Abstract
Even though alterations in platelet counts are presumed to be detrimental, their impact on the survival of patients has not been studied in large cohorts. The prevalence of thrombocytopenia and thrombocytosis was examined in a large inner city outpatient population of 36,262 individuals aged ≥65 years old. A significant association with shorter overall survival was found for both thrombocytopenia (HR=1.45; 95% CI: 1.36–1.56) and thrombocytosis (HR=1.75; 95% CI: 1.56–1.97) when compared to the survival of patients with normal platelet counts. This effect persisted across all ethnic groups. However, African-Americans (non-Hispanic Blacks) with either thrombocytopenia or thrombocytosis were at significantly lower risk compared to non-Hispanic Caucasians (HR=0.82; 95% CI: 0.69–0.96 and HR=0.70; 95% CI: 0.53–0.94, respectively). Furthermore, Hispanics with thrombocytosis were found to have a lower mortality risk compared to non-Hispanic Caucasians with thrombocytosis (HR=0.60; 95% CI: 0.44–0.81). A value of <125,000 platelets per microliter was a better prognostic marker for non-Hispanic Blacks and these subjects with this platelet count had similar overall survival to that of Caucasians with a value of <150,000 per microliter. In conclusion, thrombocytosis and thrombocytopenia are independently associated with shorter overall survival in elderly subjects and this effect is modified by ethnicity. Using different thresholds to define the association of thrombocytopenia and thrombocytosis with overall mortality risk among non-Hispanic Blacks may, therefore, be warranted.
Introduction
Platelets play a critical role in hemostasis and are also important in the development of pathological processes including atherosclerosis and arterial thrombosis.1,2 Quantification of platelets in venous blood is a common laboratory measurement, performed by automated hematology analyzers which utilize a reference range for a normal platelet count between 150,000 to 450,000 platelets/μL of blood.3 Both abnormally low (thrombocytopenia) and elevated (thrombocytosis) platelet counts are common findings in several illnesses including liver diseases, infections, autoimmune disorders and malignancies.4 Morbidity and mortality associated with abnormal platelet counts is commonly thought to be due to such underlying diseases. However, there are very few data on the prevalence of thrombocytopenia and thrombocytosis and their independent effect on survival among elderly individuals.
The number of elderly individuals ≥65 years in the United States is expected to reach 71 million by 2030.5 A similar aging trend is occurring globally and by 2030 the worldwide population aged ≥65 years is projected to reach 973 million.5 Consequently, an ever increasing number of asymptomatic elderly individuals will be found to have thrombocytopenia or thrombocytosis during routine complete blood cell count measurements. We, therefore, decided to study the prevalence and independent impact on survival of abnormal platelet counts in a large cohort of inner city, elderly outpatients comprising different ethnicities and with a variety of comorbid conditions. Previous studies have reported differences in platelet counts among different ethnicities.6,7 The present study population included a high proportion of minorities: this makes it more representative of the changing national demographics and allows for a better delineation of the racial and ethnic implications of abnormal platelet counts on the survival of the elderly.
Methods
Patients
The study cohort consisted of all patients ≥65 years of age seen at an outpatient clinic within the Montefiore medical system from January 1st 1997 to May 1st 2008 who underwent a complete blood count within 3 months of the earliest visit date. Acutely ill patients were excluded by removing from the analysis all individuals who had been recently discharged from one of our inpatient facilities within 30 days prior to the index clinic visit. Approval for all study procedures was obtained from the Institutional Review Board of the Albert Einstein College of Medicine and Montefiore Medical Center.
