Abstract
Introduction/Hypothesis
The subfornical organ, one of the central circumventricular organs, has been shown to mediate many of the effects of circulating angiotensin II (AngII). Where these signals are processed downstream is not fully understood. The SFO does indeed project to prominent cardiovascular regulatory centers such as the paraventricular nucleus (PVN), of whose neurons are activated by central AngII. We reasoned that AngII sensed at the SFO would cause neuronal activation at downstream hypothalamic areas such as the median preoptic nucleus and paraventricular nucleus, and as such would be diminished in animals with lesions of the SFO.
Materials and Methods
To test this hypothesis, groups of rats underwent either SFO lesion (SFOx) or sham operation. Five days later rats were instrumented with radiotelemetry transducers for monitoring of mean arterial pressure (MAP) and venous catheters for infusions. MAP and heart rate were measured continuously. After a 4 day control period, infusion of AngII (0.575 µg/kg/min) was begun for a period of 2 hours. Rats were then sacrificed and brains were processed for neuronal Fos expression.
Results
AngII produced Fos expression in the SFO, MnPO and PVN of sham rats. Fos expression was greatly attenuated in the PVN of SFOx rats.
Conclusion
These results support our hypothesis, suggesting that AngII sensitive neurons of the SFO can mediate neuronal activation in the PVN.
Introduction
The central nervous system monitors body fluid and adjusts its sympathetic nervous output and hormonal secretion based on circulating blood angiotensin II (AngII) concentration and osmolality (1–7). These signals are sensed by circumventricular organs (CVOs) in the brain, unique central sites that line the third and fourth ventricles and have an incomplete blood brain barrier due to the presence of fenestrated capillaries (8,9). These special characteristics of CVOs allow circulating substances, including peptide hormones such as AngII, to gain access to the brain where they are normally otherwise excluded. Of particular relevance to the present study, is the subfornical organ (SFO), which lines the third ventricle and has been implicated in the central effects of AngII (10). The SFO has a wealth of AT1 receptors (11) and has been shown to be involved in mediating both pressor (12–14) and dipsogenic (12,15) effects of AngII, as well as centrally mediated release of vasopressin (16). Lastly, we have recently demonstrated attenuated hypertensive effects in rats with lesions of the SFO during a ten day chronic infusion of AngII (17).
The central sites where sensory information is processed downstream from areas such as the SFO, and ultimately influence hindbrain activity are not fully understood. The SFO projects to the median preoptic nucleus (MnPO), a potential relay station to the paraventricular nucleus (PVN) (18–26), as well as directly projecting to the latter. Evidence supports that the MnPO has an important role in relaying and integrating information received by the SFO. For example, this nuclear region has substantial afferent inputs from the SFO (25), and its neurons have been shown to increase their metabolic activity when the SFO is electrically stimulated (22). After MnPO ablation, reductions in water intake and vasopressin output have been observed, as well as attenuated arterial pressure responses to AngII infusion (27–32). Additionally it has been shown that the ventral MnPO is critical for expression of the immediate early gene, c-Fos in the PVN following i.c.v. infusion of AngII (33). Lastly, we have recently reported reduced chronic hypertensive responses to AngII in animals with either chemical or electrolytic lesions of the MnPO, supporting a role of the MnPO neurons in this response (32,34).
With regard to the PVN, SFO neurons identified as projecting to the PVN are excited by AngII administration (35) and this is blocked by prior treatment with saralasin (36). Likewise, lesions of the PVN decrease pressor responses to SFO stimulation (37). Furthermore, PVN cells projecting to the intermediolateral cell column are excited by SFO stimulation (38). Lastly, PVN or rostral ventral lateral medulla pretreatment with an AngII antagonist blocks pressor responses seen with AngII injection at the SFO (39).
Taken together, the SFO, MnPO, and PVN are brain structures of interest that are involved in sensing AngII or participate in downstream actions of this hormone. Expression of the proto-oncogene c-fos has been shown to increase in neurons in response to a number of stimuli (40–42), and thus, increased formation of the protein product of c-fos expression, Fos, may indicate increased activity of neurons (43). In the present study, experiments were designed to test the hypothesis that the SFO is a primary site of action of AngII that leads to downstream activation of Fos in the MnPO and/or PVN, providing evidence to support a central pathway that may mediate central regulation of blood pressure. In order to test this hypothesis, Fos expression was measured in hypothalamic nuclei (MnPO and PVN) after intravenous AngII infusion in animals with or without an intact SFO.
