receptor agonists (TPO-RA) bind to the thrombopoietin receptor c-MPL (TPO-R) on the hematopoietic stem cell leading to stem cell differentiation toward the megakaryocytic lineage. This culminates in stimulation of megakaryocyte proliferation, which results in elevation of platelet counts.1 These agents have been tested extensively in patients with immune thrombocytopenia (ITP) – a condition characterized by autoantibody-mediated platelet destruction and suboptimal platelet production.2 In randomized, placebo-controlled clinical trials, romiplostim and eltrombopag demonstrated unequivocal superiority over placebo in elevating platelet counts in ITP patients. As many as 80–90% of treated patients had a substantial increase in platelet counts during treatment.3–6 Subsequent trials showed that responses to these thrombopoietic agents were generally sustained as long as treatment continued.7–9 Along with efficacy, these agents showed acceptable short-term safety profiles.
Early on, concerns were raised regarding possible induction of bone marrow fibrosis as a result of sustained stimulation of megakaryopoiesis by these agents. The relationship between hyper-stimulation of megakaryopoiesis by TPO-RA and development of marrow fibrosis has been well documented in animal models.10,11 Recent clinical trials indicate that therapeutic doses of TPO-RA may indeed induce marrow fibrosis in patients with ITP.9,12,13
Mechanistically, the process of collagen synthesis and deposition appears to be mediated through intramedullary release of pro-fibrotic cytokines such as transforming growth factor-β, which is abundantly present in megakaryocytes and platelets, as well as basic-fibroblast growth factor and platelet-derived growth factor.14,15 Trials in TPO-RA-treated ITP patients were consequently initiated to assess the risk of developing marrow fibrosis.16,17 Another concern related to continuous stimulation of the hematopoietic stem cell compartment is induction of hematologic neoplasms, which thus far has been seen primarily in patients with myelodysplastic syndrome.18 Following limited exploration of both of these issues, follow-up with bone marrow aspirates and biopsies was recommended at intervals of 1–2 years in the routine clinical setting in patients with ITP.17
The aims of this study were: first, to determine the presence, magnitude and clinical significance of reticulin and collagen fibrosis in the bone marrow of TPO-RA-treated ITP patients; second, to assess the clinical significance of higher grades of bone marrow fibrosis; and third, to ascertain the incidence of phenotypic and/or karyotypic clonal abnormalities in the bone marrow of these patients.
Methods
Patients
This single-center, partially retrospective, and later prospective study was carried out at the Unit of Platelet Disorders of Weill Cornell Medical College/New York Presbyterian Hospital, New York, USA. Eligibility criteria for inclusion into the study were: (i) diagnosis of ITP; (ii) treatment with a TPO-RA, and (iii) availability of at least one bone marrow biopsy performed after the initiation of treatment with a TPO-RA.
This study was approved by the Institutional Review Board of Weill Medical College of Cornell University. Research consent for use of the bone marrow was obtained; all marrow biopsies were performed for clinical reasons.
Bone marrow examinations
Bone marrow biopsies and aspirates for morphological examination, immunophenotyping and cytogenetic analysis were performed every 1–2 years as part of the standard follow-up procedure for patients on TPO-RA treatment at our center.
Bone marrow sections were stained by Gomori silver impregnation and trichrome for the evaluation of reticulin and collagen deposition, respectively. The biopsies were reviewed by three pathologists (JG, LB, AO) to assess the grade of fibrosis; discordant cases were reviewed together until consensus was reached. Pathologists were blinded to clinical and laboratory data. Myelofibrosis (MF) was graded according to the European Consensus Grading System19 a semi-quantitative four-tier scale scoring system as follows: MF-0, scattered linear reticulin with no intersections; MF-1, loose network of reticulin with many intersections especially in perivascular areas; MF-2, diffuse and dense increase in reticulin with extensive intersections, occasionally with (only) focal bundles of collagen and/or focal osteosclerosis; and MF-3, diffuse and dense increase in reticulin with extensive intersections, with coarse bundles of collagen, often associated with significant osteosclerosis.
Flow cytometry and cytogenetics
Four-color flow cytometric analysis was performed on bone marrow aspirates in order to assess the presence of B-cell and T-cell clonality, increased myeloid blasts and evidence of immunophenotypic abnormalities in myeloid lineage cells.