Independent variables
All clinical data, including complete blood counts and comorbidities were retrieved using Clinical Looking Glass (CLG), a quality improvement health care surveillance software (Emerging Health Information Technology, Yonkers, NY, USA). CLG is an interactive clinical information system developed at Montefiore Medical Center that integrates demographic, clinical, and administrative datasets and allows them to be reproduced in a programmable format for statistical access. Demographic data (age, gender and ethnicity) were determined by CLG based on registration information. Ethnicity was classified as either “non-Hispanic White” (non-Hispanic Caucasian), “non-Hispanic Black” (African-American), “Hispanic” or “other”. The Charlson comorbidity index (CCI), an extensively validated risk adjustment tool used for research which can measure the effect of multiple morbidities on mortality using ICD-9 diagnosis codes,8–9 was calculated using information derived from CLG. The CCI incorporates multiple comorbid conditions including myocardial infarct, congestive heart failure, peripheral vascular diseases, cerebrovascular diseases, dementia, chronic pulmonary diseases, rheumatologic diseases, peptic ulcer disease, liver disease, diabetes with or without complications, hemiplegia/paraplegia, renal disease, malignant tumors, metastatic diseases and AIDS. As previously described by Charlson et al.,8 age-adjusted CCI scores can be calculated, by weighting and scoring each comorbid condition and then assigning 1 point for each additional decade of age over 40 years old. The validity of the age-adjusted CCI for assessing the effect of comorbidities on survival rates has been studied more extensively than other similar measures and this index has been shown to perform well in outpatient primary care and community settings.10 Mortality data were matched by CLG based on the patient’s name, medical record and social security numbers. The social security death registry was used by CLG to obtain the date of death. Because anemia and neutropenia may independently affect survival, these covariates were also included in our analysis. Anemia was defined based on the World Health Organization (WHO) criteria of hemoglobin <13 g/dL in men and <12 g/dL in women. Patients were considered to have mild anemia if they met WHO criteria but had a hemoglobin level that was >10 g/dL, and severe anemia if the hemoglobin level was ≤10 g/dL. Neutropenia was defined as an absolute neutrophil count <1500/μL of blood. Neutropenia was classified as “mild” if the absolute neutrophil count ranged between 1000 to 1499/μL, “moderate” if it ranged between 500 to 999/μL and “severe” if the absolute neutrophil count was <500/μL of venous blood. Thrombocytopenia and thrombocytosis were defined as a platelet count of <150,000/μL or >450,000/μL, respectively.
Statistical analysis
The Kolmogorov-Smirnov test was applied for analysis of variance of all continuous variables. The choice of methods for statistical comparisons of continuous variables between groups was based on whether the data permitted parametric or non-parametric analysis. Categorical variables were compared using Pearson χ2 tests. Multivariate analysis was performed with binary logistic regression tests to assess the influence of patients’ demographic characteristics and comorbidities on platelet count. Because the log-minus-log survival curves did not show a violation of the proportional-hazards assumption, a Cox proportional hazards model was constructed to assess the independent association