Abstract
The majority of patients with acute myeloid leukemia will relapse, and older patients often fail to achieve remission with induction chemotherapy. We explored the possibility that leukemic suppression of innate immunity might contribute to treatment failure. Natural killer cell phenotype and function was measured in 32 consecutive acute myeloid leukemia patients at presentation, including 12 achieving complete remission. Compared to 15 healthy age-matched controls, natural killer cells from acute myeloid leukemia patients were abnormal at presentation, with downregulation of the activating receptor NKp46 (P=0.007) and upregulation of the inhibitory receptor NKG2A (P=0.04). Natural killer cells from acute myeloid leukemia patients had impaired effector function against autologous blasts and K562 targets, with significantly reduced CD107a degranulation, TNF-α and IFN-γ production. Failure to achieve remission was associated with NKG2A overexpression and reduced TNF-α production. These phenotypic and functional abnormalities were partially restored in the 12 patients achieving remission. In vitro co-incubation of acute myeloid leukemia blasts with natural killer cells from healthy donors induced significant impairment in natural killer cell TNF-α and IFN-γ production (P=0.02 and P=0.01, respectively) against K562 targets and a trend to reduced CD107a degranulation (P=0.07). Under transwell conditions, the inhibitory effect of AML blasts on NK cytotoxicity and effector function was still present, and this inhibitory effect was primarily mediated by IL-10. These results suggest that acute myeloid leukemia blasts induce long-lasting changes in natural killer cells, impairing their effector function and reducing the competence of the innate immune system, favoring leukemia survival.
Introduction
Chemotherapy induces remission in up to 80% of patients presenting with de novo acute myeloid leukemia (AML). However, disease free survival remains poor because of high mortality from remission induction failure and relapse of the majority of patients who achieve remission.1–3 In contrast to the low curative potential of chemotherapy, the graft-versus-leukemia (GVL) effect conferred by donor lymphocytes following allogeneic stem cell transplantation contributes to permanent disease eradication.4 Notably, natural killer (NK) cells have a potent GVL effect in both HLA mismatched and matched donor-recipient combinations,5–13 and rapid NK recovery is associated with improved outcome and a greater GVL effect after stem cell transplantation (SCT).14 NK cells are an important component of the innate immune system, providing first-line defense against virus-infected cells and tumors. NK cell function, which includes cytotoxicity and cytokine release, is governed by a balance between inhibitory receptors, notably the killer Ig-like receptors (KIRs) and the heterodimeric C-type lectin receptor (NKG2A), and activating receptors, in particular the natural cytotoxicity receptors (NCR) NKp46, NKp30, NKp44 and the membrane protein NKG2D.15–17
It is possible that NK cells also exert control over AML in the autologous setting. NK cells are cytotoxic to AML blasts18 and higher NK-mediated cytotoxicity has been reported to result in superior leukemia-free survival.19 While some studies suggest that NK cells may be compromised in AML,20,21 only limited data are available on the prognostic significance of these abnormalities and whether such changes normalize once patients achieve remission. To explore whether individual NK cell characteristics affect outcome in patients with AML, we examined NK phenotype and function in newly diagnosed AML patients at presentation and following remission induction (CR) using age-matched healthy controls.
We found that NK cells in AML blood at presentation have an abnormal phenotype with downregulation of the activating receptor NKp46 and upregulation of the inhibitory receptor NKG2A. We also identified impaired cytotoxic and effector cytokine function which partially corrected in remission, and could be induced in vitro in NK cells of healthy controls by co-incubation with AML blasts. These changes predicted outcome of remission-induction chemotherapy. Our findings indicate that, in patients with AML, an immuno-editing process induced by AML blasts limits NK cell control of leukemia and that abnormal NKG2A and TNF-α production predicts response to treatment for AML.
Methods
Patient consent was obtained in accordance with the Declaration of Helsinki. The local ethics board approved the study (NREC ref. 10/H0711/16).
Peripheral blood (PB) samples were collected prospectively from September 2009 to January 2012 from 32 consecutive AML patients at presentation and compared with paired remission samples in 12 patients who achieved complete remission post chemotherapy, and with PB samples from age-matched healthy controls (n=15). All samples underwent Ficoll density separation (Organon-Teknika, USA), freezing and storage in liquid nitrogen.
Surface receptor phenotyping
Cell surface analysis was performed with a BD FACS Calibur flow cytometer (BD Biosciences, Oxford, UK) and FlowJo software (Tree Star, San Carlos, CA, USA). PBMC were immune-stained with CD3 and CD56 antibodies to identify the NK population (CD56+, CD3−) and CD13, CD33 and CD34 antibodies to exclude AML blasts. NK were characterized for surface expression of NKp30, NKp44, NKp46, NKG2A, NKG2D, KIR2DL1/S1, KIR2DL2/S2, KIR3DL1 and Pan KIR. AML blasts were characterized for expression of NK ligands: DR4/5, HLA-A, B, C, MICA/B, HLA-E and Fas. Where cells were available, experiments were performed in triplicate. Controls for AML blast phenotyping included healthy-donor PBMC and Hela cells.
Cytotoxicity studies
AML blasts were separated from PBMC on a Robosep instrument (STEMCELL, Grenoble, France) using a monoclonal antibody cocktail against CD33, CD34, CD123 and CD36 (StemSep, France, modified from Le Dieu et al.22). Samples were checked to ensure a minimum purity of 90% and rested overnight in RPMI/20%FCS.
PBMC were incubated with target cells for 5 h at an optimized effector: target ratio of 1:1 based on percentage of NK frequency in PBMC. Experiments were performed in triplicate based on sample availability. Targets for cytotoxicity studies included the K562 cell line (grown in RPMI/FCS) and autologous AML blasts. PBMC were incubated without targets as the negative control and stimulated with PMA (50 ng/mL) and ionomycin (2 mg/mL, Sigma Aldrich) as positive controls.
Optimizing a previously-published23 protocol, CD107a and Fas Ligand antibodies, monensin (BD GolgiStopTM) and BFA (Brefeldin A, Sigma, UK) were added to the cultures at incubation onset. Cells were washed and stained with CD3, CD56 and KIR antibodies, fixed/permeabilized (BD Biosciences, UK) and stained with IFN-γ and TNF-α antibodies. Cells were analyzed on a BD LSRFortessa flow cytometer.