Statistical analysis
Continuous variables are expressed by median and interquartile range (IQR) or mean and standard deviation (SD). Comparisons between independent groups were made using a t-test for normally distributed variables, the Mann-Whitney U test for variables that are not normally distributed and the χ2 test for categorical variables. McNemar test was used for dependent categorical variables. P-values ≤0.05 are considered statistically significant. For the purpose of comparison the grades of fibrosis were dichotomized into MF-0/MF-1, which are considered as non-increased fibrosis and MF-2/MF-3, which represent pathological amounts of fibrosis in the bone marrow. The duration of treatment up to the last bone marrow biopsy was calculated from the date of initiation of the TPO-RA to the date of the last biopsy. In patients in whom treatment was interrupted, duration of exposure was summed.
Results
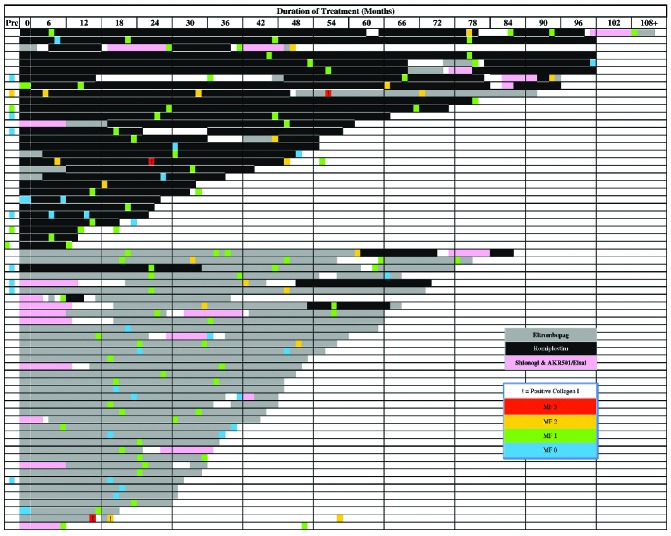
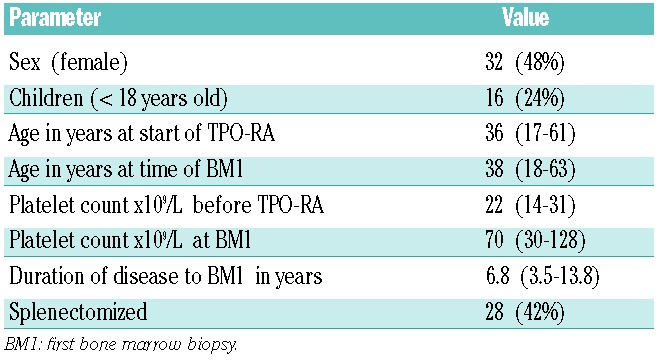
The study population comprised 66 ITP patients who had undergone one or more bone marrow biopsies performed during treatment with one or more of the following TPO-RA agents: romiplostim, eltrombopag, AKR501 (Eisai), or a now-discontinued Shionogi agent. The patients’ clinical characteristics are summarized in Table 1. The median platelet count prior to initiation of TPO-RA treatment was 22×109/L. At the time of the first on-treatment bone marrow biopsy, the patients’ median age was 38 years (IQR 18–63), their median duration of ITP was 6.8 years, and median platelet count was 70×109/L. Sixteen out of 66 (24%) patients were children <18 years old.
Table 1.
Clinical characteristics of 66 ITP patients. Continuous variables are expressed as the medians and interquartile ranges; categorical variables are expressed as numbers and percentages.
Bone marrow fibrosis
A total of 141 BM biopsies from the 66 patients were reviewed: 15 biopsies were performed prior to initiation of TPO-RA, 117 during treatment with TPO-RA, and 9 post-treatment, i.e. after discontinuation of TPO-RA. Of 66 patients, 34 had one bone marrow biopsy, 19 had two, 8 had three, 4 had four, and 1 had five biopsies while on treatment.
Table 2 summarizes the distribution of myelofibrosis grades in pre- and on-treatment biopsies. In total, 21 on-treatment bone marrow biopsies were graded as MF-0, 75 as MF-1, 18 as MF-2 and 3 as MF-3. The median duration of treatment with TPO-RA measured from the start of therapy to the first on-treatment biopsy was 1.4 years (IQR 1–1.9) and that to the most recent on-treatment bone marrow biopsy was 2.4 years (range, 0.3–8.3 years). The duration of treatment to other sets of biopsies are summarized in Table 2. At the time of the first, second, third and fourth on-treatment biopsies, median romiplostim dosages were 9, 9, 7.5, and 8 μg/kg/week and median eltrombopag dosages were 63, 75, 75, and 75 mg/day, respectively.
Table 2.