of abnormal platelet counts with subsequent mortality after controlling for comorbidities and demographic variables. The age-adjusted CCI was treated as a continuous variable in the regression model and was used as a risk-adjustment covariate. Other potential confounders such as ethnicity, gender, anemia and neutropenia were treated as categorical covariates. To assess whether there was heterogeneity in the association between abnormal platelet counts and mortality risk across the different ethnicities, an additional Cox proportional hazards model was constructed to test the interaction terms of ethnicity with platelet count status. Odds ratios (OR) and hazard ratios (HR) were derived from the logistic and Cox regression analyses respectively and are presented along with the corresponding 95% confidence intervals (95% CI). There were no missing values for the variables analyzed. To describe the nonlinear effect of platelet count on survival, LOESS regression was used to plot log relative hazards for each ethnicity against platelet count after adjusting for potential confounders (gender, anemia, neutropenia and age-adjusted CCI score). A P value of <0.05 was considered statistically significant. P values of post hoc paired comparisons were adjusted with the Bonferroni method.
Results
Baseline characteristics and prevalence of thrombocytopenia and thrombocytosis
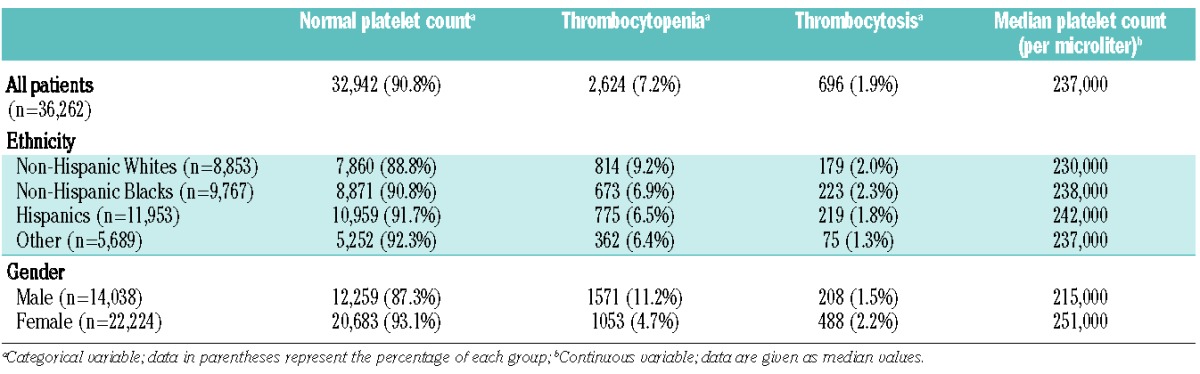
A total of 36,262 individuals (14,038 men and 22,224 women) met our inclusion criteria. The demographics and baseline characteristics, age-adjusted CCI scores, anemia and neutropenia rates of our study population are shown in Table 1. Men had significantly higher age-adjusted CCI scores compared to those of women (mean rank 18,610.8 versus 17,828.8 respectively; P<0.001). Non-Hispanic Blacks had the highest age-adjusted CCI scores compared to all other ethnicities (P values <0.01). More than half of our study population consisted of minorities with 53.3% of men and 64% of women being either non-Hispanic Blacks or Hispanics. The prevalence of abnormal platelet counts by gender and ethnicity is shown in Table 2. Non-Hispanic Whites had significantly lower median platelet counts (230,000/μL) compared to non-Hispanic Blacks (238,000/μL; P<0.001), Hispanics (242,000/μL, P<0.001) or all other ethnicities (237,000/μL; P<0.001). Hispanics had the highest median platelet counts (P<0.01 for all comparisons). In addition, non-Hispanic Whites had significantly higher thrombocytopenia rates (9.2%) compared to non-Hispanic Blacks (6.9%; P<0.01), Hispanics (6.5%; P<0.01) and other ethnicities (6.4%; P<0.01). Thrombocytosis rates did not differ significantly between non-Hispanic Whites (2%), non-Hispanic Blacks (2.3%) and Hispanics (1.8%). However, “other” ethnicities had significantly lower thrombocytosis rates (1.3%) compared to non-Hispanic Whites and non-Hispanic Blacks (P<0.01). Women had significantly higher platelet counts than men and this effect was consistent among all ethnic groups (P<0.01). Men also had significantly higher thrombocytopenia rates and significantly lower thrombocytosis rates compared to women (P values <0.01). The highest thrombocytopenia rate (13.5%) was seen among non-Hispanic White men (P<0.05 compared to men of all other ethnicities).
Table 1.
Baseline characteristics, median age, age-adjusted Charlson comorbidity index (CCI) and prevalence of anemia and neutropenia by ethnicity and gender.
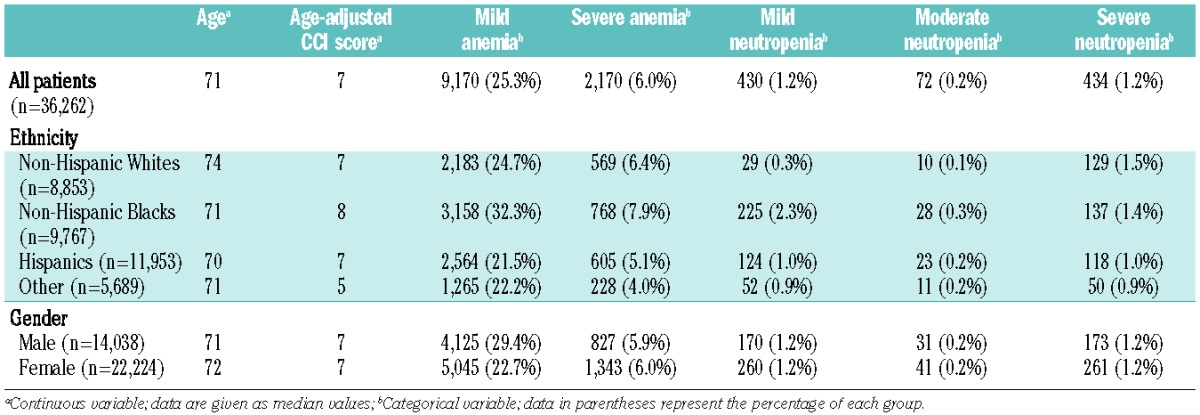
Table 2.
Prevalence of thrombocytopenia and thrombocytosis by ethnicity and gender.