Co-culture of healthy NK cells with leukemia cells
Healthy-donor NK cells were negatively selected (Miltenyi Biotec, Germany) then cultured in 96-well plates at 250,000 cells/well for 24 h, with or without primary AML blasts or in transwell devices at an NK:blast ratio of 10:1 or 1:1. Paired experiments were performed overnight with or without 100 IU/mL rhIL-2 (Proleukin, Chiron, Emeryville, CA, USA) to assess if any functional or phenotypic NK abnormalities induced by AML blasts could be reversed by IL-2. NK cells were then analyzed for cell surface receptor expression by flow cytometry, and for cytotoxicity and effector cytokine production against K562 leukemia cell targets. After centrifugation, supernatant from the co-culture experiments were evaluated for production of the immunomodulatory cytokines TGF-β and IL-10 by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions using Human TGF-beta1 Platinum ELISA (ebiosciences) assay and OptEIA human IL-10 ELISA (BD Bisosciences). Cytokine concentrations in supernatants were expressed as mean+standard deviation of triplicates.
Indoleamine 2,3-dioxygenase expression and activity
Human primary AML cells were tested for IDO mRNA expression by PCR.24 AML cells (1×105) were cultured in RPMI complete medium with 106/mL allogeneic NK cells for 24 h. Then, NK cells were collected and their effector function tested against K562 leukemia targets (ratio 1:1).
Results
Patients’ characteristics
Natural killer cells from AML patients were analyzed from 32 consecutive patients at presentation and following complete remission in 12 patients for whom remission samples were available. Results were compared with 15 age-matched healthy controls. Patients’ characteristics are detailed in Table 1,25 and include 16 with primary AML, 14 with AML secondary to antecedent hematologic disease, and 2 with therapy-related AML. Median age was 61 years (range 21–87 years). Patients were treated with a combination of daunorubicin (50–60 mg/m2 × 3 doses over 5 days) and cytarabine (100 mg/m2 × 20 doses over 10 days). In 3 patients, serial PBMC samples were taken after each course of consolidation chemotherapy and at later time points, as detailed. Patients received standard supportive care with antifungal, anti-viral and antibiotic therapy as appropriate.
Table 1.
Patients’ characteristics.
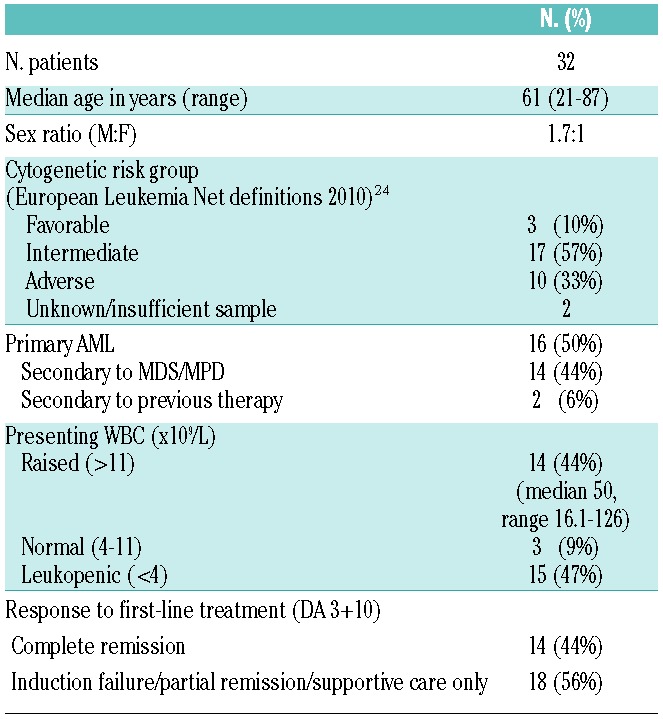
NK surface receptor phenotype is abnormal in AML and favors NK inhibition
We analyzed by flow cytometry the surface expression of NK receptors including NCRs, KIRs and C-type lectins on NK cells from 32 AML patients at diagnosis compared with NK cells from 15 age-matched healthy controls (Figure 1). Frequency of NKG2A-expressing NK cells was significantly higher in AML at diagnosis compared to healthy controls (mean 45%±4.2 vs. 32%±2.7; P=0.04) (Figure 1C). Conversely, expression of the activating receptor NKp46 was significantly lower in AML (MFI 187±15 vs. 266±q24 in healthy controls; P=0.007) (Figure 1D). We found no significant differences in the surface expression of CD56 bright and dim populations, NKG2D, KIR, NKp30 or NKp44 on NK cells in AML patients compared to healthy controls (Figure 1C–F).
Figure 1.
NK surface phenotype is abnormal in AML compared to healthy controls. NK receptor surface expression in 32 patients with AML at diagnosis compared with 15 healthy controls. (A) Gating strategy for AML-NK surface phenotype results. AML-NK were gated on CD13, CD33 and/or CD34−, CD3, CD56+ lymphocyte fraction of PBMCs. (B) Histograms showing NK surface phenotype in a representative AML patient and healthy control. Open histograms represent isotype matched monoclonal antibody staining. Filled histograms represent staining with specific PE-conjugated monoclonal antibodies. NK surface receptor phenotype of (C) C-type lectins (D) NCRs (E) KIR (F) CD56 bright and dim. Horizontal bars denote mean expression. Error bars denote standard deviation between individuals within group. KIR: killer immunoglubulin receptor; NCR: natural cytotoxicity receptor; HC: healthy control; AML: acute myeloid leukemia.