Bone marrow features, including grade of marrow fibrosis (MF), immunophenotype and karyotype.
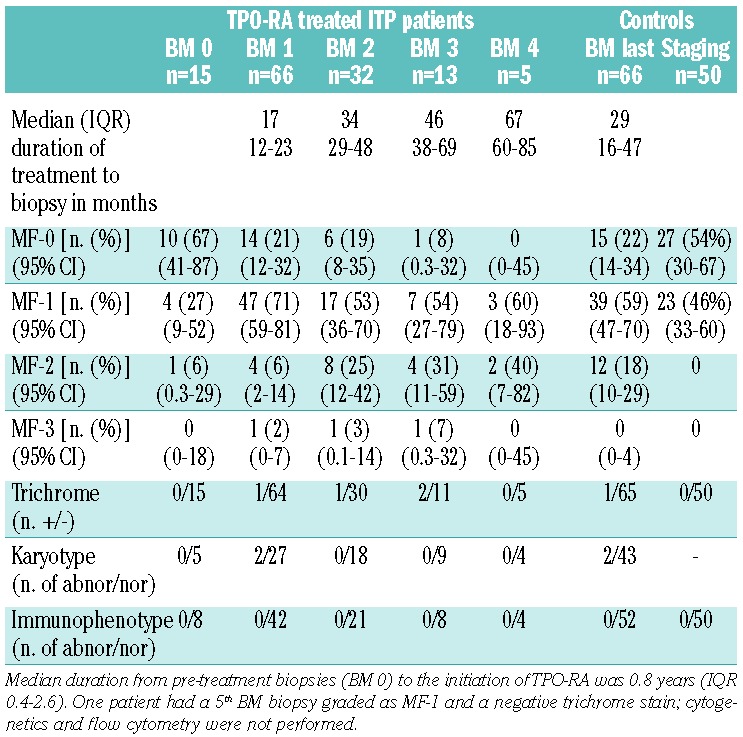
Fifty control bone marrow biopsies performed for staging purposes in lymphoma patients and determined to be uninvolved were also evaluated. These control cases were matched for age (median 42 years; range, 1–85 years) and sex (18 men/32 women) with the ITP group.
Changes in myelofibrosis grading
Pre-treatment versus on-treatment bone marrow biopsies
As shown in Figure 1, the proportion of cases of MF-0 decreased from 67% (10/15) in the pre-treatment biopsies to 22% in the 66 first on-treatment biopsies, which were largely MF-1. In the 32 patients who underwent multiple bone marrow biopsies, the second and third sets of biopsies demonstrated increasing fractions of ≥ MF-1 marrows compared to pre-treatment biopsies. In the 15 patients who had undergone pre-treatment bone marrow biopsies, there was a significantly higher number of MF-0 in the pre-treatment biopsies than in the first on–treatment biopsies (10/15 versus 3/15 biopsies, McNemar test, P=0.016).
Figure 1.
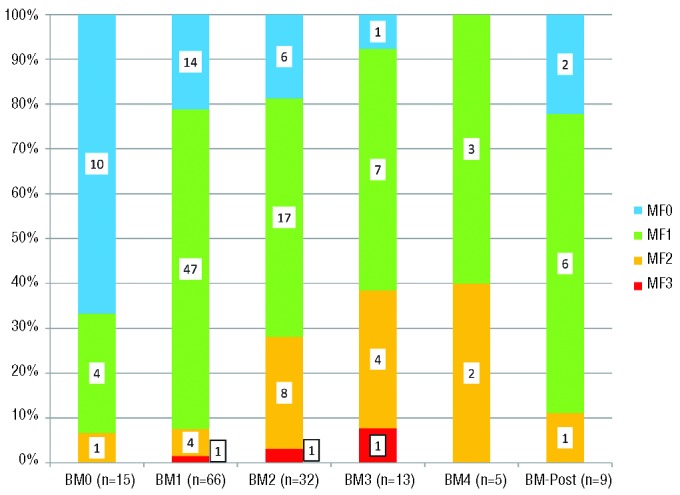
The distribution of the four myelofibrosis-grades in pre-treatment (BM0), four sets of on-treatment BM1-4) and post-treatment bone marrow biopsies.
First versus last on-treatment bone marrow biopsies
The first on-treatment bone marrow biopsy was graded as MF-1 in 24 of the 32 patients with more than one on-treatment bone marrow biopsy. In the last set of these patients’ biopsies, 8 had progressed to MF-2/3, 12 remained MF-1 and 4 had become MF-0 illustrating the uncertainty of the future state of the bone marrow from the first on-treatment bone marrow biopsy. Nonetheless, the percentage of grade MF-2/3 marrows (10/32; 31%) in the last on-treatment biopsy was significantly higher than that in the first biopsy (3/32; 9%; McNemar test; P=0.039).