Predictors of abnormal platelet counts
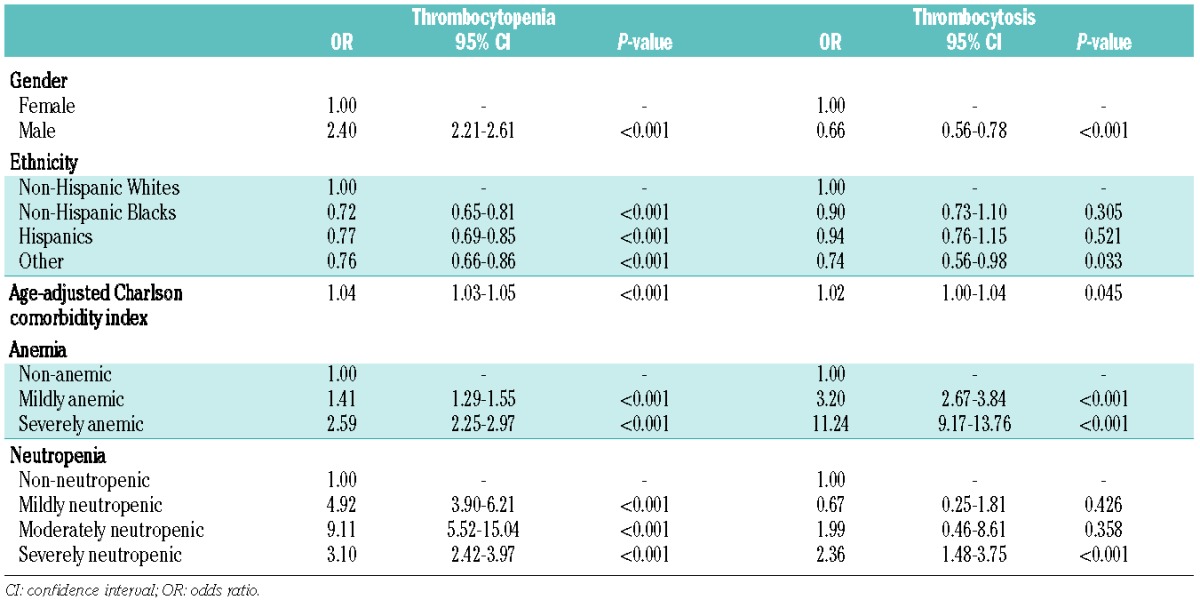
Table 3 shows the results of multivariate analyses of the independent influence of patients’ demographic factors and comorbidities on thrombocytopenia and thrombocytosis rates. The significant association of male gender with increased thrombocytopenia and with decreased thrombocytosis rates persisted in these adjusted models (P<0.001). Non-Hispanic Whites continued to be more likely to have higher thrombocytopenia rates after adjusting for covariates (P<0.001). In addition, patients with higher age-adjusted CCI scores were more likely to have thrombocytopenia or thrombocytosis (P<0.001 and P=0.045, respectively). Mild anemia was also associated with an increased risk of thrombocytopenia (OR=1.41; 95% CI: 1.29–1.55) and thrombocytosis (OR=3.20; 95% CI: 2.67–3.84) and this effect was even more pronounced among severely anemic patients (OR=2.59; 95% CI: 2.25–2.97 and OR=11.24; 95% CI: 9.17–13.76, respectively). Neutropenic patients were also significantly more likely to have thrombocytopenia and this association was strongest among moderately neutropenic patients (OR=9.11; 95% CI: 5.52–15.04). In contrast, only severe neutropenia was significantly associated with a higher risk of thrombocytosis (OR=2.36; 95% CI: 1.48–3.75).
Table 3.
Multivariate association (multivariable logistic regression) of patients’ demographics and comorbidities with thrombocytopenia and thrombocytosis.
Effect of abnormal platelet counts on overall survival
The multivariate survival analysis main effects model relating platelet counts to mortality is presented in Table 4. The median follow-up period was 3.3 years with a total of 134,132 person-years of observation. The overall mortality rate was 62.6 per 1,000 person-years. After adjusting for all covariates, a significant association with increased mortality was found for both thrombocytopenia (HR=1.45; 95% CI: 1.36–1.56) and thrombocytosis (HR=1.75; 95% CI: 1.56–1.97) when compared to normal platelet counts. Fully adjusted overall survival curves based on platelet count status are presented in Figure 1. Other variables that were independently associated with increased overall mortality were male gender (HR=1.28; 95% CI: 1.23–1.34), higher age-adjusted CCI scores (HR=1.09; 95% CI: 1.09–1.10), severe neutropenia (HR=1.75; 95% CI: 1.54–1.99) as well as both mild and severe anemia (HR=1.80; 95% CI: 1.72–1.89 and HR=3.03; 95% CI: 2.83–3.25, respectively). After adjusting for comorbidities and gender, non-Hispanic Blacks and Hispanics were significantly associated with increased overall survival rates compared to non-Hispanic Whites (HR=0.71; 95% CI: 0.67–0.76 and HR=0. 71; 95% CI: 0.67–0.75, respectively).
Table 4.
Cox regression analysis (main effects only model) of the association between platelet count and overall survival after adjusting for patients’ demographics, anemia, neutropenia and age-adjusted CCI score.
