Abstract
Erythropoietin (EPO) was hypothesized to mitigate reperfusion injury, in part via mobilization of endothelial progenitor cells (EPCs). The REVEAL trial found no reduction in infarct size with a single dose of EPO (60,000 U) in patients with ST-segment elevation myocardial infarction. In a substudy, we aimed to determine the feasibility of cryopreserving and centrally analyzing EPC levels to assess the relationship between EPC numbers, EPO administration, and infarct size. As a prespecified substudy, mononuclear cells were locally cryopreserved before as well as 24 and 48–72 h after primary percutaneous coronary intervention. EPC samples were collected in 163 of 222 enrolled patients. At least one sample was obtained from 125 patients, and all three time points were available in 83 patients. There were no significant differences in the absolute EPC numbers over time or between EPO- and placebo-treated patients; however, there was a trend toward a greater increase in EPC levels from 24 to 48–72 h postintervention in patients receiving ≥30,000 U of EPO (P = 0.099 for CD133+ cells, 0.049 for CD34+ cells, 0.099 for ALDHbr cells). EPC numbers at baseline were inversely related to infarct size (P = 0.03 for CD133+ cells, 0.006 for CD34+ cells). Local whole cell cryopreservation and central EPC analysis in the context of a multicenter randomized trial is feasible but challenging. High-dose (≥30,000 U) EPO may mobilize EPCs at 48–72 h, and baseline EPC levels may be inversely associated with infarct size.
Keywords: Erythropoietin, Endothelial progenitor cells, Myocardial infarction, Cryopreservation
Extensive preclinical literature suggests a benefit of erythropoietin (EPO) on ischemia reperfusion injury [1, 2]. Although EPO may theoretically limit myocardial injury based on anti-apoptotic and direct angiogenic signaling, EPO is also a direct mobilizer of endothelial progenitor cells (EPCs) [3, 4], which in preclinical models contribute directly to EPO-induced angiogenesis [5] and may be related to degree of myocardial salvage [6].
The Reduction of Infarct Expansion and Ventricular Remodeling With Erythropoietin After Large Myocardial Infarction (REVEAL, ClinicalTrials.gov identifier NCT00378352) trial evaluated the effectiveness of EPO to minimize infarct size in patients with ST-segment elevation myocardial infarction (STEMI) [7, 8]. Given the association of EPO-induced EPC mobilization and myocardial salvage [6], a defined secondary endpoint in REVEAL was to explore the feasibility of central EPC analysis in a multicenter acute STEMI clinical trial with the goal of assessing the relationship between EPCs, EPO dosing, and infarct size.
Methods
The design of the REVEAL study has been previously reported [7]. The investigation conforms with the principles outlined in the Declaration of Helsinki, and the protocol was approved by independent institutional review boards at each site.
All patients were required to present with STEMI, with symptom onset within 12 h. After successful primary or rescue percutaneous coronary intervention (PCI), patients were treated with a single bolus of EPO within 4 h. REVEAL included EPO dose escalation safety phases (testing doses of 15000, 30000 and 60000 U), as well as an efficacy phase in which a 60,000 U dose was tested. Patients underwent collection of 8–10 ml of blood before EPO administration, at 24 h (±12 h), and at either 48 or 72 h (±12 h) post-EPO administration.
Sites were provided with kits containing all tubes, reagents, and equipment required for EPC cryopreservation. Blood was collected into cell preparation tubes (Becton, Dickinson and Co., Franklin Lakes, NJ, USA) and centrifuged within 4 h (1,800×g for 20 min). Plasma was removed and the buffy coat isolated using a pipette. Cells were washed twice with phosphate-buffered saline containing 1 % bovine serum albumin, resuspended in 3 ml Dulbecco’s modified Eagle’s medium containing 20 % fetal bovine serum and 10 % final (vol/vol) of dimethyl sulfoxide, and frozen in cryovials at <−70 °C.
EPC analysis
Cryopreserved specimens were thawed in a 37 °C controlled temperature bath, and the numbers of intact cells were estimated under microscopic analysis. If no intact cells were observed, EPC enumeration was not performed after several experiments demonstrated that microscopic visualization could accurately predict lack of viable cells on flow cytometry.
EPC number was assayed using flow cytometry based on cell surface expression of CD133 and CD34, and on the basis of aldehyde dehydrogenase (ALDH) activity, a fundamental property of multiple stem cell types [9, 10].
