Abstract
Objectives
Major hepatic resection is now performed frequently and with relative safety, but is accompanied by significant pathophysiological changes. The aim of this review is to describe these changes along with interventions that may help reduce the risk for adverse outcomes after major hepatic resection.
Methods
The MEDLINE, EMBASE and CENTRAL databases were searched for relevant literature published from January 2000 to December 2011. Broad subject headings were ‘hepatectomy/’, ‘liver function/’, ‘liver failure/’ and ‘physiology/’.
Results
Predictable changes in blood biochemistry and coagulation occur following major hepatic resection and alterations from the expected path indicate a complicated course. Susceptibility to sepsis, functional renal impairment, and altered energy metabolism are important sequelae of post-resection liver failure.
Conclusions
The pathophysiology of post-resection liver failure is difficult to reverse and thus strategies aimed at prevention are key to reducing morbidity and mortality after liver surgery.
Introduction
Liver resection for primary and selected metastatic hepatic neoplasms can be performed safely with low rates of morbidity and mortality.1 However, major liver resection is accompanied by significant and to some extent predicable pathophysiological changes. These range from mild transient abnormalities to progressive derangements that can be life-threatening in the case of post-resection liver failure (PLF).2 Patients with pre-existent parenchymal liver disease and/or a small residual liver volume (RLV) are at greatest risk for PLF,1,2 the clinical features of which are similar to those of liver failure in other contexts and include coagulopathy, encephalopathy, jaundice and ascites. A comprehensive understanding of the pathophysiological changes that can be expected after major hepatic resection is important to enable surgeons to recognize deviations from the normal course at an early stage. The aim of this review is to describe the pathophysiological changes that occur following major hepatic resection and interventions that may prevent or reduce the risk for the development of those complications specifically associated with hepatic resection.
Materials and methods
A literature search was performed using the MEDLINE, EMBASE and CENTRAL (Cochrane Central Register of Controlled Trials) databases for articles published from January 2000 to December 2011. The medical subgroup headings (MeSH) ‘hepatectomy/’, ‘liver function/’, ‘liver failure/’ and ‘physiology/’ were used as broad search terms. The search was performed according to PRISMA (preferred reporting items for systematic reviews and meta-analyses) recommendations (Fig. 1). To outline key components of pathophysiological change after liver resection, the above MeSH terms were combined using Boolean operators with the MeSH terms ‘reperfusion injury/’, ‘liver ischaemia/’, ‘immunity/’, ‘liver circulation/’, ‘blood coagulation/’, ‘blood cell count/’, ‘metabolism/’, ‘liver function tests/’, ‘kidney function/’, ‘liver regeneration/’ and ‘liver hypertrophy/’. Additional articles were identified from the references of articles highlighted by the electronic search. Eligibility criteria required studies to have been published from January 2000 onwards, to refer to investigations in humans in which more than eight patients underwent major hepatectomy, and allowed the inclusion of randomized and non-randomized studies including observational studies without control groups, individual studies and reviews with relevance to physiological topics. Exclusion criteria discounted animal studies, studies in which only transplantation was performed, studies reported in languages other than English and studies with insufficient data on physiological topics. The selection of articles was conducted by two reviewers. Any data obtained were confirmed by at least one other reviewer.
Figure 1.
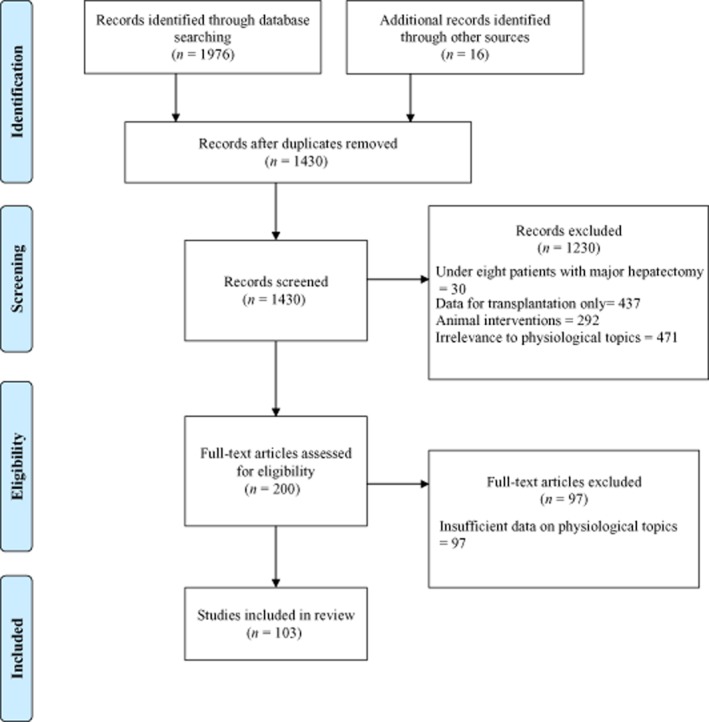
Flow diagram showing the identification and screening of studies for review in line with PRISMA criteria
Results
Biochemical liver function tests
Commonly measured biochemical parameters of hepatic function following liver resection include aspartate aminotransferase (AST) and alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), bilirubin, albumin and C-reactive protein (CRP). Time-dependent change, influencing factors and aetiologies of change are shown in Table 1.