Methods
Adult male Sprague Dawley rats (Charles River Laboratories, Wilmington, Mass) weighing 275–300g upon arrival, were used in all experiments. Rats were maintained under a 12h:12h light dark cycle and given standard rat chow and distilled water ad libitum. All procedures were conducted in accordance with the National Institutes of Health guidelines and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Surgical Procedures
Rats were randomly assigned to either a lesion of the subfornical organ (SFOx, n=6) or a sham (n=5) operation. Pentobarbital sodium (39 mg/kg, IP) was given as a preanesthetic medication with atropine (0.2mg/kg), and followed by an intramuscular injection of a combination of acetylpromazine, butorphanol tartrate, and ketamine (0.2 mg/kg; 0.15 mg/kg, 18.5 mg/kg, respectively) to achieve a surgical plane of anesthesia. An intramuscular injection of antiobiotic (gentamicin 2.5 mg) was given pre-operatively as antimicrobial prophylaxis. Anesthetized rats were then positioned in a Kopf stereotaxic apparatus. A dorsal midline incision was made through the skin of the skull. Bregma and lambda landmarks were exposed, and a 3 mm hole was drilled 1.5 mm posterior to bregma. A Teflon coated, tungsten electrode with 0.008 inches exposed at the tip was passed into the brain at 4 predetermined coordinates relative to bregma. The four coordinates caudal and ventral to bregma were −0.8, and −5.2 mm, −1.0 and −5.1 mm, −1.2 and −4.9 mm, and −1.4 and −4.7 mm, respectively. At each location, a 1 mA current was passed for 8 seconds to complete the lesion. The hole was then closed with bonewax, and the skin sutured with 3-0 silk suture. Sham operations were identical to lesion surgeries, with the exception that ventral coordinates were 1.5 mm less, and no current was passed. A subcutaneous injection of 0.075 mg of butorphanol tartrate was given post-operatively for analgesic purposes, and animals were kept on a heating pad until they recovered.
On day 5 post lesion surgery, animals were implanted with radiotelemetry blood pressure transducers (Model TA11PA-C40, Data Sciences International, St. Paul, MN) and femoral venous infusion catheters, for 24 hour sampling of mean arterial blood pressure (MAP) and heart rate (HR), and infusion of AngII, saline, or phenylephrine, respectively. Briefly, a midline abdominal incision skin was made to expose the abdominal aorta, the artery was clamped proximally, and the tip of the catheter advanced directly into the artery after a hole was made with a bent 21g needle. A patch was affixed and glued into place on the outer wall of the vessel where entry occurred, and the clamp was then removed. The body of the unit was sutured to the abdominal wall to secure it. For the infusion catheter, the inner thigh was incised and blunt dissection used to expose the femoral vein for placement of the venous catheter. Silk sutures were used to briefly occlude the vessel for catheter placement. The catheter was advanced to the level of the atrium, and sutured into place with 3.0 silk. Hemostats were used to draw the other end of the catheter subcutaneously through a scapular incision, and once exteriorized at the nape of the neck, sutured in place. Animals were fitted in jackets attached to springs enclosing the catheters. Each rat was housed singly with the spring attached to a swivel mounted above a metabolic cage. Animals were injected with antibiotics (Ampicillin 15 mg/kg) daily for 3 days, followed by heparinized saline (50 IU/ml) to maintain line patency until infusion and subsequent tissue collection for Fos immunoreactivity measurements.
Experimental Protocol
Blood pressures were monitored over 3 days. Four days after implantation surgery, animals were infused for 2 hours (1 ml saline/hour) with one of three solutions: AngII (0.575 µg/kg/min), isotonic saline, or phenylephrine (10 µg/kg/min). The dose of AngII was chosen based previous reports demonstrating centrally induced Fos expression in response to this dose (12). SFOx (n=6) or sham (n=5) animals were treated with AngII, and separate non-lesioned controls (n=3) were infused with phenylephrine (at a dose chosen to cause a similar rise in pressure as those animals infused with AngII) to control for the increase in blood pressure produced by AngII. Separate saline control animals (n=2) were examined as well as 24 hour water deprived Fos positive control rats for expression in the PVN (n=9). It is well known that water deprivation causes Fos expression in this area of the hypothalamus.
Immunohistochemistry
Within 20 minutes post infusion, all rats were anesthetized to a surgical plane, as assessed by response to noxious stimulus, then rapidly decapitated and the brains removed. Brain tissue was immersed in 0.1M acetate fixative on ice, placed on a shaker at 4° C for approximately 6 hours, transferred to 0.1M borate fixative at 4° C for approximately 2–3 days, then transferred to a 20% glycerol in 0.1M phosphate buffer solution at 4° C for 1 ½ days.
40 µm coronal slices were then collected from the SFO, MnPO, and PVN using a sliding cryotome with freezing stage set at −19° C. Tissues were transferred from PBS into cryoprotectant for storage in −20° C until immunohistochemistry was performed. Alternating slices from each nucleus were selected and processed.
After removal from cryoprotectant and rinsing with PBS, sections were reacted with 3% H2O2 and 10% methanol mixture, blocked with normal goat serum in PBS-T, and incubated overnight with anti-c-Fos (Ab-5) rabbit polyclonal antibody (1:80,000 in 2% NGS/PBS-T; Calbiochem) at 25° Celsius. After rinsing with PBS-T, sections were incubated with biotinylated goat anti rabbit IgG secondary antibody (1:400 in 2% NGS/PBS-T, Vector Labs, Burlingame, CA) for 1 hour, followed by avidin-biotin complex for 1 hour (Vector Labs), and followed by the addition of diaminobenzidine as per kit instructions (Vector Labs). Sections were mounted on gelatin-coated glass slides and coverslipped with Permount mounting media after being dehydrated with alcohol and methyl salicylate. Fos-IR (immunoreactivity) cells in the SFO, MnPO, and PVN were counted using the Metamorph Imaging System®. At least 4 slices per section for each nucleus were counted, averaged and compared between groups.
In the SFOx group, only rats with greater than 90% lesions and no or slight damage to adjacent areas were included in the analysis, as determined by visualization of the SFO by light microscopy.