Based on previous work suggesting that analysis of ALDHbr EPCs was more reliable and tightly associated with vascular injury [10, 11], ALDH activity was used as the preferred method for EPC enumeration if sample quality precluded both analyses. ALDHbr cells were identified using Aldecount, as previously described [10]. Thawed mononuclear cells (MNCs) were washed and incubated in Aldecount tubes, and a portion of the sample was transferred to a tube containing diethylaminobenzaldehyde, a potent inhibitor of ALDH activity. Cells were isolated, washed, and kept on ice until completion of flow cytometry.
When sufficient MNCs were present, EPCs were also identified after incubation with CD133-APC (Miltenyi Biotec, Cambridge, MA, USA) and CD34-FITC (Becton Dickinson) antibodies. Nonspecific binding was inhibited using FcR reagent (Becton Dickinson).
Cells were sorted on a FACSCalibur machine (Becton Dickinson) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). All analyses were completed prior to unblinding of treatment assignment. All EPCs were expressed as a percentage of MNCs.
Statistical analysis
EPCs were measured at four potential time points: baseline, 24 h, and either 48 or 72 h. For analysis purposes, the results from the 48- and 72-h time points were combined. The analyses were exploratory in nature, but a two-sided alpha level of 0.05 was considered statistically significant. P values were not adjusted for multiple comparisons. All analyses were performed in SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Summary statistics on baseline demographics, laboratory values, and medication use were produced. The raw EPC data were first inspected by reviewing the distributions, crude summary statistics, and spaghetti plots for all subjects. A right skew was detected, and a logarithmic base 2 transformation was employed to achieve a more normal distribution for analysis. The results were back transformed for interpretability on the raw scale. REVEAL patients who had an evaluable EPC result for at least two of the three time points were included in the analysis.
Associations between EPC values at baseline and patient characteristics were evaluated using the Wilcoxon–Kruskal–Wallis test for categorical variables and the Spearman rank correlation coefficient for continuous variables. The association between baseline EPC levels and cardiac magnetic resonance (CMR) endpoints were evaluated using the Spearman rank correlation coefficient.
The absolute changes in EPC levels from baseline to 24 h, from baseline to 48/72 h, and from 24 to 48/72 h were calculated by treatment group. Between-group comparisons for each EPC type were performed with analysis of variance.
The change in EPC levels over all three time points was evaluated using a mixed model, and the least square means were produced by time point for each treatment group and graphically displayed.
Results
Sample flow
REVEAL enrolled and treated a total of 222 patients at 22 sites, of whom 47 patients were from six sites not participating in the EPC substudy, leaving 175 eligible patients (Fig. 1). Of these, at least one EPC sample was obtained in 163 patients (477 total samples).
Fig. 1.
Study flow. EPC endothelial progenitor cell, ALDH aldehyde dehydrogenase
The EPC core laboratory received 161, 159, and 152 baseline, 24-h, and 48/72-h samples, respectively. Analysis of ALDHbr cells was possible in 318 samples (93 from baseline, 114 from 24 h, and 111 from 48/72 h), while CD133/CD34-expressing cells were enumerated in 182 samples (58 from baseline, 63 from 24 h, and 61 from 48/72 h). ALDHbr and CD133/34 were analyzed in 15 and 14 patients, respectively, treated with 15,000 U of EPO; in nine and eight patients, respectively, treated with 30,000 U of EPO; in 41 and 17 patients, respectively, receiving 60,000 U; and in 60 and 30 patients, respectively, randomized to placebo. EPC analysis was not performed uniformly across the trial, due to the activation of high-enrolling sites that did not participate in the EPC study during late stages of enrollment.
Eight of the 12 sites that attempted to obtain EPCs on all patients provided suitable samples in over 50 % of cases. Of the 163 participants’ EPC samples collected, 54 were of sufficient quality to assess EPCs based on both cell surface markers and ALDH activity at a minimum of two time points.
Baseline characteristics
The baseline demographics and medication use of the patients who contributed samples to the EPC substudy are shown in Tables 1 and 2. These demographics are similar to those observed in the overall REVEAL study [8].
Table 1.
Patient demographics and laboratory values
| Variable | Median | Range |
|---|---|---|
| Age (years) | 57.0 | 31–90 |
| Weight (kg) | 88.5 | 58–142 |
| BMI (kg/m2) | 28.5 | 21–55 |
| Infarct size (% LV mass) | 12.8 | 0–53.6 |
| Baseline CK-MB (ratio to ULN) | 10.2 | 0.2–119.3 |
| Peak CK-MB (ratio to ULN) | 23.7 | 0.8–157.3 |
| Baseline troponin I (ng/ml) | 9.5 | 0.01–484.9 |
| Baseline troponin T (ng/ml) | 0.8 | 0.01–25 |
| Hematocrit (%) | 39.8 | 30.3–51.2 |
| WBC count (109/L) | 10.9 | 4.6–24.3 |
| hs-CRP (mg/dl) | 0.3 | 0.01–13.1 |
BMI body mass index, CK-MB creatine kinase-MB, hs-CRP high-sensitivity C-reactive protein, LV left ventricular, ULN upper limit of normal, WBC white blood cell
Table 2.