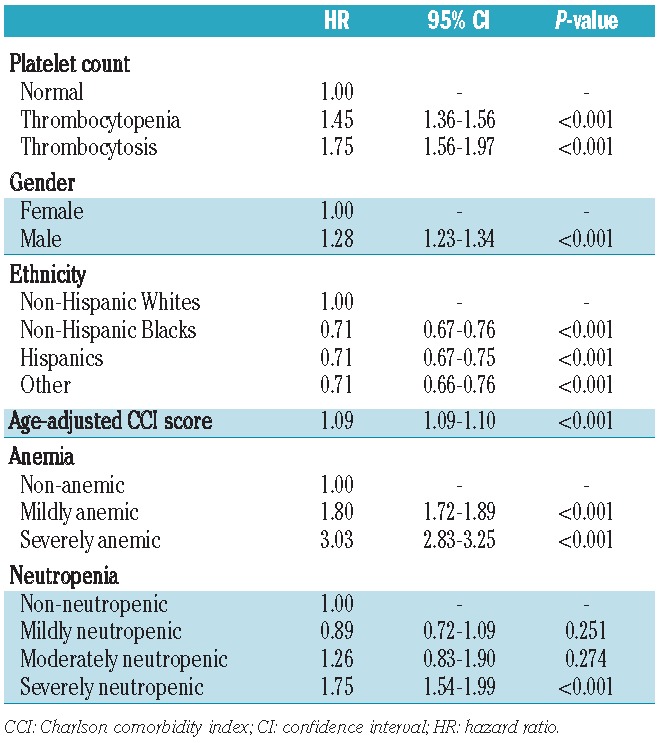
Figure 1.
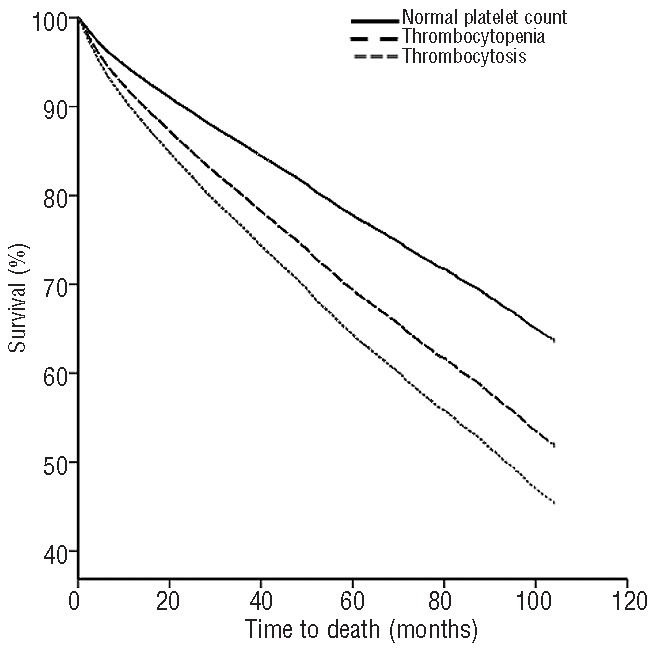
Overall survival curves stratified by platelet count after adjustment for all covariates including gender, ethnicity, anemia, neutropenia and age-adjusted Charlson comorbidity index. Both thrombocytopenia and thrombocytosis independently and significantly decreased overall survival compared to that of patients with normal platelet counts (P<0.01).
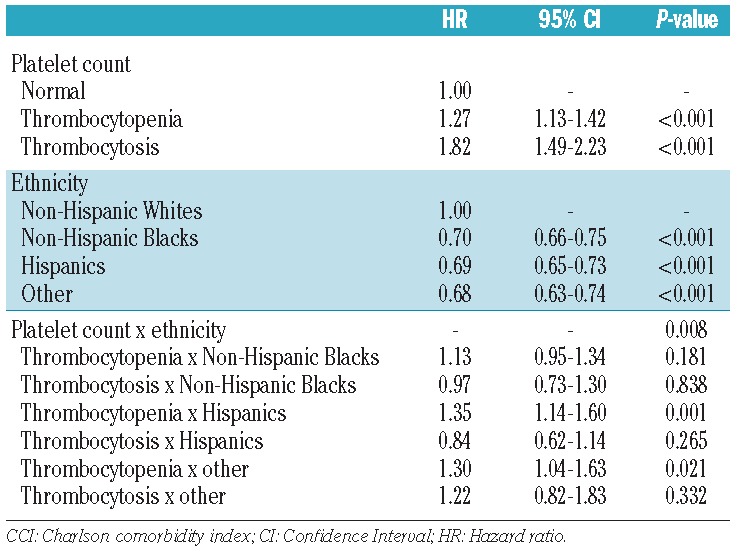
The Cox regression model including the interaction terms between patients’ ethnicity and platelet count status is shown in Table 5. Because the interaction between ethnicity and platelet count was statistically significant (P=0.008), stratified models by platelet count status were constructed which showed that non-Hispanic Blacks with either thrombocytopenia or thrombocytosis were at significantly lower risk compared to non-Hispanic Whites (HR=0.82; 95% CI: 0.69–0.96 and HR=0.70; 95% CI: 0.53–0.94, respectively). In addition, Hispanics with thrombocytosis had significantly lower mortality rates compared to non-Hispanic Whites with thrombocytosis (HR=0.60; 95% CI: 0.44–0.81).