Analysis
Fos positive nuclei were identified under normal brightfield illumination. Statistical analysis between lesioned, sham, and phenylephrine treated groups was performed by one way ANOVA using the statistical packages NCSS (NCSS, Kaysville, UT) and Abacus concepts, Inc. Between group comparisons of Fos counts were done with sham and lesion animals and within group comparisons between baseline and two hour MAP and HR were performed after AngII infusion (Microsoft Excel, Microsoft Corp, Redmond, WA). All values were presented as means ± SE.
Results
Effects of AngII infusion on blood pressure and heart rate
Table 1 shows resting baseline levels (measured as average values during the 60 minutes prior to the start of infusion) of MAP and HR, and the changes in these parameters induced by intravenous infusion of AngII or phenylephrine for a period of two hours. MAP showed an abrupt and sustained increase with acute AngII infusion that was not lessened by lesion of the SFO (SFOx, n=6; 124±7 mmHg) in comparison with sham animals (n=5; 129±6 mmHg). Phenylephrine infusion (n=3) raised MAP to similar levels as seen in both sham and SFOx lesion groups that received AngII. Baseline HR were similar in all three groups and treatments produced similar bradycardic responses in all 3 groups (see Table 1).
Table 1.
Absolute values of mean arterial pressure and heart rate before and after treatments (either AngII or phenylephrine) in sham or SFOx rats. (HR=heart rate, MAP=mean arterial pressure, SFOx=rats with lesion of the SFO).
| Sham AngII group | SFOx AngII group | Phenylephrine group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Pre | Post | n | Pre | Post | n | Pre | Post | |
| MAP (mmHg) | 5 | 102±7 | 129±6 | 6 | 98±3 | 124±7 | 3 | 99±8 | 124±12 |
| HR (bpm) | 5 | 430±15 | 335±27 | 6 | 428±22 | 353±11 | 3 | 431±18 | 327±22 |
Effects of AngII infusion on Fos production
AngII infusion induced Fos expression in the SFO, MnPO, and PVN in addition to other brain nuclei sites, while saline and phenyleprhine infused controls resulted in more limited responses. Figure 1 shows a typical example of a positive control animal demonstrating Fos expression in the PVN of an animal that was water deprived for 24 hours. Figure 2 displays examples of Fos expression in the SFO, MnPO and PVN in SFOx animals after stimulation with AngII over two hours time compared with sham animals. Additionally, Fos expression in these areas is shown in the phenylephrine treated group. Averages of number of Fos expressing cells in SFO, MnPO and PVN are shown in figure 3 for all 3 groups (AngII-sham, AngII-SFOx and phenylephrine treated).
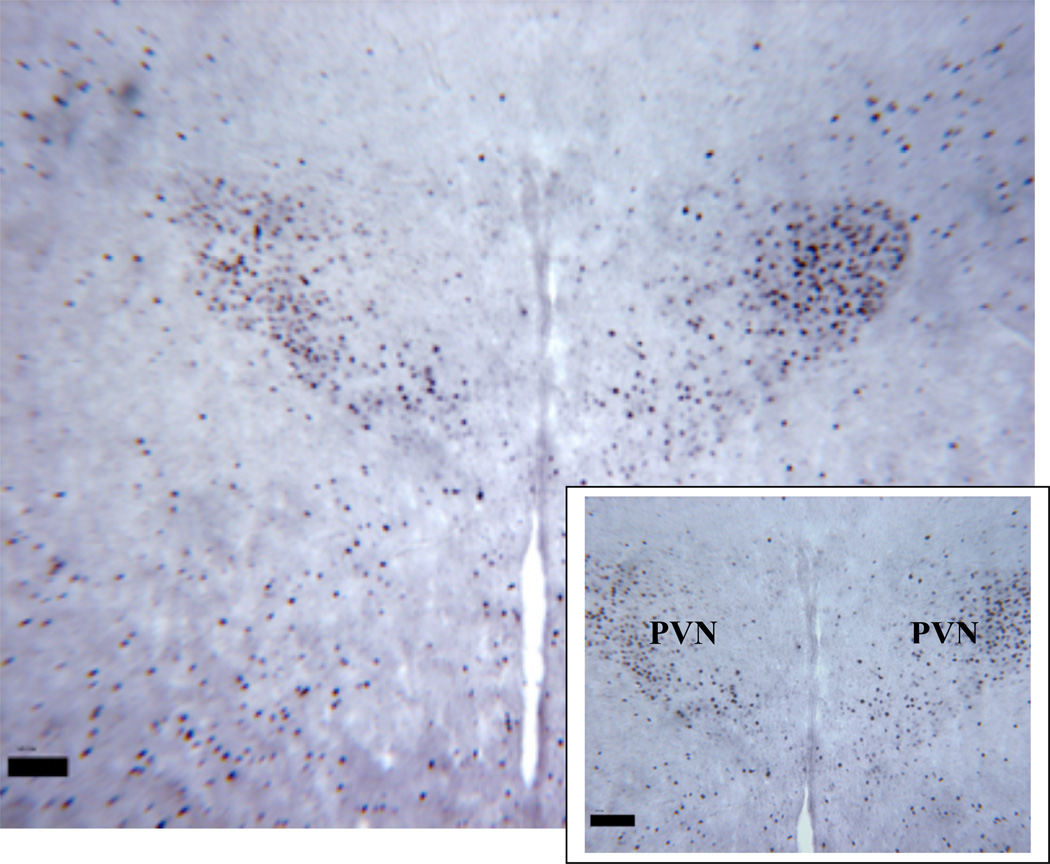
Figure 1.