Patient demographics
| Characteristic | Frequency (%) |
|---|---|
| Male sex | 80.9 |
| Race | |
| White | 78.3 |
| Non-hispanic | 95.7 |
| Treatment assignment | |
| 15,000 U EPO | 11.3 |
| 30,000 U EPO | 7.8 |
| 60,000 U EPO | 35.7 |
| Placebo | 45.2 |
| Diabetes | 20.0 |
| Hypertension | 53.9 |
| Hyperlipidemia | 38.3 |
| Cancer (<5 years) | 1.7 |
| Tobacco use | |
| Never | 36.3 |
| Current | 41.6 |
| Previous | 22.1 |
EPO erythropoietin
Baseline EPC levels
We explored the association of EPC levels at baseline (prior to study intervention) with a variety of clinical factors, including baseline demographics, cardiac biomarker levels, and use of cardiac medications. Most of the patient characteristics were not associated with EPCs in any consistent manner, although a few associations are noteworthy (Table 3).
Table 3.
Association of baseline clinical factors with endothelial progenitor cells (EPCs)
| Variable | CD133 |
CD34 |
CD133/CD34 |
ALDHbr |
||||
|---|---|---|---|---|---|---|---|---|
| P | r | P | r | P | r | P | r | |
| Categorical | ||||||||
| Gender | 0.54 | 0.08 | 0.47 | 0.39 | ||||
| Diabetes | 0.84 | 0.20 | 0.12 | 0.32 | ||||
| Hypertension | 0.28 | 0.24 | 0.48 | 0.03a | ||||
| Hyperlipidemia | 0.06 | 0.44 | 0.34 | 0.37 | ||||
| Smoking | 0.40 | 0.34 | 0.19 | 0.20 | ||||
| LAD IRA | 0.52 | 0.39 | 0.52 | 0.03a | ||||
| Continuous | ||||||||
| Age | 0.81 | 0.66 | 0.62 | 0.45 | ||||
| Weight | 0.03 | 0.28 | 0.08 | 0.47 | 0.39 | |||
| BMI | 0.10 | 0.79 | 0.34 | 0.61 | ||||
| Infarct size | 0.05 | −0.28 | 0.006 | −0.39 | 0.03 | −0.32 | 0.20 | |
| Baseline CK-MB | 0.18 | 0.57 | 0.39 | 0.37 | ||||
| Peak CK-MB | 0.21 | 0.62 | 0.16 | 0.12 | ||||
| Baseline troponin I | 0.46 | 0.82 | 0.65 | 0.44 | ||||
| Baseline troponin T | 0.99 | 0.69 | 0.77 | 0.16 | ||||
| Hematocrit | 0.01 | −0.34 | 0.53 | 0.92 | 0.26 | |||
| WBC | 0.68 | 0.43 | 0.98 | 0.67 | ||||
| hs-CRP | 0.93 | 0.95 | 0.53 | 0.04 | −0.25 | |||
| Time from symptom onset | 0.77 | 0.62 | 0.59 | 0.39 | ||||
Correlation coefficients (or direction of association) is shown if the P value was <0.05 ALDHbr aldehyde dehydrogenase-bright, BMI body mass index, CK-MB creatine kinase-MB, hs-CRP high-sensitivity C-reactive protein, LAD IRA left anterior descending infarct-related artery, WBC white blood cell
Higher EPCs in patients with hypertension and non-LAD infarct size
CD133+ cells were correlated with baseline weight (r = 0.28, P = 0.03), and baseline hematocrit (r = −0.34, P = 0.01). There were higher levels of ALDHbr cells in patients with hypertension (P = 0.03), and in patients with non-left anterior descending coronary artery infarcts (P = 0.03). CD34+ cells were numerically higher in males (P = 0.08), while there was a trend toward higher CD133+ cells in patients with hyperlipidemia (P = 0.06). There was no relationship between EPCs and use of aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or lipid-lowering therapy on study entry.