Comparing the first to the last on-treatment bone marrows, the grade of fibrosis increased in 11 cases, remained the same in 15 and decreased in 6 (Figure 2A). Slightly different numbers were seen comparing the myelofibrosis grade of the first marrow to the highest myelofibrosis grade found in an on-treatment biopsy (Figure 2B).
Figure 2.
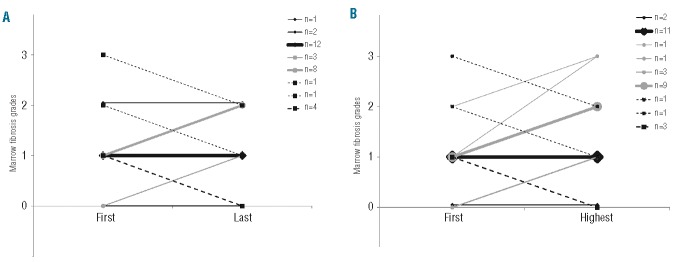
Changes in the grade of fibrosis from (A) first to last and (B) first to highest subsequent bone marrow grading in 32 patients who had multiple biopsies.
In ten cases the grade of myelofibrosis on-treatment increased to MF-2. Treatment was stopped in half of these patients (5/10); after discontinuation, the myelofibrosis grade decreased to MF-1 in two patients, remained the same in one, and two patients are awaiting a follow-up bone marrow biopsy. Three additional patients whose myelofibrosis grade increased on treatment later returned to normal (MF-0/1) despite continuing TPO-RA treatment. These data provide evidence that the effects of TPO-RA are inconsistent but that TPO-RA treatment will generally increase the degree of fibrosis over time.
A positive trichrome stain was seen in three patients (4/117 on-treatment biopsies, 3%). However in two of these three patients, the trichrome stain became negative at the last bone marrow biopsy despite the fact that they continued treatment with TPO-RA.
Post-treatment bone marrow biopsies
In the nine post-treatment biopsies performed after the discontinuation of TPO-RA, fibrosis was graded as MF-0 in two, MF-1 in six and MF-2 in one. The median (IQR) duration from end of treatment to post-treatment bone marrow biopsy was 7 months (3.5–29). Compared to the last on-treatment bone marrow biopsy, after discontinuation of TPO-RA the grade of fibrosis decreased from MF-2 to MF-1 in two patients and from MF-1 to MF-0 in one.
Pre-treatment and on-treatment bone marrow biopsies versus controls
All control cases were graded as either MF-0 (54%) or MF-1 (46%). Comparing the pre-treatment bone marrow biopsies from ITP patients with biopsies from controls, no difference was found in the proportion of MF-0/1 versus MF-2/3 cases. However, there was a significant increase in the proportion of MF-2/3 graded marrows in the last bone marrow biopsies as compared to in the control bone marrow biopsies (χ2; P<0.001).
Factors associated with increased bone marrow fibrosis at last bone marrow biopsy
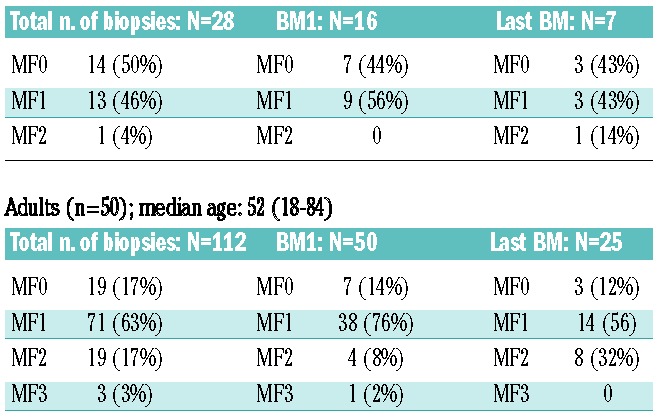
Age
Of the multiple clinical factors examined (Table 3), only older age was associated with higher grades of bone marrow fibrosis. Age was significantly higher at time of last biopsy in those with MF-2/3 (mean 57 years) as compared to those with MF-0/1 (mean 38 years) (t-test; P=0.01). The three patients who showed positive trichrome stains for collagen were all females over the age of 60 years. While five adult patients started with MF-2/3, none of the 16 children started with MF-2/3 (Table 4). Among those children with two or more on-treatment biopsies, only one out of seven with subsequent on-treatment biopsies had MF-2 at their last biopsy, while eight out of 25 (32%) adults had MF-2 (P=0.4). Overall, however, children (n=16), while tending to have lower degrees of reticulin fibrosis, were not statistically different from adults (n=50) (Table 4).