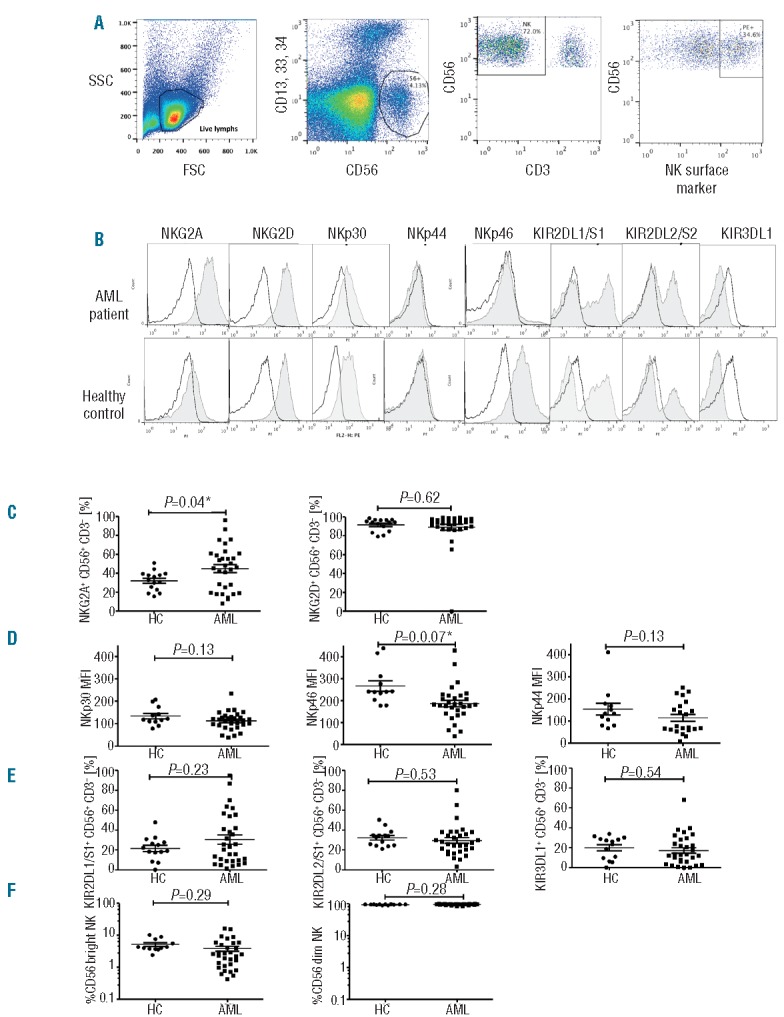
NK phenotypic abnormalities in AML are partially restored following induction chemotherapy
We next examined whether alterations in activating and inhibitory receptors normalized in patients achieving remission (Figure 2). Following induction chemotherapy, mean NKp46 expression increased significantly from 122±56 at presentation to 242±152 (P=0.012) (Figure 2A), reaching values found in healthy controls. In contrast, mean NKG2A expression continued to increase after induction chemotherapy from 45%±24 at presentation to 61%±25 in remission (P=0.008) (Figure 2B). NKp46 and NKG2A expression was assessed in 3 patients at diagnosis (T0), after induction chemotherapy (T1), and following repeated courses of consolidation chemotherapy (T2 and T3). Interestingly, whereas NKp46 expression remained within normal limits at T2 (MFI, 287) and T3 (MFI, 382), NKG2A expression remained higher than in healthy controls and continued to increase following consolidation chemotherapy to 77% at T2 and 84% at T3 (P=0.05) (Figure 2C).
Figure 2.
NK phenotypic abnormalities in AML are partially restored following induction chemotherapy. (A) NKp46 expression is restored following induction chemotherapy to levels consistent with those of healthy controls. (B) NKG2A expression remains elevated. P values report significance of paired t-tests comparing AML T0 (at presentation) and T1 (following induction chemotherapy) and unpaired t-tests comparing AML with healthy controls. (C) NKp46 and NKG2A expression in a representative healthy control (left column) and AML patient at serial time points (right column): diagnosis (T0), following induction chemotherapy (T1), following consolidation chemotherapy #1 (T2), following consolidation chemotherapy #2 (T3). Horizontal bars denote mean expression. Error bars denote standard deviation between individuals within group. HC: healthy control; AML: acute myeloid leukemia.
NK phenotypic abnormalities are associated with impaired cytotoxicity and effector cytokine function
AML-NK cells had significantly reduced CD107a degranulation (5% vs. 11%; P=<0.001), TNF-α (1% vs. 3%; P=0.008) and IFN-γ production (1% vs. 5%; P=<0.001) compared to healthy controls (Figure 3A). We also tested the ability of NK cells from AML patients to exert effector function against autologous blasts. NK cells from the majority of patients with AML at presentation failed to mount significant cytotoxicity or effector cytokine production against autologous AML blasts.
Figure 3.
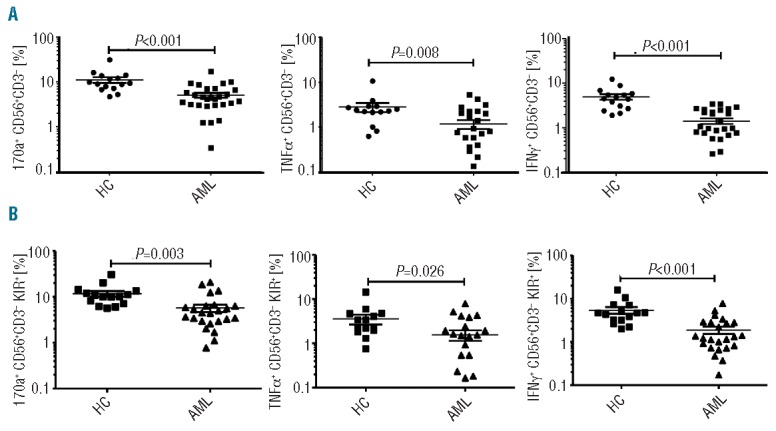
NK cytotoxicity and effector function in AML patients at diagnosis compared to healthy controls. (A) Total NK (CD56+ CD3−) cytotoxicity (CD107a degranulation), TNF-α and IFN-γ production against K562 leukemia targets in 32 patients with AML and 15 healthy controls; (B) KIR specific NK (CD56+ CD3− KIR+) cytotoxicity and effector cytokine function against K562 leukemia targets in 32 patients with AML and 15 healthy controls. Horizontal bars denote mean expression. Error bars denote standard deviation between individuals within group. HC: healthy control; AML: acute myeloid leukemia.
Since KIRs are important in both NK licensing and in mediating NK cytotoxicity against AML,26–29 we compared the effector function of KIR+ and KIR− subsets against K562 leukemia targets. As expected, KIR+ subsets displayed greater cytotoxicity and effector function compared to KIR- subsets in AML patients and in controls (Online Supplementary Figure S1). Pan KIR+ NK cells derived from AML patients at diagnosis displayed significantly less cytotoxicity (CD107a degranulation, 6% vs. 12%; P=0.003) and effector cytokine function (TFN-α production 2% vs. 4%; P=0.026; IFN-γ production 2% vs. 5%; P=<0.001) against K562 leukemia targets compared to control NK cells (Figure 3B). A similar pattern was seen in KIR2DS1/DL1+, KIR2DS2/DL2+ and KIR3DS1/DL1 expressing NK subsets (Online Supplementary Figure S1). Impaired cytotoxicity and effector function in AML-NK cells was observed in both KIR+ and KIR− subsets with no significant difference between different KIR expressing NK subsets (Online Supplementary Figure S1).