Table 5.
Cox regression analysis showing the interaction terms between ethinicity and platelet count after also adjusting for gender, anemia, neutropenia and age-adjusted CCI score.
Using a different threshold of 125,000 platelets/μL to define thrombocytopenia among non-Hispanic Blacks (as compared to the threshold of 150,000 platelets/μL used for non-Hispanic Whites), abrogated the overall survival difference between non-Hispanic Blacks and non-Hispanic Whites (HR=0.85; 95% CI: 0.69–1.06). Furthermore, using a threshold of >525,000 platelets/μL to define thrombocytosis in non-Hispanic Blacks (versus >450,000/μL for thrombocytosis in non-Hispanic Whites) also mitigated the survival disparity between thrombocytotic Blacks and thrombocytotic non-Hispanic Whites (HR=0.78; 95% CI: 0.55–1.10). On the other hand, the survival advantage of thrombocytotic Hispanics compared to thrombocytotic non-Hispanic Whites was not alleviated by multiple different thresholds, up to 600,000 platelets/μL, tested in regression models (data not shown).
Figure 2 presents the LOESS model generated to describe the non-linear effect of platelet count on survival by ethnicity after adjusting for gender, anemia, neutropenia and age-adjusted CCI score. The association of platelet count with mortality was U-shaped for all ethnicities, with both lower and higher platelet levels showing a statistically significant association with higher mortality. Pairwise comparisons of the LOESS curves by ethnicity confirmed that non-Hispanic Whites had significantly higher mortality rates, regardless of platelet count, compared to either non-Hispanic Blacks (HR=1.42; 95% CI: 1.34–1.50) or Hispanics (HR=1.44; 95% CI: 1.34–1.55). On the other hand, there was no significant difference in overall survival between non-Hispanic Blacks and Hispanics (HR=1.01; 95% CI: 0.94–1.10).
Figure 2.
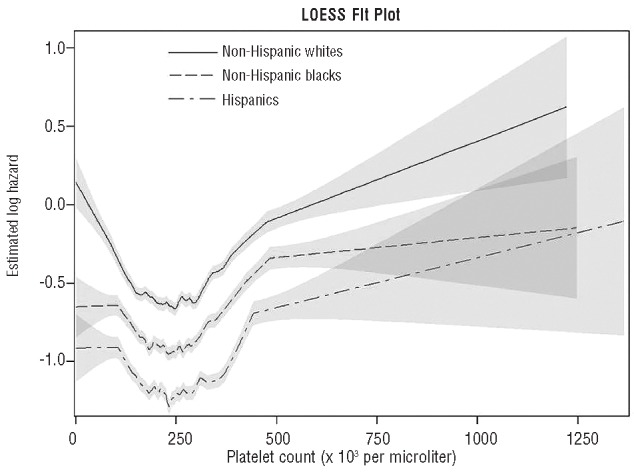
LOESS regression model showing the association between platelet count (horizontal axis) and the log relative hazard ratio for overall mortality (vertical axis) for each ethnicity after adjusting for gender, anemia, neutropenia and age-adjusted Charlson comorbidity index. The shaded areas around each curve delimit the 95% confidence intervals.
Discussion
Previous reports have suggested an association between abnormal platelet counts with adverse clinical outcomes in various clinical conditions such as patients presenting with acute myocardial infarction,11,12 community-acquired pneumonia13 and solid cancers.14–17 However, very few studies have investigated the effect of platelet counts on the survival of healthy populations.18–20 The present report describes the largest investigation of the independent effects of abnormal platelet counts on overall survival to date. Our study took advantage of the availability of a very large database that incorporates clinical and laboratory data from an inner city, outpatient population of elderly patients comprising different ethnicities and with multiple comorbid conditions. We were thus able to determine that platelet counts have a U-shaped association with mortality after adjusting for comorbidities. Of note, the CCI is the most extensively studied and validated comorbidity index currently available.10,21 In addition, our study’s diverse population better elucidated the effect of ethnicity on mortality rates associated with abnormal platelet counts.