Table 3.
Clinical characteristics of patients and blood values stratified according to grade of fibrosis in the last biopsy dichotomized into MF-0/1 and MF-2/3. Continuous variables are expressed as mean (standard deviation).
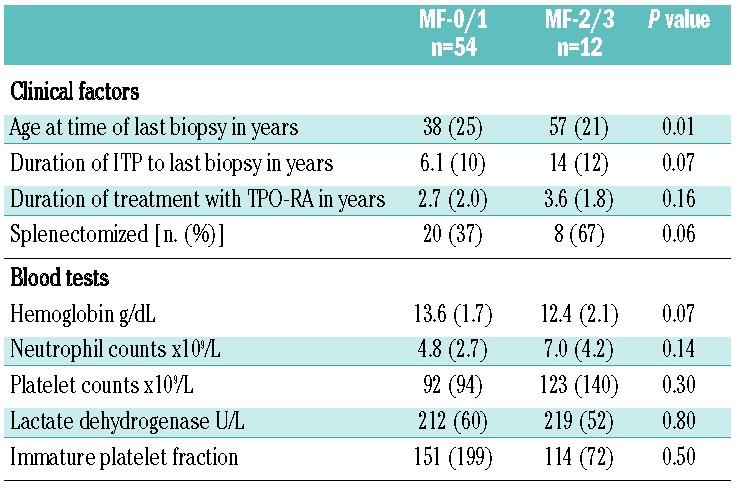
Table 4.
Comparison between the distribution of myelofybrosis-grades between children (<18 years) and adults.
Children (n=16); median age: 13 (1–17)
Duration of treatment
Patients with MF-2/3 tended to have been treated for longer than patients with MF-0/1, although the difference was not statistically significant. Figure 3 illustrates, by individual, the grades of bone marrow fibrosis plotted against the duration of treatment with TPO-RA. Of 16 patients treated with a TPO-RA for at least 4 years, fibrosis was graded as MF-0 in two, MF-1 in ten, and MF-2 in four. Although several patients on romiplostim had been treated longer than any of those on eltrombopag, the pattern of myelofibrosis grades for these two TPO-RA were not different.
Figure 3.
The grade of bone marrow fibrosis in relation to the duration of exposure to and type of TPO-RA agent in 66 patients.
Other factors
Splenectomized patients tended to have more MF-2/3 more frequently than MF-0/1. No relationship was identified between the grade of fibrosis and any of the following factors: initial marrow fibrosis grade, duration of disease, immature platelet fraction, the dose of TPO-RA (Mann–Whitney U test; P=0.48), or the type of TPO-RA agent (χ2 test; P=0.6). Of 12 patients with MF-2/3, eight were on eltrombopag and four on romiplostim suggesting that fibrosis could occur with either agent.
Clinical significance of increased reticulin fibrosis in the bone marrow
Median hemoglobin concentrations, absolute neutrophil counts, platelet counts and lactate dehydrogenase levels in patients with MF-0/1 and MF-2/3 are shown in Table 3. Although not statistically significant, hemoglobin concentration tended to be lower in patients at the time of bone marrow biopsies showing MF-2/3, and platelet counts tended to be higher (Table 3).
Concurrent peripheral blood smears (± 3 months) from patients with MF-2/3 bone marrow biopsies were evaluated for the presence of leukoerythroblastic changes (nucleated red blood cells and immature granulocytes) and anisopoikilocytosis (dacryocytes and schistocytes). One or more of these features were detected in three of the 13 available smears, including two with evidence of leukoerythroblastosis. In contrast, only one of 13 patients with MF-0/1 who were randomly chosen showed anisopoikilocytosis and none showed leukorythroblastic changes.
Flow cytometry and cytogenetic analysis
Flow cytometric immunophenotyping of bone marrow aspirates was performed in 89 examinations. None of the bone marrow biopsies showed an increase in blasts. No aberrant antigen expression was detected on myeloid precursors, monocytes or lymphocytes. T-lymphocytes had no antigen loss and there was no increase in the number of T-large granular lymphocytes or natural killer cells. There was no evidence of B-lymphocyte monoclonality. Cytogenetic analysis was performed on 72 bone marrow biopsies. All but two showed normal karyotypes: one patient had Turner syndrome (45,X) and one showed loss of Y chromosome in a subset of metaphases, considered a non-specific finding due to advanced age (the patient was 79 years old).