Interestingly, AML-NK cells were not significantly different to NK from healthy donors in their ability to degranulate and produce TNF-α and IFN-γ in response to stimulation with PMA and ionomycin (Online Supplementary Figure S2). This is in marked contrast to the reduced cytotoxicity and effector function after stimulation by leukemic targets (Figure 3). These data indicate that NK dysfunction in AML is likely related to impaired NK receptor-ligand interaction, without affecting protein kinase C dependent PMA- and/or ionomycin-induced degranulation.30
Restoration of NK cytotoxicity and effector cytokine function following induction chemotherapy
We assessed NK effector function and cytotoxicity in the 12 patients who achieved remission following induction chemotherapy. AML-NK cells derived from patients at remission tested against K562 leukemia target cells displayed CD107a degranulation and TNF-α production levels comparable to that of healthy donor NK cells (9% vs. 11%; P=0.4) and (2% vs. 3%; P=0.3), respectively. In contrast, IFN-γ production, although significantly improved from diagnosis (1% vs. 3%; P=0.009), only partially normalized (3% at AML remission vs. 5% in healthy donors; P=0.044) (Figure 4A). We assessed whether NK cells from AML patients in remission recognized and mediated cytotoxicity against autologous blasts collected at diagnosis. Although there was some response in a minority of patients, NK cells from the majority of patients in remission failed to degranulate or produce effector cytokines when stimulated with autologous AML blasts in vitro (Figure 4B).
Figure 4.
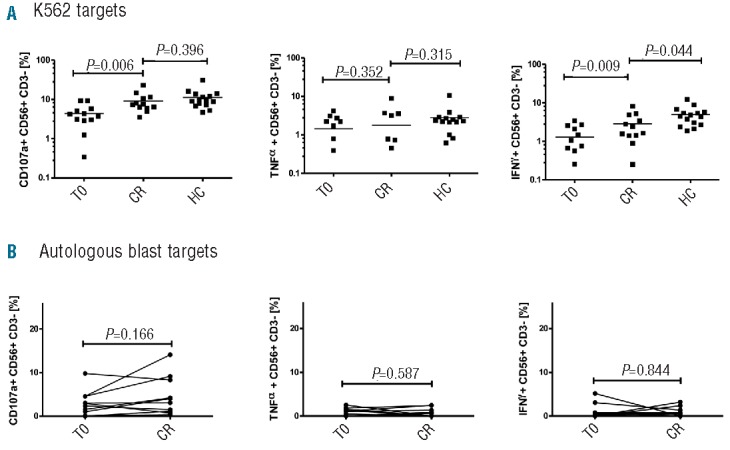
NK cytotoxicity and effector function at remission. (A) NK (CD56+ CD3−) CD107a degranulation and effector cytokine production against K562 in 12 AML patients at remission compared to diagnosis and to 15 healthy controls. (B) NK CD107a degranulation and effector cytokine function against autologous blasts in 12 AML patients at remission compared to diagnosis. P values report significance of paired T tests comparing AML at T0 and remission and unpaired t-tests comparing AML with healthy controls. Horizontal bars denote mean expression. Error bars denote standard deviation between individuals within group. CR: complete remission; T0: diagnosis; HC: healthy control; AML: acute myeloid leukemia.
NK phenotypic abnormalities in AML correlate with impaired cytotoxicity and predict response to chemotherapy
We next correlated NK surface receptor phenotype with NK effector function and cytotoxicity, and response to chemotherapy. We observed 2 groups of patients: a high NKG2A-expressing and a low NKG2A-expressing group. Those with higher NKG2A expression (> median 32.6%) had impaired TNF-α production (P=0.003) (Figure 5A) and were significantly less likely to achieve CR post chemotherapy compared to those with lower NKG2A expression (CR rate of 31% vs. 78%; P=0.041) (Figure 5C). In contrast, whereas low NKp46 expression (< median MFI) was associated with significantly reduced CD107a degranulation and IFN-γ production (P=0.004 and P=0.047, respectively) (Figure 5B), we found no significant correlation between NKp46 expression level at presentation and the probability of CR post induction chemotherapy (data not shown), suggesting that NKG2A may be a more clinically relevant marker for disease response than NKp46. Interestingly, individuals with NKG2AhiNKp46lo NK cells displayed the poorest NK effector function against leukemia targets in vitro (Online Supplementary Figure S3). NK effector function at diagnosis was associated with the probability of achieving CR after chemotherapy. AML patients presenting with TNF-α production above the median all achieved CR, compared to only 39% (9 of 23) of patients with TNF-α production below the median (P=0.041) (Figure 5D).
Figure 5.
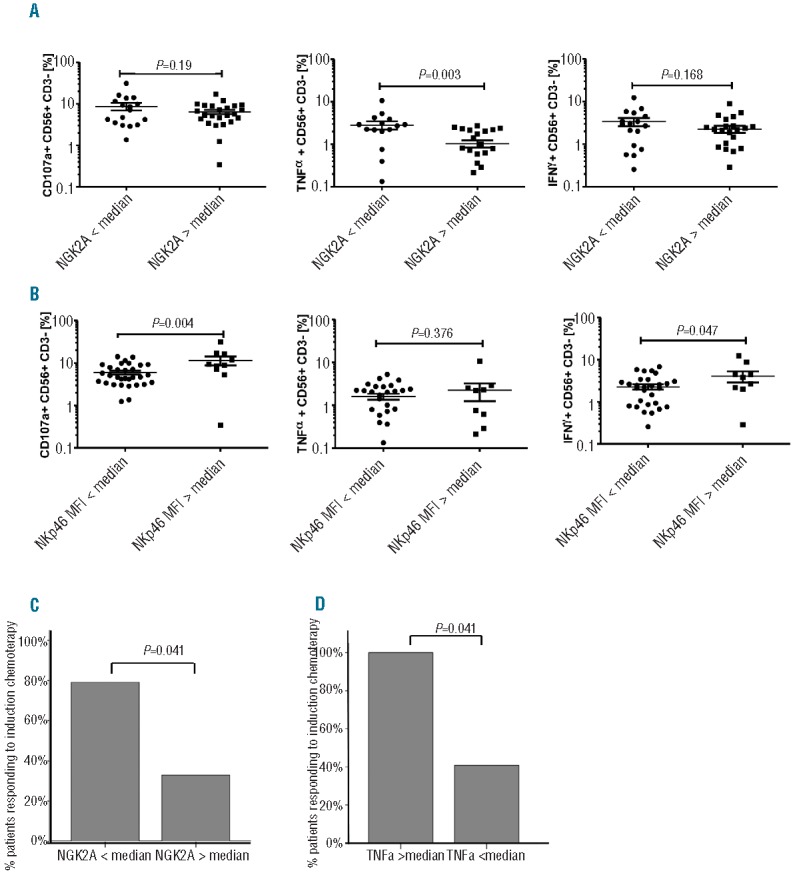
NK phenotypic and functional abnormalities at diagnosis and response to chemotherapy. (A) NK cells in individuals with low NKp46 expression (< median MFI) exhibited impaired cytotoxicity and IFN-γ production against K562. (B) NK cells in individuals with high NKG2A expression (> median) had impaired TNF-α production against K562. (C) High NKG2A expression (> median) at presentation was associated with a poor response to chemotherapy. (D) Low TNF-α production against K562 at presentation (< median) was associated with a poor response to chemotherapy. Horizontal bars denote mean expression. Error bars denote standard deviation between individuals within group. P values denote significance of unpaired t-tests.