It should be noted that, to our knowledge, no other epidemiological study has reported the survival effect of thrombocytopenia or thrombocytosis among Hispanics. Interestingly, we found that the association of thrombocytosis with increased mortality was stronger among non-Hispanic Whites than among Hispanics or non-Hispanic Blacks. In addition, non-Hispanic Blacks with thrombocytopenia had a lower mortality risk compared to non-Hispanic Whites with thrombocytopenia. Previous work demonstrated a strong genetic component to the variations between platelet counts among individuals and different ethnicities.22,23 Our results indicate that such genetic factors (or occult ethnicity-associated environmental factors) may also influence the effect of platelet abnormalities on overall survival. In addition, further exploratory analysis of our population cohort revealed that platelet counts of 125,000/μL and 525,000/μL may be more appropriate lower and upper platelet limits for defining the association of thrombocytopenia and thrombocytosis, respectively, with overall mortality risk among non-Hispanic Blacks. Indeed, these thresholds confer similar prognostic value with regards to overall survival among non-Hispanic Blacks aged ≥65 years old as do the commonly used values of <150,000/μL and >450,000/μL in non-Hispanic Whites. Of note, while the use of ethnic-specific thresholds may be required when determining the overall long-term prognosis of patients, the present study did not investigate whether different thresholds may be warranted to define thrombocytopenia or thrombocytosis in relation to specific clinical disorders such as idiopathic thrombocytopenic purpura, myelodysplastic syndrome or leukemias. In addition, thrombocytotic Hispanics in our study had a survival advantage compared to non-Hispanic Whites irrespectively of the definition of thrombocytosis used. This may indicate the presence of unknown protective factors in the Hispanic population that are independent of platelet counts.
Although we have clearly shown that both thrombocytopenia and thrombocytosis are associated with decreased overall survival, the mechanisms mediating this effect remain to be elucidated. It is likely that in at least some of these cases, further clinical investigation may reveal occult disease processes that can affect mortality. It is also conceivable that abnormal platelet counts may play a direct role in pathophysiological processes (e.g. via platelet involvement in atherosclerotic plaque formation). The linearity of the respective survival curves (Figure 1) and the fact that thrombocytopenia and thrombocytosis were independently associated with increased mortality over a long period may indicate that these conditions are associated with long-term pathophysiological changes rather than acting as proxies for other unmeasured disorders. Whether treatment of thrombocytopenia or thrombocytosis would have any effect on outcomes remains to be determined.
Our study was conducted in a general elderly population with the only exclusion criterion being recent hospitalization in order to avoid potential biases caused by the inclusion of acutely ill individuals. Thus, the present results are applicable to other outpatient populations and reflect the natural history and mortality effects of incidentally discovered abnormal platelet counts among the elderly. Racial and gender disparities were noted in our study population as men and non-Hispanic Blacks were more likely to have higher CCI scores. In our analysis, non-Hispanic Whites and males had lower platelet counts compared to all other ethnicities and females, respectively. These observations are consistent with prior reports of gender and racial differences in platelet counts6,22 and further support the generalizability of our findings. Notably, we also found that an alarmingly high proportion of elderly non-Hispanic White males (13.5%) is thrombocytopenic. Further investigation will be required to elucidate the causes of this observed difference in platelet counts. As expected, higher age-adjusted CCI scores were independently associated with increased mortality. Furthermore, our results replicated the well-established finding that, in developed countries, elderly women have longer overall survival than elderly males.24 We also found that a considerable proportion of our patients had other hematologic abnormalities such as anemia and severe neutropenia which were also independently associated with shorter overall survival in our population. Future investigation should thus also focus on the mechanisms associated with these cell line abnormalities and their independent effect on mortality.
A number of variables were shown in the present study to be independently associated with abnormal platelet counts. Our findings confirm the significant association between male gender and non-Hispanic White ethnicity with higher rates of thrombocytopenia even after adjusting for all other covariates. Furthermore, increasing comorbidity scores were found to be predictive of higher rates of thrombocytopenia and thrombocytosis. This finding is consistent with the well-known associations between abnormal platelet counts and the diverse clinical conditions accounted for in the present study including cancer, liver diseases and rheumatologic diseases.4 Both anemia and neutropenia were found to be associated with thrombocytopenia, indicating the presence of bone marrow failure syndromes in such patients. Anemia and severe neutropenia were also related to thrombocytosis, which in most of these cases is likely reactive in nature and secondary to a variety of disorders including neoplasms, iron deficiency and immune-mediated diseases.4
The present study is limited by its observational nature which prevents the determination of causality. Although this report is unique in its extensive control of comorbidities known to influence platelet count, we do not have specific information on the etiology of the platelet count abnormalities. Furthermore, we were unable to determine the specific causes of death of the patients in our cohort. Baseline thrombocytopenia and thrombocytosis were defined based on a single measurement, although it should be noted that a large amount of evidence points to the considerable intra-individual stability of steady state platelet counts.22,25 The present study also has a number of strengths. It analyzed data from a very large and diverse population of elderly patients which facilitated multivariate analysis. In addition, the study patients were seen at various inner city outpatient facilities within the Montefiore medical system which encompasses a broad range of treating physicians and settings, consequently making our results more generalizable.