Low and high magnification of a typical coronal section of PVN from a Fos positive (black dots represent counts of Fos production) control animal. Water was withheld for 24 hours prior to tissue collection to induce Fos expression. One such control animal was performed with every group to verify DAB staining intensity (PVN=paraventricular nucleus).
Magnification bar = 100 µm
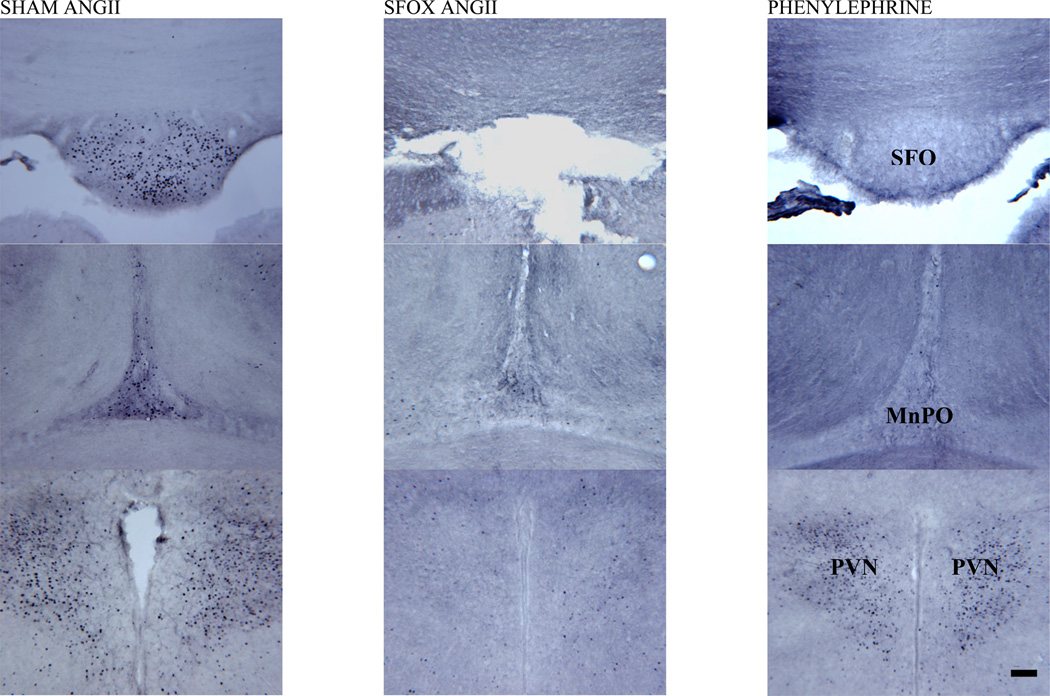
Figure 2.
Representative coronal sections from subfornical organ (SFO; top row), dorsal median preoptic nucleus (MnPO; middle row) and paraventricular nucleus (PVN; bottom row) in animals following 2 hours of intravenous infusion of AngII in sham (first column) and SFOx (second column) animals, as well as phenylephrine (final column) treated control rats. Midline structures of the lamina terminalis are labeled in the third column as SFO and MnPO, as well as the bilaterally paired areas of the hypothalamic PVN.
Magnification bar = 100 µm.
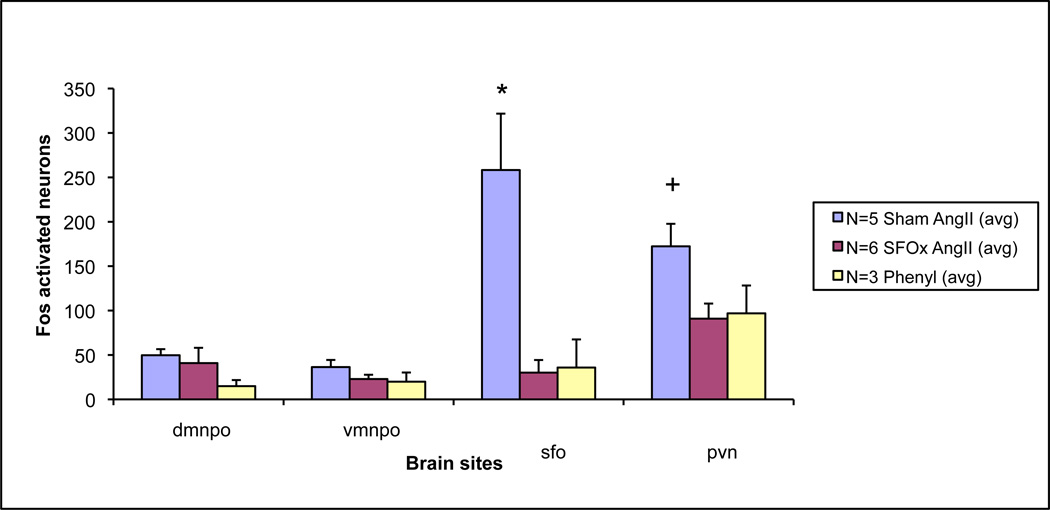
Figure 3.