Our findings that abnormal NK phenotype and effector function at diagnosis can predict treatment response were independent of other known AML prognostic factors such as white blood count or karyotypic abnormality (data not shown), suggesting that the effect of AML on NK cells occurs irrespective of the patient’s AML disease characteristics.
NK cells co-cultured with AML cells display prompt impairment of cytolytic activity
Ex vivo selected NK cells from healthy controls were co-incubated with primary AML blasts from patients at a 10:1 ratio for 24 h and their phenotype assessed in 4 independent experiments. There was no significant difference in the expression of NKG2A or NKp46 in control NK cells after co-culture with leukemia cells in the presence or absence of IL-2 (200 iU/mL) compared to NK cells incubated for 24 h in the absence of AML blasts +/− IL-2 (200 iU/mL) (data not shown). The effect of AML blasts on cytotoxicity and effector function of peripheral blood NK cells from 3 healthy controls was then tested. After 24 h co-incubation of NK cells with primary AML blasts (at a ratio of 10:1) in the presence or absence of IL-2 (200 iU/mL), NK cells were purified and their effector function was assessed against K562 leukemia targets. Co-incubation of NK cells with AML blasts for 24 h resulted in upregulation of NKG2A expression, associated with significant impairment in NK cytotoxicity and effector function with marked reduction in TNF-α production (P=0.02), IFN-γ production (P=0.01), and a trend to reduced CD107a degranulation (P=0.07) against K562 leukemia targets (Figure 6A–C). In 2 experiments, sufficient patient material was available to test the dose effect of AML blasts on NK dysfunction. NK cells were co-incubated with AML blasts at two ratios of 1:1 and 10:1. NK effector abnormalities were even more pronounced following co-incubation with AML blasts at the higher ratio of 1:1 for 24 h (Figure 7A and B).
Figure 6.
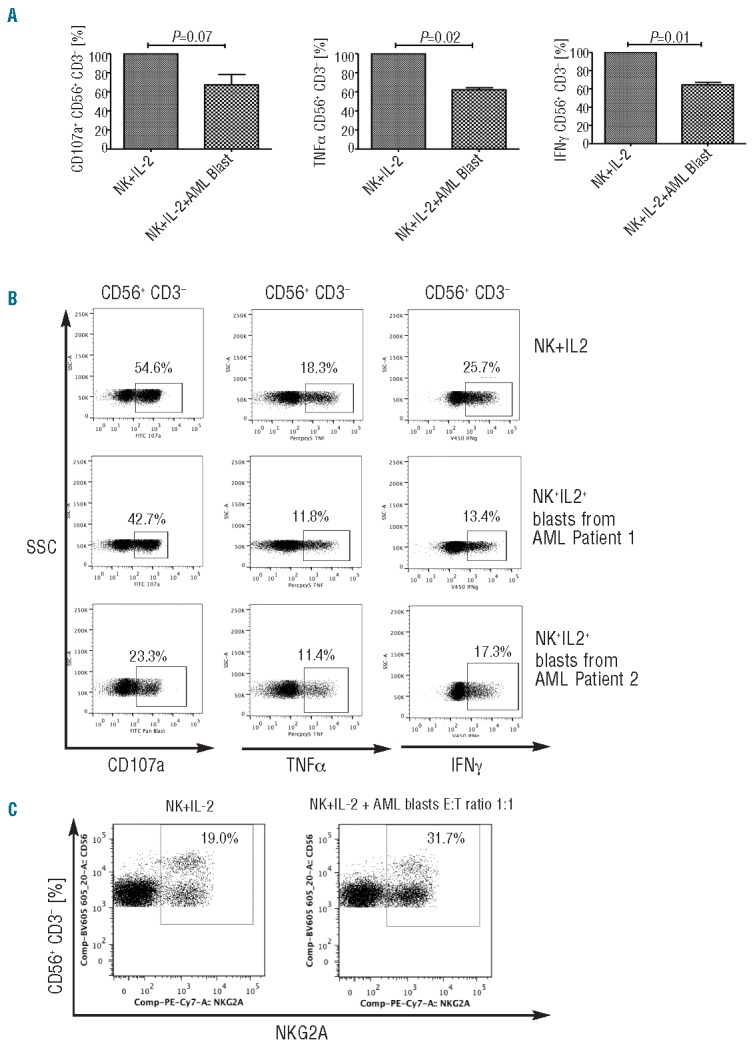
AML cells induce impairments in NK effector function in vitro. (A) Effect of AML blast co-culture on NK cell CD107a degranulation, TNF-α and IFN-γ production against K562. (B) Representative FACS plots showing the effect of co-incubating AML blasts from 2 different patients on healthy donor NK cell cytotoxicity and effector function are presented. After 24 h of healthy donor NK co-culture with primary AML blasts (ratio of 10:1) + IL-2 (200 iU/mL), the effector function of NK cells was assessed against K562 leukemia targets (ratio 1:1). Plots are gated on CD56+CD3− NK cells. (C) A dose effect with increasing concentrations of blasts exhibiting greater inhibition of healthy NK function.
Figure 7.
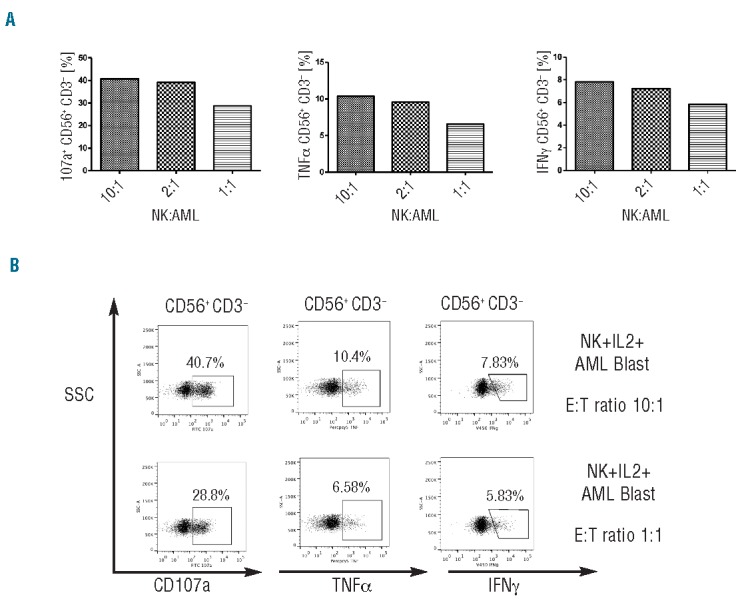
(A) FACS plots showing greater inhibition of NK effector function when healthy NK were exposed to higher concentrations of blasts. (B) Analysis of the effect of AML blast co-culture on the expression of NKG2A.