In conclusion, the present study demonstrated that both thrombocytosis and thrombocytopenia independently predicted mortality in a large, outpatient cohort of inner city, elderly subjects and this effect was heterogeneous across different ethnicities. Future areas of investigation should determine the mechanisms by which platelet count abnormalities are associated with decreased overall survival.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Stokes KY, Granger DN. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590(Pt 5):1023–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology Am Soc Hematol Educ Program. 2011;2011:51–61 [DOI] [PubMed] [Google Scholar]
- 3.Van den Bossche J, Devreese K, Malfait R, Van de Vyvere M, Wauters A, Neeis H, et al. Reference intervals for a complete blood count determined on different automated haematology analysers: Abx Pentra 120 Retic, Coulter Gen-S, Sysmex SE 9500, Abbott Cell Dyn 4000 and Bayer Advia 120. Clin Chem Lab Med. 2002;40(1):69–73 [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. From the Centers for Disease Control and Prevention. Public health and aging: trends in aging–United States and worldwide. JAMA. 2003;289(11):1371–3 [PubMed] [Google Scholar]
- 6.Segal JB, Moliterno AR. Platelet counts differ by sex, ethnicity, and age in the United States. Ann Epidemiol. 2006;16(2):123–30 [DOI] [PubMed] [Google Scholar]
- 7.Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49(8):664–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83 [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9 [DOI] [PubMed] [Google Scholar]
- 10.Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10(2):134–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, et al. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial). Am J Cardiol. 2007; 99(8):1055–61 [DOI] [PubMed] [Google Scholar]
- 12.Turakhia MP, Murphy SA, Pinto TL, Antman EM, Giugliano RP, Cannon CP, et al. Association of platelet count with residual thrombus in the myocardial infarct-related coronary artery among patients treated with fibrinolytic therapy for ST-segment elevation acute myocardial infarction. Am J Cardiol. 2004;94(11):1406–10 [DOI] [PubMed] [Google Scholar]
- 13.Mirsaeidi M, Peyrani P, Aliberti S, Filardo G, Bordon J, Blasi F, et al. Thrombocytopenia and thrombocytosis at time of hospitalization predict mortality in patients with community-acquired pneumonia. Chest. 2010;137(2):416–20 [DOI] [PubMed] [Google Scholar]
- 14.Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9(3):287–91 [DOI] [PubMed] [Google Scholar]
- 15.O’Keefe SC, Marshall FF, Issa MM, Harmon MP, Petros JA. Thrombocytosis is associated with a significant increase in the cancer specific death rate after radical nephrectomy. J Urol. 2002;168(4 Pt 1):1378–80 [DOI] [PubMed] [Google Scholar]
- 16.Hernandez E, Donohue KA, Anderson LL, Heller PB, Stehman FB. The significance of thrombocytosis in patients with locally advanced cervical carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78(2):137–42 [DOI] [PubMed] [Google Scholar]
- 17.Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9(9):1826–30 [DOI] [PubMed] [Google Scholar]
- 18.Stasi R, Amadori S, Osborn J, Newland AC, Provan D. Long-term outcome of otherwise healthy individuals with incidentally discovered borderline thrombocytopenia. PLoS Med. 2006;3(3):e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Bom JG, Heckbert SR, Lumley T, Holmes CE, Cushman M, Folsom AR, et al. Platelet count and the risk for thrombosis and death in the elderly. J Thromb Haemost. 2009;7(3):399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaulow E, Erikssen J, Sandvik L, Stormorken H, Cohn PF. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation. 1991;84(2):613–7 [DOI] [PubMed] [Google Scholar]
- 21.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–9 [DOI] [PubMed] [Google Scholar]
- 22.Biino G, Balduini CL, Casula L, Cavallo P, Vaccargiu S, Parracciani D, et al. Analysis of 12,517 inhabitants of a Sardinian geographic isolate reveals that predispositions to thrombocytopenia and thrombocytosis are inherited traits. Haematologica. 2011;96(1):96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qayyum R, Snively BM, Ziv E, Nalls MA, Liu Y, Tang W, et al. A meta-analysis and genome-wide association study of platelet count and mean platelet volume in african americans. PLoS Genet. 2012;8(3):e1002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buvinic M, Medici A, Fernandez E, Torres AC. Gender Differentials in Health. 2006 [PubMed] [Google Scholar]
- 25.Buckley MF, James JW, Brown DE, Whyte GS, Dean MG, Chesterman CN, et al. A novel approach to the assessment of variations in the human platelet count. Thromb Haemost. 2000;83(3):480–4 [PubMed] [Google Scholar]