Bar graph representing counts of Fos activation in SFO, MnPO and PVN in animals following intravenous infusion of AngII in sham and SFOx animals, as well as phenylephrine treated control rats.
* = p<0.05 between groups in SFO; + = p<0.05 between groups in PVN.
While having no effect on the acute blood pressure response, lesions of the SFO (SFOx) suppressed Fos activation in the PVN compared to sham animals in response to AngII infusions (figure 3). Fos expression was still present in both the MnPO and PVN in SFOx animals, but was significantly decreased in the PVN of SFOx rats.
SFO
As presented in Figure 3, following AngII infusion, Fos activation in the SFO of sham animals was marked (258±64). Phenylephrine treated control animals had a paucity of activation here compared to AngII infused shams (36±32). Separate saline infused controls (both sham and SFOx animals) had few Fos positive neurons (4±1 and 3±3 respectively). Figure 2 shows representative examples of the SFO (top row) in the treated groups.
MnPO
AngII infusion in sham animals produced Fos activity in both dorsal (d=50±7) and ventral (v=36±8) MnPO regions. As shown figure 3, lesion of the SFO did not reduce the numbers of Fos activated cells in SFOx rats (d=41±17; v=23±5) compared with sham animals. Phenylephrine treated animals also demonstrated some activation in all regions of the MnPO (d=15±7; v=20±10). Fos expression in separate saline infused controls were as follows: sham; d=26±7, v=17±12 and SFOx; d=19±8, v=4±0.1. Figure 2 (middle row) shows representative examples of the dorsal region of the MnPO in the treated groups.
PVN
Dense groups of neurons were activated in the PVN after Ang II infusion in sham animals (172±25). As shown in figure 3, there was however, a marked and significant reduction in the number of Fos activated cells seen in AngII treated SFOx animals (91±17). Phenylephrine treated animals had similarly less activation noted in this area (97±31). Fos counts in the PVN of saline infused controls (sham and SFOx) were 92±9 and 78±12, respectively. Figure 2 (bottom row) shows the Fos expression in the PVN after AngII infusion in both SFOx and sham animals, as well as phenylephrine treated controls.
Discussion
Many of the central actions of AngII are thought to be mediated through central nervous system sites known as CVOs (40, 45). Of these, the SFO and its role in the actions of AngII has been studied in the past, and the results have been consistent regarding the ability of AngII to induce SFO neuronal activation (46, 47). The SFO is abundant in AT1 receptors (48, 49), and is involved in both the central dipsogenic and pressor responses to AngII (50). We have recently demonstrated a role of the SFO in the long term hypertensive effects of AngII as well (17). Once AngII is sensed at the SFO, it is of interest what downstream central sites may be further activated. It has been reported that many SFO neurons containing AT1 receptors project to the PVN (51), and is therefore logical that AngII activation in the SFO might be mediated and/or integrated at this downstream site.
Neuroanatomical studies have shown that the SFO sends axonal projections to the PVN and supraoptic nucleus (SON) (24, 25), via two main efferent pathways (25), either synapsing directly or relaying at the MnPO before reaching these hypothalamic nuclei. The present study was conducted to test the hypothesis that the SFO is a primary site of action of AngII which leads to downstream activation of Fos in the MnPO and/or PVN via central effects. As such, the results of the present study demonstrate that after SFO ablation, there is significant suppression of neuronal stimulation in response to IV AngII administration in the PVN. Rats were placed in two main groups: SFOx or sham. The SFO was removed to test if Fos expression in the downstream MnPO or PVN would be lessened in response to AngII. Animals were infused for 2 hours with one of three possible solutions: AngII (0.575 µg/kg/min), isotonic saline, or phenylephrine (10 µg/kg/min). Controls infused with phenylephrine were used to control for any pressure induced baroreceptor input/activation produced by the acute pressor effects of circulating AngII. Our results, (as seen in Figure 2) indicate diminished neuronal activation of the PVN in response to AngII infusion in SFOx animals, whereas Fos expression in the MnPO remained unchanged.
The novel results of the present study extend the findings of McKinley et al, in that while intravenous phenylephrine and AngII infusions both elicited Fos responses in neurons of the PVN, we further demonstrated a significant reduction of PVN neuronal Fos expression in animals with SFO ablations after AngII infusion, confirming the central effects of AngII at the SFO in this pathway. Interestingly, McKinley et al observed that Fos activation occurred with AngII and phenylephrine infusion in the PVN, as well as another CVO, the area postrema (AP), yet was diminished in the MnPO after phenylephrine treatment (18). While we observed less Fos expression in the MnPO of both SFOx-AngII as well as phenylephrine treated animals compared to sham-AngII, neither of these differences were statistically significant. Overall, these studies complement previous findings and further suggest a role of the SFO in relation to AngII induced Fos activation in the PVN. Additionally, these findings are in agreement with earlier studies using both lesion and electrophysiological recording methods, which indicated that the SFO was crucial in the central effects of AngII on PVN neuronal activity (51–53).
Baseline HR and MAP were similar in all 3 groups. Additionally, while MAP and HR changed significantly within groups in response to treatments of AngII or phenylephrine, the changes in these paramenters in response to these treatments were not different between groups (Table 1). The change in pressure in the phenylephrine group was similar to the AngII treated groups and therefore used as a control for the pressor effects of AngII apart from its direct central effects. Furthermore, SFOx had no effect on the acute cardiovascular responses to AngII at this dose.