In clinical protocols of NK adoptive therapy, systemic IL-2 is administered to support NK expansion. We also examined whether IL-2 can reverse the immune-editing effects of AML blasts on NK cells. A similar pattern of NK inhibition was seen in the presence of IL-2, indicating that IL-2 alone cannot reverse AML-induced NK dysfunction.
IL-10, but not TGF-β or indoleamine 2,3-dioxygenase, are involved in AML-mediated modulation of NK activity
To investigate the mechanisms responsible for the inhibitory effects of AML blasts on NK function, co-culture experiments were performed in transwells. As shown in Figure 8A, under transwell conditions, the inhibitory effect of AML blasts on NK cytotoxicity and effector function was still present. These results suggested that AML blasts constitutively release soluble factor(s) capable of interfering with NK effector function. In an attempt to discover the soluble factor responsible for this phenomenon, supernatants from co-culture experiments were evaluated for production of the immunomodulatory cytokines TGF-β and IL-10, previously shown to induce expression of CD94/NKG2A on the surface of NK cells,31 and play a role in the escape of tumor cells from NK-mediated immune surveillance.32 Although, we did not detect significant levels of TGF-β in the supernatants collected from NK-AML co-cultures, IL-10 levels were significantly increased in the supernatants collected from NK-AML co-cultures after 24 h of culture, irrespective of whether cells were in direct contact or separated by a transwell membrane (Figure 8B). Interestingly, the greatest degree of AML-induced NK dysfunction was seen in co-cultures with the highest levels of IL-10, which may reflect distinct immunosuppressive properties of the leukemic blasts from patients with varying subtypes of AML.
Figure 8.
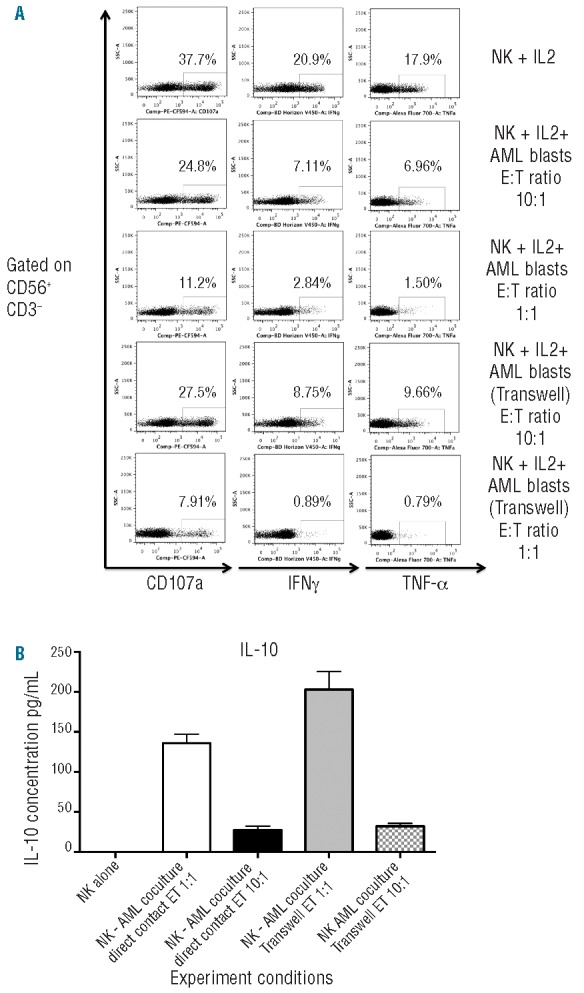
(A) Healthy donor NK cells were cultured for 24 h in IL-2 (200 iU/mL), either alone or with primary AML blasts at a 1:10 or 1:1 ratio in the same well (NK + AML) or in transwell devices and effector function of NK cells was assessed against K562 leukemia targets (ratio 1:1). Plots are gated on CD56+CD3− NK cells. (B) After centrifugation, supernatant from the co-culture experiments were evaluated for production of the immunomodulatory cytokines TGF-β and IL-10 by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions using the Human TGF-beta1 Platinum ELISA (ebiosciences) assay and OptEIA human IL-10 ELISA set from BD Bisosciences. Cytokine concentrations in supernatants were performed in duplicates.
Recent studies suggest that abnormal expression of the immunosuppressive tyrosine-converting enzyme IDO may be an important mechanism in melanoma-induced NK dysfunction.33 To investigate whether IDO may also play a part in AML-induced NK impairment, we measured IDO expression in AML blasts from patients at diagnosis. As previously observed, IDO was over-expressed in blasts from a subset of patients. We next co-cultured NK cells with AML blasts from patients with high versus no expression of IDO for 24 h. We found no correlation between the inhibitory effect of AML blasts on NK function and IDO expression (Online Supplementary Figure S4). Taken together, these studies support a role for IL-10, but not TGF-β or IDO, in AML-mediated modulation of NK activity.
Discussion
In this study, we showed that the NK cells of patients presenting with AML have phenotypic abnormalities, with increased expression of the inhibitory receptor NKG2A and downregulation of the activating receptor NKp46. Following remission induction chemotherapy, NKp46 expression, but not NKG2A, normalized. These changes were associated with impaired NK effector function and cytotoxicity, and were induced in normal NK cells by incubating with AML blasts, suggesting that NK cells are subject to immune-editing by AML cells.