Table 1.
Changes to biochemical markers following hepatic resection
| Test | Peak or trough | Influencing factors | Marker of: |
|---|---|---|---|
| Transaminases (AST/ALT)1,4,92 | Peak at day 1 followed by exponential decay | Time of surgery and hepatic ischaemia Bilirubin level |
Hepatocellular injury |
| Bilirubin2–4,93 | Peak at day 3 (variable), biphasic Falls at 3–12μmol/day Early postoperative period: Unconjugated/conjugated ratio: >1 Late postoperative period: Unconjugated/conjugated ratio: <1 |
Day 1: preoperative bilirubin, duration of surgery, blood transfused Days 1–7: volume hepatic resection, hepatic ischaemic time >Day 7: sepsis |
Preload of haem Hepatocellular function Rate of biliary excretion Sepsis |
| C-reactive protein4,94 | Peak at day 3 | Rise is inversely proportional to extent of resection (days 1–5) | Synthetic function Hepatic failure following major hepatic resection (<32 mg/l at day 1) Complication (days 3–7) |
| Albumin4,94 | Trough (>day 7) then slowly recovers | Proportional to extent of resection Presence of complication (days 1–5) |
Synthetic function Complication (days 1–7) |
| ALP, GGT93 | Trough to approximately 7% of initial value then slowly climbs at rate 14 U/l/day GGT greater variability |
Presence of biliary obstruction (can be diagnosed by confirming elevated cholesterol level) Recovery correlates with normalization of prothrombin activity |
Regeneration |
| α-GST95 | Peaks at 15–30 min after reperfusion | Preconditioning can be protective | Ischaemia Postoperative hepatic dysfunction (>490 μg/l at 2 h post-resection) |
| Glucose20,23,24,96 | Rises within minutes of reperfusion; elevation can persist for up to 16 h | Chronic liver disease or fibrosis Hormonal manipulation (insulin, cortisol, glucagon, noradrenaline) Portal clamping |
Glycogenolysis Gluconeogenesis |
| Lactate20–22,92,93,97 | Rises within minutes of reperfusion and returns to normal within 24 h Represents adequate capacity of remnant |
Degree of ischaemia (correlates with AST rise) Pre-existing liver disease |
Ischaemia–reperfusion injury Anaerobic hepatic metabolism Systemic levels of lactate but not intestinal lactate levels Level >4–5 mmol/l associated with hepatic failure (although time course may be more important) |
| Serum creatinine45,46 | Little change in immediate postoperative period Patients with a preoperative serum creatinine of ≥ 155 mmol/l are less predictable Peaks at days 2–3 |
Raised preoperative serum creatinine Impaired neurohumoral autoregulation (diabetes, ischaemic heart disease, hypertension) Perioperative hypotension (low CVP) Late sepsis/multi-organ failure, post-resection liver failure |
Acute kidney injury Haemofiltration requirement |
| Phosphate/Ca2+16–19 | Hypophosphataemia almost universal (mean drop 47%) Severe in up to 20% of patients Nadir seen on days 2–3 and recovers by day 7 Ca2+ reaches nadir 24 h earlier than PO4− |
Size of fall inversely proportional to hepatic remnant volume and rise in ALP Inversely proportional to prothrombin level |
Increased urinary loss of phosphate: mechanism unclear |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; GGT, γ-glutamyl transpeptidase; α-GST, α-glutathione S-transferase.