Discussion
The first study reporting long-term usage of a TPO-RA, romiplostim, in patients with ITP indicated that nine patients had developed reticulin fibrosis.9 This initial finding raised the level of concern regarding bone marrow fibrosis in patients being treated with TPO-RA. A pathological basis had been well established in animal models of TPO-RA overdose although at much higher doses per body weight than TPO-RA doses given to humans.11
Available data describing the extent of marrow reticulin fibrosis in ITP patients treated with TPO-RA are still limited. Serial examinations in the EXTEND study of eltrombopag suggested that the degree of higher-grade fibrosis is minimal and may be linked to those with an abnormal underlying bone marrow.13 Previous studies from our group suggested that the grade of reticulin might increase after initiation of TPO-RA in patients with ITP.12,16 Thus far, other clinical data are only available in abstract form. The study reported here addresses the following unanswered questions: does continuous exposure to therapeutic doses of TPO-RA induce progressive fibrosis? If so, is it clinically significant¿ Is fibrosis reversible if it occurs¿ Does continued exposure increase the risk of developing clonal changes in the bone marrow¿
This study describes the findings from serial surveillance bone marrow examinations of 66 ITP patients treated with thrombopoietic agents for a median duration of 2.4 years (maximum exposure 8.3 years). The data suggest that therapeutic doses of TPO-RA increase the extent of reticulin fibrosis in a subset of patients with ITP as supported by the following findings. First, the number of patients whose marrows were graded MF-0 decreased from 67% in the pretreatment biopsies to 22% in the first set of on-treatment bone marrow biopsies to <10% in subsequent sets of biopsies. The increase in the grade of fibrosis was statistically significant in the 15 patients in whom pre-treatment and on-treatment bone marrow biopsies were performed (P=0.016). Second, the proportion of MF-0 and MF-1, which are considered as normal grades of fibrosis, decreased from 92% in the first set of on-treatment bone marrows to 72% in the second set and to 62% in the third set. Third, in those patients with multiple on-treatment biopsies (n=32) the proportion of MF-2/3 increased from 9% in the first on-treatment biopsy to 32% in the last on-treatment biopsy (P=0.039).
However, other results of this study also emphasize that most patients do not develop progressive marrow fibrosis while on TPO-RA. First, the MF grade remained unchanged in 15 of 32 patients with multiple on-treatment biopsies and even decreased in another six (Figure 2A). Second, the number of bone marrow biopsies with MF-3 did not increase upon sustained exposure to TPO-RA. In the three patients with MF-3, the grade of fibrosis decreased to MF-2 despite ongoing treatment; collagen fibrosis, detected in three patients (4%), reverted to negative in two despite ongoing treatment. Third, three quarters of patients treated for at least 4 years with TPO-RA had MF-0/1 and there was no significant association between either the duration of treatment or the dose of TPO-RA with the degree of fibrosis. Overall, it appeared that certain patients were susceptible to developing higher grades of reticulin in their marrow but that the majority of patients were not. The development of MF-2/3 could seemingly occur with any duration of treatment from less than 1 year to 8.5 years.
Two studies specifically designed to address the bone marrow issue including pre-treatment samples are ongoing. Two recently published long-term studies of the use of romiplostim and eltrombopag in ITP also considered this topic although the romiplostim study only had bone marrow data for 40 out of more than 200 patients.20 The long-term study on safety and efficacy of eltrombopag (the EXTEND study) reported findings in which the initial results were in line with ours after 1 year of treatment (first set of bone marrow biopsies, Table 2), showing that 92% of patients had MF-0/MF-1 and 8% had MF-2.13 Also similar to our findings, Brynes et al., in a study of 45 patients in which pre-treatment biopsies were compared to those conducted after 1 and 2 years of treatment with eltrombopag, found a decrease in the proportion of MF-0 marrows from 90% in pre-treatment biopsies to 60% after 1 year of treatment; however, unlike the study reported here, the proportion of MF-0 increased again to just over 80% after 2 years of treatment.21 One difference between these studies and ours is that the duration of ITP before the first set of bone marrow biopsies was much longer in our study. Another difference that may explain the lower proportion of MF-2/3 in these studies may be related to the shorter observation time in the two eltrombopag studies since, as shown in Figure 3, ten of 14 MF-2/3 graded bone marrows emerged only after at least 24 months of TPO-RA treatment. Since patients with chronic ITP initiating TPO-RA treatment may be on these agents for as long as 5 to 10 years, longer follow-up is needed to find out whether prolonged exposure to TPO-RA would result in advanced and possibly clinically relevant grades of fibrosis in a higher percentage of patients.