The natural cytotoxicity receptors (NCRs) are a group of activating glycoprotein receptors restricted to NK cells. Recently, a number of cellular ligands for the NCRs have been reported. These include ligands expressed on the surface of virally-infected cells as well as tumor cells.34–38 NCRs have been described as crucial receptors for target cell recognition and induction of NK cell-mediated cytotoxicity towards cancer cells.39–41 In keeping with previous reports, we found significant downregulation of NKp46 expression in patients with AML at presentation.20,21 In contrast to the study by the Costello group,21 we did not observe a reduction in the surface expression of NKp30. Previous studies have reported distinct non-overlapping activities of NKp46 and NKp30 in decidual NK (dNK) cells. Moreover, NKp46-mediated dNK cytotoxicity was negatively controlled by specific co-engagement of NKG2A inhibiting receptor.42 Whether this is involved in the selective downregulation of NKp46 expression in NK-AML cells remains to be determined. Our data support previous reports of synergy among NK receptors and the requirement for a 2-stage process of activation and triggering for target cell lysis.43–45
Another possible cause for this discrepancy may be due to differences in phenotyping techniques. Because CD56 is often aberrantly expressed on AML blasts, we first gated out blasts expressing CD13, 33 or 34 to separate these from NK-AML cells in our study. The Costello group21 defined and excluded AML blasts using CD45high expression. They also excluded patients with a presenting white blood cell count of more than 50×109/L, whereas this population comprised 16% (5 of 32) of our patients. Furthermore, NK cell analysis in the study by the Costello group was performed on freshly-selected NK cells or NK cells treated in vitro with interleukin-2. In our study, PBMC collected from individual patients were frozen and batched prior to analysis to allow accurate assessment of the kinetics and comparison of NK receptor expression over time.
At presentation, NK-AML cells had impaired effector function and cytotoxicity against autologous AML blasts as well as MHC-class-I-deficient leukemia targets. As expected, KIR-expressing NK cells exhibited more cytotoxicity and effector cytokine function against the MHC class I deficient K562 cell line than their KIR-negative counterparts, further supporting a role for KIR immunogenetics in shaping the immune response to leukemia.7,13 However, there were no significant differences in effector function of specific KIR-expressing NK cell subsets against K562. Our analysis of effector function of KIR-expressing NK subsets was limited by a number of factors: not all the monoclonal antibodies used could distinguish activating and inhibitory KIR receptors. Furthermore, due to the stochastic expression of KIR on NK cells, the gated populations were likely to co-express multiple KIR receptors.
Altered NKp46 and NKG2A expression was associated with impaired NK effector function against autologous AML blasts, supporting a role for tumor editing of NKp46 and NKG2A. Interestingly, there was no significant difference in NK cytotoxicity between patients with primary or those with secondary AML (Online Supplementary Figure S5) suggesting that the process of immune-editing by blasts is relevant in both de novo and secondary AML.
We found that in AML patients achieving remission following chemotherapy, NK cells displayed normal NKp46 expression whereas NKG2A expression remained increased. A similar observation was reported in melanoma patients after chemotherapy.46 We hypothesize that higher frequencies of NKG2A+ NK cells are induced by AML cells as part of immune-editing to facilitate tumor survival. Indeed, our in vitro studies confirmed that NKG2A expression is up-regulated following 24 h of in vitro co-incubation with AML blasts.
Following remission induction, there was a complete restoration of NK cytotoxicity and partial recovery of effector cytokine production against K562 leukemia target cells. However, autologous leukemic blasts remained resistant to lysis by NK cells derived from patients at remission, suggesting that AML cells have evolved a mechanism of escape from NK-cell mediated recognition. In assessing NK receptor ligands on AML blasts, we found no significant downregulation of HLA class I expression. However, our study was limited by the use of a pan class I mAb and the expression of individual HLA class I molecules could not be ascertained. We also assessed the expression by AML of the “non-classical” HLA-E ligand of NKG2A. Although HLA-E expression was down-regulated on leukemic blasts in some patients, there was no significant association of HLA-E with NKG2A expression or outcome (data not shown). Another possible explanation for the failure of NK cells to recognize autologous blasts could be downregulation of as yet unidentified NCR target cell ligands on the surface of AML cells.20,47 Since remission reversed some of the abnormal phenotypic and functional changes in AML-NK cells, we explored the ability of primary AML blasts to modify NKp46 and NKG2A expression and effector function in healthy-control NK cells. Co-culture of healthy-donor NK cells with AML blasts induced upregulation of NKG2A and impaired NK effector function and cytotoxicity. Moreover, transwell experiments showed that this effect is independent of cell-to-cell contact, suggesting that AML blasts may release soluble factors to avoid NK-mediated killing. In this context, a number of cytokines, growth factors, and enzymes synthesized by tumor and/or stromal cells have been reported to exert suppressive effects on cells involved in immune responses.48 For example, a recent study suggested abnormal expression of the immunosuppressive tyrosine-converting enzyme IDO as a mechanism for reduced NK cytotoxicity in melanoma patients,33 as well as impaired T-cell function in AML.24 However, we failed to detect a significant correlation between AML blast IDO expression and NK-AML effector function. Similarly, we failed to detect significant production of the immunomodulatory cytokine TGF-β in the supernatants collected from NK-AML co-cultures (data not shown). IL-10 has been shown to mediate immunosuppression49 and a recent study suggests that release of IL-10 by AML cells can directly diminish granule mobilization, cytotoxicity, and interferon-γ production of human NK cells.50 Accordingly, we found increased levels of IL-10 in NK-AML culture supernatants, which correlated with the degree of AML-induced NK dysfunction. Taken together, these findings suggest that while NK cells from healthy donors can exert anti-leukemia effects, the release of soluble factors with immunomodulatory properties such as IL-10 by AML blasts may ultimately limit the immunotherapeutic benefit of NK cell therapy.
In summary, in AML patients, increased frequency of NKG2A-expressing NK cells and downregulation of the activating receptor NKp46 appear to alter the balance of receptor signals towards inhibition of NK cells and are associated with impaired lytic and effector function. Increased frequency of NKG2A-expressing NK cells and impaired TNF-α production are associated with failure to achieve remission after induction chemotherapy. Our studies identify a negative influence of AML cells on the ability of the innate immune system to control leukemia proliferation. This has implications for the ability of the individual to achieve remission with standard induction chemotherapy, while the degree of NK functional recovery in remission may influence long-term survival. Impaired TNF-α production and increased NKG2A expression at diagnosis, therefore, represent new prognostic markers for chemotherapy response in AML. Future studies will seek to better define the prognostic significance of NK phenotypic and functional markers in AML treatment outcome and identify mechanisms through which AML cells suppress NK cell function.
Footnotes
The online version of this article has a Supplementary Appenix.