In contrast to Rowland et al (21), we found that SFO lesions did not completely abolish neuronal activity in the MnPO or OVLT (not shown) and that phenylephrine infusions, dosed at levels that mimicked the blood pressure and heart rate effects of AngII treatment, caused some Fos-like activity in all regions we studied except for the SFO itself. Reasons for divergent results between our work and that of Rowland et al are not clear, but possibilities include activation from neuronal pathways originating from other CVOs, including the area postrema (AP), which has been previously and clearly shown to be significant in the central hypertensive effect of AngII (54). While Rowland et al was unable to detect Fos-like activity in the AP, we and others (18) have observed activity in this region that could account for these differences. Additionally, a notable difference was that a lower dose of AngII was used in our experiments that could have altered activity seen in this hindbrain region. Indeed, the AP was the first CVO thought to mediate acute central actions of AngII with dose dependant effects (44). While Fink et al (54) previously showed that the AP was necessary for the chronic hypertensive actions of AngII in a high salt model, recent results from our laboratory appear to diminish the role of the AP as a critical mediator of the chronic hypertensive effect of intravenous AngII during normal sodium intake (55).
Lastly, it should be noted that Fos activation in saline treated control animals was observed in all regions we monitored, however to a much lesser extent than other treatment groups. This is actually in agreement with previous studies where it was also noted that isotonic saline causes Fos activation in the PVN (20). Moreover, in that study they noted there was a significant difference between the saline infused sinoaortic denervated (SAD) animals and the AngII infused SAD animals, indicating AngII's greater effect than saline on Fos expression, regardless of baroreceptor input.
In the present study, we have demonstrated AngII induced Fos activation in the hypothalamus and most notably the PVN. These results validate previous work by others examining this pathway (18,19), and more importantly report the observation of reduced Fos expression in the PVN in animals with lesions of the SFO in response to AngII treatment. SFOx had no effect on Fos expression in the MnPO of AngII treated animals. These results provide further support for the role of neurons of the SFO projecting to and providing input to the PVN in response to and modulating the central effects of increased circulating AngII.
Acknowledgements
This study was supported by National Heart, Lung, and Blood Institute Grant RO1 HL-072180. The authors wish to thank Dr. Trasida Ployngam for assistance in the statistical analyses used throughout these studies.
References
- 1.Cerasola G, Cottone S, D’Ignoto G, et al. Effects of enalapril maleate on blood pressure, renin- angiotensin- aldosterone system, and peripheral sympathetic activity in essential hypertension. Clin Ther. 1987;9:390–399. [PubMed] [Google Scholar]
- 2.Matsukawa T, Gotoh E, Minamisawa K, et al. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. Am J Physiol. 1991;261:R690–R696. doi: 10.1152/ajpregu.1991.261.3.R690. [DOI] [PubMed] [Google Scholar]
- 3.Ota M, Crofton JT, Liu H, Festavan G, Share L. Increased plasma osmolality stimulates peripheral and central vasopressin release in male and female rats. Am J Physiol. 1994;267:R923–R928. doi: 10.1152/ajpregu.1994.267.4.R923. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt H, Schmitt H. Increased activity in sympathetic nerves induced by angiotensin. Rev Can Biol. 1968;27:255–257. [PubMed] [Google Scholar]
- 5.Scrogin KE, Grygielko ET, Brooks VL. Osmolality: A physiological long-term regulator of lumbar sympathetic nerve activity and arterial pressure. Am J Physiol. 1999;276:R1579–R1586. doi: 10.1152/ajpregu.1999.276.6.R1579. [DOI] [PubMed] [Google Scholar]
- 6.Tobey JC, Fry HK, Mizejewski CS, Fink GD, Weaver LC. Differential sympathetic responses initiated by angiotensin and sodium chloride. Am J Physiol. 1983;245:R60–R68. doi: 10.1152/ajpregu.1983.245.1.R60. [DOI] [PubMed] [Google Scholar]
- 7.Weekley LB. Angiotensin-II acts centrally to alter renal sympathetic nerve activity and the intrarenal renin-angiotensin system. Hypertension. 1987;9:355–361. doi: 10.1093/cvr/25.5.353. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson AV, Donevan SD, Papas S, Smith PM. Circumventricular structures: CNS sensors of circulating peptides and autonomic control centres. Endocrinol Exp. 1990;24:19–27. [PubMed] [Google Scholar]
- 9.Simpson JB. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology. 1981;32:248–256. doi: 10.1159/000123167. [DOI] [PubMed] [Google Scholar]
- 10.McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ. Interaction of circulating hormones with the brain: The roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol Suppl. 1998;25:S61–S67. doi: 10.1111/j.1440-1681.1998.tb02303.x. [DOI] [PubMed] [Google Scholar]