EPC time course in acute myocardial infarction
To better understand the natural time course of circulating EPCs in the acute myocardial infarction setting, we assessed the levels of EPCs over the 48/72-h sampling period in placebo-treated patients (Fig. 2; Table 4). The baseline EPC samples were collected 6.4 (±3.0) h (median = 6.2) after the onset of symptoms. The 24- and 48/72-h samples were collected at 28.6 ± 4.8 (28.8) h and 55.2 ± 9.3 (53.5) h, respectively. We observed a trend, but no statistically significant differences, with EPC levels highest on presentation and a gradual decrease over time. This trend was observed consistently among all cell types analyzed.
Fig. 2.
Time course of endothelial progenitor cells in pooled placebo patients. Symbols represent means, and error bars demonstrate 95 % confidence intervals for the estimate
Table 4.
Mean endothelial progenitor cell levels over time in pooled placebo patients
| Baseline | At 24 h | At 48/72 h | |
|---|---|---|---|
| CD133+ | 0.144 | 0.124 | 0.122 |
| CD34+ | 0.312 | 0.259 | 0.259 |
| CD133+/CD34+ | 0.032 | 0.027 | 0.029 |
| ALDHbr | 0.066 | 0.042 | 0.042 |
ALDHbr aldehyde dehydrogenase-bright
Effect of EPO on change in EPC levels
We next assessed the change in EPC types by EPO treatment. We observed a drop in levels of all EPCs over the first 24 h. In contrast to what was observed in placebo patients, most cell types, especially with higher EPO doses, showed a rebound at the 48/72-h time point. While the absolute levels of EPCs were not significantly different between EPO- and placebo-treated patients, this trend was consistently observed for each of the EPC types investigated.
The primary prespecified EPC endpoint in the REVEAL trial was change in EPC numbers between follow-up time points and baseline in treated versus untreated patients. We calculated the absolute change from baseline to 24 h, from baseline to 48/72 h, and from 24 to 48/72 h (Table 5).
Table 5.
Changes in endothelial progenitor cell numbers (as a percentage of mononuclear cells) between individual time points based on comparison of pooled placebo subjects with those treated with 60,000 U of erythropoietin (EPO; top rows), any EPO (middle rows), and ≥30,000 U of EPO (bottom rows)
| Time point | CD133+ |
CD34+ |
CD133+/34+ |
ALDHbr |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPO | Pooled placebo |
P value | EPO | Pooledp lacebo |
P value | EPO | Pooled placebo |
P value | EPO | Pooled placebo |
P value | |
| 60,000 U | ||||||||||||
| BL to 24 h | −0.020 | −0.014 | 0.575 | −0.145 | −0.100 | 0.381 | −0.006 | −0.003 | 0.891 | −0.036 | −0.020 | 0.810 |
| BL to 48/72 h | 0.010 | −0.020 | 0.423 | −0.063 | −0.112 | 0.744 | −0.002 | −0.002 | 0.746 | −0.009 | −0.020 | 0.780 |
| 24 to 48/72 h | 0.030 | 0.000 | 0.234 | 0.092 | 0.011 | 0.400 | 0.000 | 0.003 | 0.761 | 0.008 | 0.002 | 0.219 |
| Pooled active (any EPO) | ||||||||||||
| BL to 24 h | −0.016 | −0.014 | 0.626 | −0.088 | −0.100 | 0.542 | −0.003 | −0.003 | 0.747 | −0.034 | −0.020 | 0.869 |
| BL to 48/72 h | 0.010 | −0.020 | 0.247 | −0.063 | −0.112 | 0.959 | 0.000 | −0.002 | 0.981 | −0.007 | −0.020 | 0.311 |
| 24 to 48/72 h | 0.011 | 0.000 | 0.792 | 0.056 | 0.011 | 0.371 | 0.007 | 0.003 | 0.588 | 0.012 | 0.002 | 0.199 |
| 30,000 and 60,000 U | ||||||||||||
| BL to 24 h | −0.020 | −0.015 | 0.762 | −0.101 | −0.100 | 0.360 | −0.005 | −0.003 | 0.928 | −0.032 | −0.021 | 0.901 |
| BL to 48/72 h | 0.013 | −0.020 | 0.122 | −0.038 | −0.112 | 0.589 | 0.010 | −0.002 | 0.594 | −0.007 | −0.021 | 0.304 |
| 24 to 48/72 h | 0.030 | 0.000 | 0.099 | 0.092 | 0.011 | 0.049 | 0.011 | 0.003 | 0.437 | 0.012 | 0.002 | 0.099 |
ALDHbr aldehyde dehydrogenase-bright, BL baseline
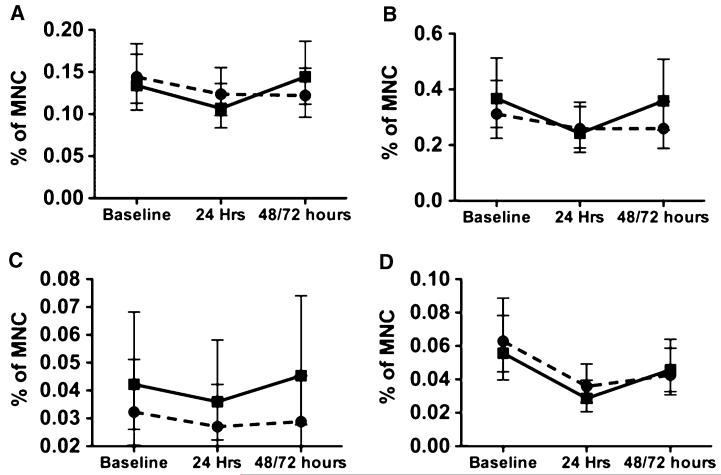