Funding
This research is supported in part by the MD Anderson Cancer Center Leukemia SPORE Grant CA100632, Leuka registered charity (286231) and a British Society for Haematology start-up grant. The sponsors of this study are public or non-profit organizations that support science in general. They had no role in gathering, analyzing, or interpreting the data.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Farag SS, Archer KJ, Mrozek K, Ruppert AS, Carroll AJ, Vardiman JW, et al. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006a;108(1):63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1312–20 [DOI] [PubMed] [Google Scholar]
- 3.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–33 [PubMed] [Google Scholar]
- 4.Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol. 2008;142(6):877–88 [DOI] [PubMed] [Google Scholar]
- 5.Beelen DW, Ottinger HD, Ferencik S, Elmaagacli AH, Peceny R, Trenschel R, et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood. 2005;105(6):2594–600 [DOI] [PubMed] [Google Scholar]
- 6.Cook MA, Milligan DW, Fegan CD, Darbyshire PJ, Mahendra P, Craddock CF, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004;103(4):1521–6 [DOI] [PubMed] [Google Scholar]
- 7.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102(3):814–9 [DOI] [PubMed] [Google Scholar]
- 9.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105(12):4878–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–9 [PubMed] [Google Scholar]
- 11.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002; 295(5562):2097–100 [DOI] [PubMed] [Google Scholar]
- 12.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stringaris K, Adams S, Uribe M, Eniafe R, Wu CO, Savani BN, et al. Donor KIR Genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant. 2010;16(9):1257–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007a;21(10):2145–52 [DOI] [PubMed] [Google Scholar]
- 15.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11(7):237–44 [DOI] [PubMed] [Google Scholar]
- 17.Marsh SG, Parham P, Dupont B, Geraghty DE, Trowsdale J, Middleton D, et al. Killer-cell immunoglobulin-like receptor (KIR) nomenclature report, 2002. Tissue Antigens. 2003;62(1):79–86 [DOI] [PubMed] [Google Scholar]
- 18.Lotzova E, Savary CA, Herberman RB. Inhibition of clonogenic growth of fresh leukemia cells by unstimulated and IL-2 stimulated NK cells of normal donors. Leuk Res. 1987;11(12):1059–66 [DOI] [PubMed] [Google Scholar]
- 19.Lowdell MW, Craston R, Samuel D, Wood ME, O’Neill E, Saha V, et al. Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol. 2002;117(4):821–7 [DOI] [PubMed] [Google Scholar]
- 20.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99(10):3661–7 [DOI] [PubMed] [Google Scholar]
- 21.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109(1):323–30 [DOI] [PubMed] [Google Scholar]
- 22.Le Dieu R, Taussig D, Lister TA, Gribben JG. Negative immunomagnetic selection of T cells from peripheral blood of presentation AML specimens. J Immunol Methods. 2009b;348(1–2):95–100 [DOI] [PubMed] [Google Scholar]
- 23.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22 [DOI] [PubMed] [Google Scholar]
- 24.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. 2007;109(7):2871–7 [DOI] [PubMed] [Google Scholar]
- 25.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74 [DOI] [PubMed] [Google Scholar]
- 26.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–42 [DOI] [PubMed] [Google Scholar]
- 27.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105(11):4416–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–13 [DOI] [PubMed] [Google Scholar]
- 29.Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaelsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115(6):1166–74 [DOI] [PubMed] [Google Scholar]
- 30.Bonnema JD, Karnitz LM, Schoon RA, Abraham RT, Leibson PJ. Fc receptor stimulation of phosphatidylinositol 3-kinase in natural killer cells is associated with protein kinase C-independent granule release and cell-mediated cytotoxicity. J Exp Med. 1994;180(4):1427–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertone S, Schiavetti F, Bellomo R, Vitale C, Ponte M, Moretta L, et al. Transforming growth factor-beta-induced expression of CD94/NKG2A inhibitory receptors in human T lymphocytes. Eur J Immunol. 1999;29(1):23–9 [DOI] [PubMed] [Google Scholar]
- 32.Castriconi R, Dondero A, Bellora F, Moretta L, Castellano A, Locatelli F, et al. Neuroblastoma-derived TGF-beta1 modulates the chemokine receptor repertoire of human resting NK cells. J Immunol. 2013;190(10):5321–8 [DOI] [PubMed] [Google Scholar]
- 33.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012;72(6):1407–15 [DOI] [PubMed] [Google Scholar]
- 34.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31(9):2680–9 [DOI] [PubMed] [Google Scholar]
- 35.Chisholm SE, Howard K, Gomez MV, Reyburn HT. Expression of ICP0 is sufficient to trigger natural killer cell recognition of herpes simplex virus-infected cells by natural cytotoxicity receptors. J Infect Dis. 2007;195(8):1160–8 [DOI] [PubMed] [Google Scholar]
- 36.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409(6823):1055–60 [DOI] [PubMed] [Google Scholar]
- 37.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baychelier F, Sennepin A, Ermonval M, Dorgham K, Debre P, Vieillard V. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood. 2013;122(17):2935–42 [DOI] [PubMed] [Google Scholar]
- 39.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21(5):228–34 [DOI] [PubMed] [Google Scholar]
- 40.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190(10):1505–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, et al. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998;188(5):953–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Costa H, Casemayou A, Aguerre-Girr M, Rabot M, Berrebi A, Parant O, et al. Critical and differential roles of NKp46- and NKp30-activating receptors expressed by uterine NK cells in early pregnancy. J Immunol. 2008;181(5):3009–17 [DOI] [PubMed] [Google Scholar]
- 43.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on rest ing NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.North J, Bakhsh I, Marden C, Pittman H, Addison E, Navarrete C, et al. Tumor-primed human natural killer cells lyse NK-resistant tumor targets: evidence of a two-stage process in resting NK cell activation. J Immunol. 2007;178(1):85–94 [DOI] [PubMed] [Google Scholar]
- 45.Sabry M, Tsirogianni M, Bakhsh IA, North J, Sivakumaran J, Giannopoulos K, et al. Leukemic priming of resting NK cells is killer Ig-like receptor independent but requires CD15-mediated CD2 ligation and natural cytotoxicity receptors. J Immunol. 2011;187(12):6227–34 [DOI] [PubMed] [Google Scholar]
- 46.Fregni G, Perier A, Pittari G, Jacobelli S, Sastre X, Gervois N, et al. Unique functional status of natural killer cells in metastatic stage IV melanoma patients and its modulation by chemotherapy. Clin Cancer Res. 2011;17(9):2628–37 [DOI] [PubMed] [Google Scholar]
- 47.Nowbakht P, Ionescu MC, Rohner A, Kalberer CP, Rossy E, Mori L, et al. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105(9):3615–22 [DOI] [PubMed] [Google Scholar]
- 48.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–74 [DOI] [PubMed] [Google Scholar]
- 50.Baessler T, Charton JE, Schmiedel BJ, Grunebach F, Krusch M, Wacker A, et al. CD137 ligand mediates opposite effects in human and mouse NK cells and impairs NK-cell reactivity against human acute myeloid leukemia cells. Blood. 2010;115(15):3058–69 [DOI] [PubMed] [Google Scholar]