Factors examined for possible relation with the degree of fibrosis included duration of disease, platelet count, type and dose of agent, immature platelet fraction, splenectomy status, age at time of biopsy and age at time of TPO-RA initiation. Only age showed a statistically significant association with the grade of fibrosis. In general, the amount of fibrosis in the bone marrow is known to increase with age,22 but the 23% of cases of MF-2/3 found in this study is unlikely to be explained by age alone. A higher grade of fibrosis also tended to be related both to having undergone splenectomy and to a longer duration of disease. This inconsistent evolution in reticulin fibrosis suggests that there may be individual susceptibility in certain ITP patients which triggers the development of fibrosis in some patients. The underlying mechanism of this phenomenon is currently unclear. Finally, since MF-2/3 was encountered in patients on both eltrombopag and romiplostim, fibrosis is clearly a class-effect rather than an agent-specific effect.
Unlike primary myelofibrosis, a clonal disorder characterized by progressive fibrosis, cellular abnormalities, altered cytokine release and worsening cytopenia,23 TPO-RA-induced fibrosis is a secondary process generally not associated with anemia, neutropenia, clonal abnormalities or alterations of biochemical parameters (e.g. lactate dehydrogenase). In this study, hemoglobin concentration was 1 g/dL lower in patients with MF-2/3 in the last set of bone marrow biopsies but this difference was not significant and the patients with MF-2/3 were almost 20 years older. Thus, the increase in the grade of bone marrow fibrosis, although morphologically evident, was not enough to be clinically significant. It is also noteworthy that all of the 12 patients with pathologically increased bone marrow fibrosis had MF-2 in their last bone marrow biopsy; further progression to MF-3, if it occurred, may have had a more substantial impact on peripheral blood counts. Morphological changes in blood smears usually associated with patients with primary myelofibrosis, such as dacryocytes or schistocytes, could only occasionally be detected in about 25% of the cases showing MF-2/MF-3 fibrosis in the corresponding bone marrow biopsy.
There is no evidence-based knowledge and very limited experience on how to manage patients who develop increasing fibrosis during treatment with TPO-RA, although we believe that discontinuation of TPO-RA will allow the degree of reticulin deposition to decrease. Our current monitoring policy is to perform a bone marrow biopsy under conscious sedation in children and adults 1–1 ½ years after TPO-RA treatment is initiated and then to continue with a second biopsy 6–24 months later, depending on the grade of myelofibrosis observed in the first biopsy. When MF-2 or MF-3 is encountered, we consider stopping TPO-RA, using another therapy to support the platelet count, and repeating the biopsy after 6 months, before resuming TPO-RA. One reason for the failure to stop TPO-RA in all patients with MF-2/3 was that in several cases the myelofibrosis grade was reclassified (upgraded) when reassessed for this study, indicating the need for consistency and experience in assessing bone marrow biopsies.14 Although our results do not support an indiscriminate requirement for serial bone marrow biopsies because of the limited number of patients who progress to MF-2 and the lack of overt clinical implications, we are continuing to pursue the same follow-up strategy until these results are clarified by other studies and by longer follow-up. There is currently no reliable marker that can be used to monitor bone marrow fibrosis non-invasively. Procollagen III N-terminal peptide, a previously suggested seromarker, has not been shown to correlate with the grade of bone marrow fibrosis in TPO-RA-treated patients.16
No clonal abnormalities in the immunophenotype or karyotype emerged in any of the patients during treatment with TPO-RA. Although these results are reassuring, the exposure time may have been too short to induce clonal abnormalities; longer follow-up is needed to ensure the safety of these agents in this regard as well.
This study has some limitations. First, bone marrow biopsies were not performed systematically in all patients on an annual or biannual basis; however, in most cases the schema described was followed. Second, pre-treatment biopsies were often unavailable, but the use of serial examinations in 32 of the patients was informative regarding the course of the bone marrow findings. Third, treatment with TPO-RA was interrupted between biopsies in a small number of patients (see Figure 3), which may have affected the degree of fibrosis in the subsequent biopsy. Finally, it should be noted that findings from approximately 25% of the bone marrow biopsies in this study were previously reported16; however, the previously reported data in this study were updated to include the longer duration of treatment and additional bone marrow biopsies.