We initially defined treated patients using two different approaches: (1) a pooled active group including all patients who received EPO at any dose (15000, 30000, or 60000 U), and (2) patients who received the highest acceptable dose of EPO (60,000 U). There was no statistically significant difference between treated and untreated patients in change of EPC numbers between any time points. However, in contrast to placebo patients, the numbers of EPCs increased at the 48-h time point in the high-dose (≥30,000 U) EPO-treated patients across each of the independent cell types (P = 0.099 for CD133+, 0.049 for CD34+, and 0.099 for ALDHbr cells) (Fig. 3; Table 5).
Fig. 3.
Time course of endothelial progenitor cells (EPCs) in placebo and pooled (30,000 and 60,000 U) erythropoietin (EPO) groups. Solid line with squares indicates high-dose EPO (≥30,000 U) groups. Dotted line with circles indicates pooled placebo patients. a CD133+ cells. b CD34+ cells. c CD133+/CD34+ cells. d aldehyde dehydrogenase-bright cells. Symbols represent means, and error bars demonstrate 95 % confidence intervals for the estimate
Correlation of baseline EPC levels and primary REVEAL endpoints
The primary endpoint of the REVEAL study was CMR-assessed infarct size (expressed as percentage of left ventricular [LV] mass) at 2–6 days after study medication. We assessed the association of baseline EPC levels with this and other CMR findings. Baseline levels of all EPCs defined by cell surface markers (CD133, CD34, CD133+/CD34) were correlated inversely with the primary endpoint of infarct size at 48–144 h (r = −0.28, P = 0.05; r = −0.39, P = 0.006; and r = −0.32, P = 0.03, respectively), while ALDHbr cells showed a similar trend (r = −0.15, P = 0.20).
Baseline EPC levels (CD133+, CD34+, and CD133+/CD34+ cells) were also correlated with CMR-determined infarct size at 3 months (CD133+: r = −0.38, P = 0.01; CD34+: r = −0.30, P < 0.05; and CD133+/CD34+: r = −0.37, P = 0.01). In addition, remodeling assessed on 3-month LV volumes, using the baseline values as covariates, also demonstrated associations with baseline CD133+/CD34+ cells, such that higher EPC numbers were associated with lower volumes (r = −0.37, P = 0.01). While correlation of other EPC types did not reach statistical significance, all EPC types correlated negatively with indices of remodeling (higher EPCs were associated with lower volumes).
Discussion
The REVEAL EPC substudy demonstrated that although local cryopreservation of intact cellular specimens for subsequent flow cytometric analysis proved challenging, a majority of sites were able to proffer usable specimens in over 50 % of cases. In cases where analysis was possible, there were limited correlations of baseline EPC levels with clinical factors; however, high doses (>30,000 U) of EPO may be associated with EPC mobilization, and baseline EPC levels were inversely related with infarct size, the primary REVEAL endpoint, as well as indices of left ventricular remodeling. These findings have the following implications: (1) central EPC analysis is feasible, but the process by which EPCs are collected in the context of a multicenter clinical trial involving acutely ill patients needs to be refined; (2) EPC mobilization is a potential target of therapy in patients with large STEMI; and (3) any pleiotropic effect of EPO that is manifest through EPC number and function remains to be confirmed in the clinical setting, as the REVEAL trial demonstrated no reduction in infarct size with single-dose EPO administration.
EPC collection and processing
EPCs are commonly assessed locally within a short time frame of blood draw. Any degradation in sample quality can significantly affect this analysis, as expression of cell surface markers may change as cells undergo injury.