- 11.Lenkei Z, Corvol P, Llorens-Cortes C. The angiotensin receptor subtype AT1A predominates in rat forebrain areas involved in blood pressure, body fluid homeostasis and neuroendocrine control. Brain Res. 1995;30:53–60. doi: 10.1016/0169-328x(94)00272-g. [DOI] [PubMed] [Google Scholar]
- 12.Mangiapane ML, Simpson JB. Subfornical organ: fore-brain site of pressor and drinking actions of angiotensin II. American Journal of Physiology. 1980;239:R382–R389. doi: 10.1152/ajpregu.1980.239.5.R382. [DOI] [PubMed] [Google Scholar]
- 13.Simpson JB, Epstein AN, Camardo JS. Localization of receptors for dipsogenic action of angiotensin II in subfornical organ of rat. J. Comp. Physiol. Psychol. 1978;92:581–608. doi: 10.1037/h0077503. [DOI] [PubMed] [Google Scholar]
- 14.Mangiapane ML, Simpson JB. Subfornical organ lesions reduce the pressor effect of intravenous angiotensin. Neuroendocrinology. 1980;31:380–384. doi: 10.1159/000123107. [DOI] [PubMed] [Google Scholar]
- 15.Collister JP, Hendel MD. Role of the subfornical organ in the chronic hypotensive response to losartan in normal rats. Hypertension. 2003;41:576–582. doi: 10.1161/01.HYP.0000058002.67558.6E. [DOI] [PubMed] [Google Scholar]
- 16.Mangiapane ML, Simpson JB. Subfornical organ lesions reduce the pressor effect of systemic angiotensin II. Neuroendocrinology. 1980;31:380–384. doi: 10.1159/000123107. [DOI] [PubMed] [Google Scholar]
- 17.Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2005;288:H680–H685. doi: 10.1152/ajpheart.00823.2004. [DOI] [PubMed] [Google Scholar]
- 18.McKinley MJ, Badoer E, Oldfield BJ. Intravenous angiotensin II induces fos-immunoreactivity in circumventricular organs of the lamina terminalis. Brain Res. 1992;594:295–300. doi: 10.1016/0006-8993(92)91138-5. [DOI] [PubMed] [Google Scholar]
- 19.Oldfield BJ, Badoer E, Hards DK, McKinley MJ. Fos production in retrogradely labeled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience. 1994;60:255–262. doi: 10.1016/0306-4522(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 20.Potts PD, Hirooka Y, Dampney RA. Activation of brain neurons by circulating angiotensin II: Direct effects and baroreceptor-mediated secondary effects. Neuroscience. 1999;90:581–594. doi: 10.1016/s0306-4522(98)00572-7. [DOI] [PubMed] [Google Scholar]
- 21.Rowland NE, Li BH, Rozelle AK, Fregly MJ, Garcia M, Smith GC. Localization of changes in immediate early genes in brain in relation to hydromineral balance: Intravenous angiotensin II. Brain Res Bull. 1994;33:427–436. doi: 10.1016/0361-9230(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 22.Ciriello J, Gutman MB. Functional identification of central pressor pathways originating in the subfornical organ. Can J Physiol Pharmacol. 1991;69:1035–1045. doi: 10.1139/y91-154. [DOI] [PubMed] [Google Scholar]
- 23.Gutman MB, Ciriello J, Mogenson GJ. Electrophysiological identification of forebrain connections of the subfornical organ. Brain Res. 1986;382:119–128. doi: 10.1016/0006-8993(86)90118-6. [DOI] [PubMed] [Google Scholar]
- 24.Lind RW, Van Hoesen GW, Johnson AK. An HRP study of the connections of the subfornical organ of the rat. J Comp Neurol. 1982;210:265–277. doi: 10.1002/cne.902100306. [DOI] [PubMed] [Google Scholar]
- 25.Miselis RR. The efferent projections of the subfornical organ of the rat: A circumventricular organ within a neural network subserving water balance. Brain Res. 1981;230:1–23. doi: 10.1016/0006-8993(81)90388-7. [DOI] [PubMed] [Google Scholar]
- 26.Stocker SD, Toney GM. Median Preoptic neurons projecting to the hypothalamic paraventricular nucleus responds to osmotic, circulating angiotensin II and baroreceptor input in the rat. J Physiol. 2005;568:599–615. doi: 10.1113/jphysiol.2005.094425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind RW, Johnson AK. Subfornical organ-median preoptic connections and drinking and pressor responses to angiotensin II. J Neurosci. 1982;2:1043–1051. doi: 10.1523/JNEUROSCI.02-08-01043.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangiapane ML, Thrasher TN, Keil LC, Simpson JB, Ganong WF. Deficits in drinking and vasopressin secretion after lesions of the nucleus medianus. Neuroendocrinology. 1983;37:73–77. doi: 10.1159/000123518. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham JT, Beltz T, Johnson RF, Johnson AK. The effects of ibotenate lesions of the median preoptic nucleus on experimentally-induced and circadian drinking behavior in rats. Brain Res. 1992;580:325–330. doi: 10.1016/0006-8993(92)90961-8. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham JT, Sullivan MJ, Edwards GL, Farinpour R, Beltz TG, Johnson AK. Dissociation of experimentally induced drinking behavior by ibotenate injection into the median preoptic nucleus. Brain Res. 1991;554:153–158. doi: 10.1016/0006-8993(91)90183-v. [DOI] [PubMed] [Google Scholar]
- 31.Gardiner TW, Stricker EM. Impaired drinking responses of rats with lesions of nucleus medianus: circadian dependence. Am J Physiol. 1985;248:R224–R230. doi: 10.1152/ajpregu.1985.248.2.R224. [DOI] [PubMed] [Google Scholar]