The primary strengths of the study are the large number of patients, the large number of bone marrow biopsies, the relatively long duration of follow-up, and a single center experience resulting in a consistent approach to the patients in the study. The long duration of exposure to TPO-RA provides data on the effect of TPO-RA on the bone marrow for more than 6 years in 13 patients. Since all bone marrow biopsies were reviewed by three hematopathologists from the same center, there is greater consistency of both staining and interpretation of the biopsies than would arise from a multicenter study. Because we were able to collect bone marrow under conscious sedation, patients only rarely refused a request for a biopsy. This is also the first report of bone marrow fibrosis findings in children with ITP who were treated with TPO-RA.
Several questions were raised at the beginning of the discussion. First, as discussed, it appears that continuous exposure to therapeutic doses of TPO-RA induces fibrosis in a subset of patients and, once induced, fibrosis may progress. A critical debate is whether this progression is clinically significant; even more data with longer follow-up are required to answer this question definitively. Our anecdotal experience is that fibrosis decreases if TPO-RA is stopped. Continued exposure to TPO-RA did not result in development of clonal changes in the bone marrow in this study.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kuter DJ. Biology and chemistry of thrombopoietic agents. Semin Hematol. 2010; 47(3):243–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9664):641–8 [DOI] [PubMed] [Google Scholar]
- 4.Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355(16):1672–81 [DOI] [PubMed] [Google Scholar]
- 5.Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–47 [DOI] [PubMed] [Google Scholar]
- 6.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403 [DOI] [PubMed] [Google Scholar]
- 7.Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363(20):1889–99 [DOI] [PubMed] [Google Scholar]
- 8.Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377(9763):393–402 [DOI] [PubMed] [Google Scholar]
- 9.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113(10):2161–71 [DOI] [PubMed] [Google Scholar]
- 10.Chagraoui H, Komura E, Tulliez M, Giraudier S, Vainchenker W, Wendling F. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002;100(10):3495–503 [DOI] [PubMed] [Google Scholar]
- 11.Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood. 2009;114(18):3748–56 [DOI] [PubMed] [Google Scholar]
- 12.Boiocchi L, Orazi A, Ghanima W, Arabadjief M, Bussel JB, Geyer JT. Thrombopoietin receptor agonist therapy in primary immune thrombocytopenia is associated with bone marrow hypercellularity and mild reticulin fibrosis but not other stromal abnormalities. Mod Pathol. 2012;25(1):65–74 [DOI] [PubMed] [Google Scholar]
- 13.Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013;121(3):537–45 [DOI] [PubMed] [Google Scholar]
- 14.Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol. 2007;139(3):351–62 [DOI] [PubMed] [Google Scholar]
- 15.Kuter DJ, Gminski DM, Rosenberg RD. Transforming growth factor beta inhibits megakaryocyte growth and endomitosis. Blood. 1992;79(3):619–26 [PubMed] [Google Scholar]
- 16.Ghanima W, Junker P, Hasselbalch HC, Boiocchi L, Geyer JT, Feng X, et al. Fibroproliferative activity in patients with immune thrombocytopenia (ITP) treated with thrombopoietic agents. Br J Haematol. 2011;155(2):248–55 [DOI] [PubMed] [Google Scholar]
- 17.Ghanima W, Bussel JB. Thrombopoietic agents in immune thrombocytopenia. Semin Hematol. 2010;47(3):258–65 [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian H, Fenaux P, Sekeres MA, Szer J, Platzbecker U, Kuendgen A, et al. Treatment with the thrombopoietin (TPO)-receptor agonist romiplostim in thrombocytopenic patients (Pts) with low or intermediate-1 (int-1) risk myelodysplastic syndrome (MDS): follow-up AML and survival results of a randomized, double-blind, placebo (PBO)-controlled study. Blood. 2012:120;421 [Google Scholar]
- 19.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–32 [PubMed] [Google Scholar]
- 20.Kuter DJ, Bussel JB, Newland A, Baker RI, Lyons RM, Wasser J, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161(3):411–23 [DOI] [PubMed] [Google Scholar]
- 21.Brynes RKO, Wong A, Bakshi RS, Bailey K, Brainsky CK. A longitudinal prospective study evaluating the effects of eltrombopag treatment on bone marrow in patients with chronic immune thrombocytopenia: interim analysis at 1 year. Blood. 2012:Abstract 2195 [Google Scholar]
- 22.Bauermeister DE. Quantitation of bone marrow reticulin--a normal range. Am J Clin Pathol. 1971;56(1):24–31 [DOI] [PubMed] [Google Scholar]
- 23.Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–70 [DOI] [PMC free article] [PubMed] [Google Scholar]