We considered a variety of approaches to EPC analysis in this trial, which would enroll acutely ill patients during off-hours, including weekends. As most sites do not have the facility or personnel to perform local EPC analysis, implementation of a central laboratory was deemed necessary.
Two approaches to central analysis were considered: (1) expedited shipment of whole blood to the core laboratory or (2) local cryopreservation of MNCs for shipment and batch analysis.
Shipment of whole blood samples might minimize local requirements for sample processing that required additional training; however, it could incur a delay of up to 96 h between sample acquisition and arrival at the core laboratory for analysis, and require shipment of samples on 3 consecutive days for most enrolled patients. In addition, whole blood shipment is subject to significant variability in sample viability [12]. We did not test what such a delay would do to the reliability of EPC analysis, but we have observed significant degradation of cell quality 24 h after sample acquisition.
Based on these concerns, we developed a technique that would allow sites to obtain and cryopreserve samples with provided equipment and solutions [11]. We demonstrated good correlation between EPC numbers in fresh and processed samples based on both cell surface marker expression (r = 0.59, P < 0.001) or ALDH activity (r = 0.90, P < 0.001) [11]. While easily implemented in experience laboratories, this technique proved challenging in a multicenter clinical trial; however, most sites were able to produce samples in a majority of patients (66.7 %), suggesting that with further refinement, such analysis may be possible.
Levels of EPCs during acute STEMI
Although levels of EPCs in acute infarction have been the subject of considerable interest, few of these studies monitored EPCs over time. While select smaller studies suggest that EPCs are maximally mobilized either at 3–4 [13] or 7 [14] days postinfarction, our observations corroborate a majority of observations which find that EPC levels peak on presentation and fall in the days following myocardial infarction [15-18]. This rapid EPC mobilization post-ischemia mirrors what is observed after bypass surgery, where EPC sampling can occur before and after the ischemic insult [19].
During study planning, little was known about the time course of EPC mobilization in the period surrounding acute infarction, or the time course for EPC mobilization after EPO administration. We elected to sample EPCs at baseline and at 24 and 72 h; however, due to changing practice patterns, many patients were being discharged before the 72-h time point, and a decision was made to change the final collection time to 48 h.
We analyzed the association of EPC levels at baseline, drawn after PCI and a mean of 6.4 h after symptom onset, with a variety of clinical factors and found few associations. However, we observed a consistent association of multiple EPCs with smaller infarct size at 48–144 h. Given the number of associations tested, the possibility of type I error must be considered. Nonetheless, a similar association was observed previously [20], and consideration should be given to the fact that inadequate acute stem cell mobilization may adversely affect recovery after myocardial injury. In addition, multiple EPC types were significantly correlated with indices of remodeling and infarct size at 3 months, findings that have also been reported in smaller patient cohorts [20]. The consistency of these results both across multiple EPCs assessed as well as with previous studies suggests that EPC mobilization may be a key determinant of myocardial salvage and remodeling post-STEMI, and that developing strategies which enhance EPC mobilization may be of clinical interest.
Various factors may have contributed to the lack of association of EPC levels with baseline clinical factors. First, degree of acute injury, variability in time to presentation, territory at risk, acute ischemic burden, and other factors may lead to variability in EPC mobilization that exceeds small differences due to chronic baseline conditions. Second, variability in the assay on cryopreserved specimens may be greater than that observed between patients [11]. Third, associations reported in the literature may represent type I error in studies in which many assessments are performed.
Effect of EPO on circulating EPC numbers
While we did not observe statistically significant differences in the change in EPC levels between various time points, there are consistent trends in multiple cell types toward an increase in EPC levels between 24 and 48/72 h in high-dose EPO-treated patients when EPC levels in the placebo patients continued to fall. The consistency across EPC types and association with EPO dose lend credence to this observation.
The lack of a stronger association between EPO administration and change in EPC levels may be due to experimental limitations discussed previously or the possibility that single bolus EPO may not maximally affect EPC levels. Indeed, the effect of EPO on erythropoiesis is not observed unless multiple doses of EPO are administered [21]. The doses used in preclinical studies were also significantly higher when normalized to weight, an observation that may explain some of the discrepancies observed between preclinical and clinical results [16]. These observations may also explain the lack of effect of EPO in REVEAL, which showed no benefit on infarct size or indices of LV remodeling over time [8].
Limitations
Limitations of this study include the uneven enrollment of patients into the EPC cohort, the lack of viability of a proportion of samples, and the possibility that variable handling at the sites and differing quality of the samples contributed to the variability observed in the study, affecting our ability to detect associations between EPCs and clinical factors. These limitations point to the difficulty in implementing procedures such as EPC sampling into multisite clinical protocols and may inform future attempts at flow cytometric analyses in clinical studies.