- 32.Ployngam TP, Collister JP. An intact median preoptic nucleus is necessary for chronic angiotensin II-induced hypertension. Brain Res. 2007;1162:69–75. doi: 10.1016/j.brainres.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, Herbert J. Regional suppression by lesions in the anterior third ventricle of c-fos expression induced by either angiotensin II or hypertonic saline. Neuroscience. 1995;67:135–147. doi: 10.1016/0306-4522(95)00050-s. [DOI] [PubMed] [Google Scholar]
- 34.Ployngam T, Collister JP. Role of the Median Prepotic Nucleus in Chronic Angiotensin II-Induced Hypertension. Brain Res. 2008;1238:75–84. doi: 10.1016/j.brainres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Gutman MB, Ciriello J, Mogenson GJ. Effects of plasma angiotensin II and hypernatremia on subfornical organ neurons. American Journal of Physiology. 1988;254:R746–R754. doi: 10.1152/ajpregu.1988.254.5.R746. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka J, Kaba H, Saito H, Seto K. Subfornical organ neurons with efferent projections to the hypothalamic paraventricular nucleus: an electrophysiological study in the rat. Brain Research. 1985;346:151–154. doi: 10.1016/0006-8993(85)91106-0. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson AV, Renaud LP. Hypothalamic paraventricular nucleus lesions decrease pressor responses to subfornical organ stimulation. Brain Research. 1984;305:361–364. doi: 10.1016/0006-8993(84)90443-8. [DOI] [PubMed] [Google Scholar]
- 38.Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. American Journal of Physiology. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- 39.Ku YH, Jia YF, Chang YZ. Mechanisms underlying pressor response of subfornical organ to angiotensin II. Peptides. 1999;20:171–176. doi: 10.1016/s0196-9781(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 40.Dragunow M, Faull R. The use of c-fos as metabolic marker in neuronal pathway tracing. J. Neurosci. Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson JT, Millhorn DE. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anesthetized rats. Brain Res. 1991;567:11–24. doi: 10.1016/0006-8993(91)91430-9. [DOI] [PubMed] [Google Scholar]
- 42.Felix D, Schlegel W. Angiotensin receptive neurones in the subfornical organ. Structure-activity relations. Brain Res. 1978;149:107–116. doi: 10.1016/0006-8993(78)90591-7. [DOI] [PubMed] [Google Scholar]
- 43.Oldfield BJ, Bicknell RJ, McAllen RM, Weisinger RS, McKinley MJ. Intravenous hyertonic saline induces fos immunoreactivity in neurons throughout the lamina terminalis. Brain Res. 1991;561:151–156. doi: 10.1016/0006-8993(91)90760-s. [DOI] [PubMed] [Google Scholar]
- 44.Gross PM, Weindl A. Peering through the windows of the brain. J Cereb Blood Flow Metabol. 1987;7:662–671. doi: 10.1038/jcbfm.1987.120. [DOI] [PubMed] [Google Scholar]
- 45.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka J, Saito H, Kaba H, Seto K. Subfornical organ neurons act to enhance the activity of paraventricular vasopressin neurons in response to intravenous angiotensin II. Neurosci Res. 1987;4:424–427. doi: 10.1016/0168-0102(87)90008-3. [DOI] [PubMed] [Google Scholar]
- 47.Gutman MB, Ciriello J, Mogenson GJ. Effects of plasma angiotensin II and hypernatremia on subfornical organ neurons. Am J Physiol. 1988 May;254:R746–R754. doi: 10.1152/ajpregu.1988.254.5.R746. [DOI] [PubMed] [Google Scholar]
- 48.Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am. J. Physiol. 1991;261:R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- 49.Shigematsu K, Saavedra JM, Plunkett LM, Kurihara M, Correa FMA. Angiotensin II binding site in the anteroventral-third ventricle (AV3V) area and related structures of the rat brain. Neurosci. Lett. 1986;67:37–41. doi: 10.1016/0304-3940(86)90204-1. [DOI] [PubMed] [Google Scholar]
- 50.Mangiapane ML, Simpson JB. Subfornical organ: forebrain site of pressor and dipsogenic action of angiotensin II. Am J Physiol. 1980;239(8):R382–R389. doi: 10.1152/ajpregu.1980.239.5.R382. [DOI] [PubMed] [Google Scholar]
- 51.Bain JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am. J. Physiol. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- 52.Ferguson A. Systemic angiotensin acts at the subfornical organ to control the activity of paraventricular nucleus neurons with identified projections to the median eminence. Neuroendocrinology. 1988;47:489–497. doi: 10.1159/000124960. [DOI] [PubMed] [Google Scholar]
- 53.Ferguson AV. Neurophysiological analysis of mechanisms for subfornical organ and area postrema involvement in autonomic control. Progress in brain research. 1992;91:413–421. doi: 10.1016/s0079-6123(08)62361-4. [DOI] [PubMed] [Google Scholar]
- 54.Fink GD, Bruner CA, Mangiapane ML. Area postrema is critical for angiotensin-induced hypertension in rats. Hypertension. 1987;9:355–361. doi: 10.1161/01.hyp.9.4.355. [DOI] [PubMed] [Google Scholar]
- 55.Nahey D, Collister JP. AngII-induced hypertension and the role of the area postrema during normal and increased dietary salt. Am J Physiol Heart Circ Physiol. 2007;292(1):H694–H700. doi: 10.1152/ajpheart.00998.2005. [DOI] [PubMed] [Google Scholar]