Conclusions
EPC collection in a multisite clinical study enrolling during off-hours is feasible but, as performed in the REVEAL study, poses logistical challenges. We observed no statistically significant differences in EPC levels over time or in the absolute levels of EPCs between EPO- and placebo-treated patients; however, high-dose EPO administration (≥30,000 U) may increase EPC levels from 24 h to later time points. Baseline EPC levels were associated with infarct size, suggesting that future research into the relationship between reparative capacity and injury in acute infarction is warranted.
Acknowledgments
The authors would like to thank Peter Hoffmann for thoughtful editorial and logistical support. The REVEAL study was supported by Intramural Research Program contract HHS-N-260-2005-00010-C from the National Institute on Aging, Bethesda, MD, USA. The first author was the recipient of a Duke Pepper Older Americans Independence Center Research Career Development Program in Aging Research Award (5P30AG028716), Durham, NC, USA.
Dr. Povsic has received grants from Baxter International, RegadoBiosciences, and Theragen. Dr. Najjar has received research funding from HeartWare. Dr. Hasselblad has received salary support via Grants from Eli Lilly and Medicure Inc. administered through Duke University. Dr. Heitner reported that New York Methodist Hospital received compensation for his work as a principal investigator for a study; also, he has received compensation for his expert testimony in an individual malpractice case, Grant support from the Empire Clinical Research Investigator Program, and compensation for serving on a steering committee for a trial. Dr. Raman has received Grant support from the National Institutes of Health and from Siemens Corporation. Dr. Barsness has been a consultant for Hoffmann-La Roche, Inc. and Baxter Healthcare Corporation, and has received research funding from Gilead Sciences, Inc. Dr. Patel has served as a board member for Genzyme’s advisory board and Bayer Healthcare, and as a consultant for Ikaria. Dr. Kim was an inventor on a U.S. patent for Delayed Enhancement MRI, which is owned by Northwestern University. Dr. Harrington’s complete listing of disclosure information is available at https://www.dcri.org/about-us/conflict-of-interest/COI-Harrington_2012.pdf. Dr. Rao received research funding from Novartis, Cordis Corporation, and Ikaria; and was a consultant for Sanofi-Aventis, Bristol-Meyers Squibb, Astra-Zeneca, Daiichi Sankyo-Lilly, and Terumo USA.
Footnotes
Conflict of interest The other authors report no disclosures.
Contributor Information
Thomas J. Povsic, Duke Clinical Research Institute, Duke University Medical Center, Box 103208, Durham, NC 27710, USA; Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA; Durham Veterans Affairs Medical Center, Durham, NC, USA
Samer S. Najjar, MedStar Health Research Institute, Washington, DC, USA; Intramural Research Program, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA
Kristi Prather, Duke Clinical Research Institute, Duke University Medical Center, Box 103208, Durham, NC 27710, USA.
Jiying Zhou, Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA.
Stacie D. Adams, Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA
Katherine L. Zavodni, Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA
Francine Kelly, Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA.
Laura G. Melton, Duke Clinical Research Institute, Duke University Medical Center, Box 103208, Durham, NC 27710, USA
Vic Hasselblad, Duke Clinical Research Institute, Duke University Medical Center, Box 103208, Durham, NC 27710, USA.
John F. Heitner, New York Methodist Hospital, Brooklyn, NY, USA
Subha V. Raman, Division of Cardiovascular Medicine, Ohio State University, Columbus, OH, USA
Gregory W. Barsness, Mayo Clinic, Rochester, MN, USA
Manesh R. Patel, Duke Clinical Research Institute, Duke University Medical Center, Box 103208, Durham, NC 27710, USA; Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA
Raymond J. Kim, Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA
Edward G. Lakatta, Intramural Research Program, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA
Robert A. Harrington, Duke Clinical Research Institute, Duke University Medical Center, Box 103208, Durham, NC 27710, USA; Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA
Sunil V. Rao, Duke Clinical Research Institute, Duke University Medical Center, Box 103208, Durham, NC 27710, USA; Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA; Durham Veterans Affairs Medical Center, Durham, NC, USA
References
- 1.Moon C, Krawczyk M, Paik D, Lakatta E, Talan M. Cardioprotection by recombinant human erythropoietin following acute experimental myocardial infarction: dose response and therapeutic window. Cardiovasc Drugs Ther. 2005;19:243–250. doi: 10.1007/s10557-005-3189-6. [DOI] [PubMed] [Google Scholar]
- 2.Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, Arcasoy MO. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J. 2004;18:1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- 3.Bahlmann FH, de Groot K, Spandau J-M, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, Fliser D. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 4.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 5.Westenbrink BD, Lipsic E, van der Meer P, van der Harst P, Oeseburg H, Du Marchie Sarvaas GJ, Koster J, Voors AA, van Veldhuisen DJ, van Gilst WH, Schoemaker RG. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28:2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- 6.Prunier F, Pfister O, Hadri L, Liang L, del Monte F, Liao R, Hajjar RJ. Delayed erythropoietin therapy reduces post-MI cardiac remodeling only at a dose that mobilizes endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2007;292:H522–H529. doi: 10.1152/ajpheart.00357.2006. [DOI] [PubMed] [Google Scholar]
- 7.Melloni C, Rao SV, Povsic TJ, Melton L, Kim RJ, Kilaru R, Patel MR, Talan M, Ferrucci L, Longo DL, Lakatta EG, Najjar SS, Harrington RA. Design and rationale of the Reduction of Infarct Expansion and Ventricular Remodeling with Erythropoietin after Large Myocardial Infarction (REVEAL) trial. Am Heart J. 2010;160:795–803. doi: 10.1016/j.ahj.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, Barsness GW, Prather K, Heitner JF, Kilaru R, Gruberg L, Hasselblad V, Greenbaum AB, Patel M, Kim RJ, Talan M, Ferrucci L, Longo DL, Lakatta EG, Harrington RA. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA. 2011;305:1863–1872. doi: 10.1001/jama.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentry T, Foster S, Winstead L, Deibert E, Fiordalisi M, Balber A. Simultaneous isolation of human BM hematopoietic, endothelial and mesenchymal progenitor cells by flow sorting based on aldehyde dehydrogenase activity: implications for cell therapy. Cytotherapy. 2007;9:259–274. doi: 10.1080/14653240701218516. [DOI] [PubMed] [Google Scholar]
- 10.Povsic T, Zavodni K, Kelly F, Zhu S, Goldschmidt-Clermont P, Dong C, Peterson E. Circulating progenitor cells can be reliably identified on the basis of aldehyde dehydrogenase activity. J Am Coll Cardiol. 2007;50:2243–2248. doi: 10.1016/j.jacc.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Povsic T, Adams SD, Zavodni KL, Kelly F, Melton L, Rao S, Najjar S, Harrington R, Peterson E. Aldehyde dehydrogenase activity allows reliable EPC enumeration in stored peripheral blood samples. J Thromb Thrombolysis. 2009;28:259–265. doi: 10.1007/s11239-009-0306-6. [DOI] [PubMed] [Google Scholar]
- 12.Olson W, Smolkin M, Farris E, Fink R, Czarkowski A, Fink J, Chianese-Bullock K, Slingluff C. Shipping blood to a central laboratory in multicenter clinical trials: effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function. J Transl Med. 2011;9:26. doi: 10.1186/1479-5876-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundmann F, Scheid C, Braun D, Zobel C, Reuter H, Schwinger R, Müller-Ehmsen J. Differential increase of CD34, KDR/CD34, CD133/CD34 and CD117/CD34 positive cells in peripheral blood of patients with acute myocardial infarction. Clin Res Cardiol. 2007;96:621–627. doi: 10.1007/s00392-007-0543-7. [DOI] [PubMed] [Google Scholar]
- 14.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 15.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 16.Paczkowska E, Larysz B, Rzeuski R, Karbicka A, Jałowiński R, Kornacewicz-Jach Z, Ratajczak MZ, Machaliński B. Human hematopoietic stem/progenitor-enriched CD34(+) cells are mobilized into peripheral blood during stress related to ischemic stroke or acute myocardial infarction. Eur J Haematol. 2005;75:461–467. doi: 10.1111/j.1600-0609.2005.00536.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Johnsen HE, Mortensen S, Bindslev L, Ripa RS, Haack-Sorensen M, Jorgensen E, Fang W, Kastrup J. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92:768–774. doi: 10.1136/hrt.2005.069799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 19.Scheubel RJ, Zorn H, Silber R-E, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Leone AM, Rutella S, Bonanno G, Abbate A, Rebuzzi AG, Giovannini S, Lombardi M, Galiuto L, Liuzzo G, Andreotti F, Lanza GA, Contemi AM, Leone G, Crea F. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26:1196–1204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishnan R, Cheung WK, Wacholtz MC, Minton N, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after single and multiple doses in healthy volunteers. J Clin Pharmacol. 2004;44:991–1002. doi: 10.1177/0091270004268411. [DOI] [PubMed] [Google Scholar]