Serum levels of bilirubin are dependent on the pre-hepatic load of haem, hepatocellular function and rate of biliary excretion, all of which can be altered in the postoperative period. A landmark study by Balzan et al.,3 carried out in 2005, found that serum bilirubin of >50 μmol/l combined with prothrombin time (PT) of <50% of normal time (the so-called ‘50–50’ criteria) at day 5 was associated with an odds ratio (OR) of 216 [95% confidence interval (CI) 54–861] for predicting postoperative mortality with an accuracy of 97.7%. The presence of either of these criteria alone at any time after surgery was also associated with marked increases in postoperative mortality.3
Albumin and CRP are synthesized by the liver and their postoperative change has been studied in detail (Table 1). At day 1, median albumin levels were found to be reduced from 42 g/l [interquartile range (IQR): 38–46 g/l] and 44 g/l (IQR: 40–46 g/l) to 26 g/l (IQR: 22–31 g/l) and 24 g/l (IQR: 21–26 g/l) in patients submitted to minor and major hepatic resections, respectively.4 At day 7, median values of albumin appeared to remain significantly lower than preoperative values at 32 g/l (IQR: 28–36 g/l) and 31 g/l (IQR: 28–33 g/l) in patients undergoing minor and major hepatic resections, respectively.4 Acute changes in serum albumin reflect the movement of albumin into the interstitial space in response to tissue injury, rather than reduced liver synthesis, and are exaggerated by systemic complications such as sepsis. The CRP peaked at day 3 at 138 mg/l (IQR: 104–166 mg/l) and 86 mg/l (IQR: 60–125 mg/l) in patients submitted to minor and major hepatic resections, respectively.4
Levels of ALP and GGT rise during the first week after major resection and remain elevated during the period of liver regeneration. The increases in ALP and GGT may be exaggerated in pathological situations such as biliary obstruction or small-for-size syndrome, in which intrahepatic cholestasis occurs.5
The administration of pre- and perioperative methyl prednisone to patients undergoing hepatic resection has been shown to reduce postoperative peaks in bilirubin and CRP levels, but no differences have been noted in transaminase levels or the occurrence of complications.6
Coagulation changes after hepatic resection
The homeostasis of the coagulation system is closely related to hepatic function because the liver is responsible for the synthesis of factors involved in the coagulation and fibrinolytic pathways, as well as regulation of their activators and inhibitors. The liver is also, via the reticuloendothelial system, largely responsible for the clearance of breakdown products of activated clotting factors.7 Thus, in patients with underlying cirrhosis, preoperative abnormalities in the clotting and fibrinolytic systems may coexist.7 The platelet count is also reduced in portal hypertension. Patients with prolonged obstructive jaundice may develop vitamin K deficiency that results in alterations in both the clotting system via factors II, VII, IX and X and the anticoagulant system via proteins C and S.7 The magnitude of the changes to the coagulation profile have been correlated with the extent of hepatic resection (weight of resected specimen),8,9 amount of blood loss8 and time of surgery.9 In addition, the degree of change in the level of any individual factor will be governed not only by impaired synthesis, but also by the rate of consumption and half-life dependent decay.
Activated partial thromboplastin time (aPPT) and PT, or the international normalized ratio (INR), are the most commonly measured parameters of coagulation following hepatic surgery (Table 2). In assessing the frequency at which these changes (aPPT, INR) occur, it is important to take into consideration the proportion of patients undergoing major hepatic resection,10,11 the extent of blood loss11 and the amount of i.v. fluid administered11 in association with the resection. In a series of 124 patients with normal underlying hepatic function, in whom the mean ± standard deviation (SD) weight of the hepatic resection was 667 ± 443 g, PT was prolonged in 43% of patients at day 3 and in 22% at day 7,9 with a mean ± SD maximal value of 18 ± 3.8 s (normal range: 11–16 s). It is also worth noting that some of the immediate postoperative changes reflect the diluting effect of the administration of i.v. fluid, whereas those at 24 h and later reflect the amount of liver resected.11
Table 2.
Summary of changes in coagulation system following hepatic resection
| Factor | Test | Main findings |
|---|---|---|
| II, VII, IX, X8–11 | INR/PT | Immediate rise, peaks at days 1–3, normalizes at day 5 |
| I, II, V, VIII, IX–XII8,10 | aPPT | Small immediate rise, peaks (40–50 s) within 24 h and normalizes within 48 h |
| Platelets8,9,11 | Platelets | Non-clinically significant fall with nadir at day 3 returning to normal at day 5 |
| Antithrombin III10,12 | AT-3 | Extent of nadir proportional to extent of hepatic resection Level of <60% of baseline is associated with procoagulant state |
| Protein C12 | Protein C | Fall proportional to fall in AT-3 |
| Protein S12 | Protein S | Transient fall returning to normal within 24 h (extrahepatic synthesis) |
| Fibrinogen13 | Fibrinogen | Dampened rise post-resection proportional to magnitude of resection |
INR, international normalized ratio; PT, prothrombin time.
Three studies8,9,11 have looked at the effect on platelets following hepatic resection (Table 2). As expected, given that platelets are not synthesized in the liver, the level of reduction was correlated with the amount of blood loss and the amount of intraoperative fluid administered, but not with the amount of liver resected.11
Antithrombin III (AT-III) is a serine protease inhibitor that is synthesized in the liver.10 It acts to neutralize thrombin and factor Xa.10 Bezeaud et al. showed a fall to <50% of baseline, which persisted to day 5 and coincided with similarly sized reductions in levels of the coagulation inhibitor protein C.12 The same study12 measured levels of the prothrombotic marker thrombin-antithrombin. The level of plasma thrombin–antithrombin complexes indicate the degree of intravascular thrombin formation.12 In this study,12 despite the administration of prophylactic low molecular weight heparin, a 10-fold increase in plasma levels of thrombin–antithrombin complexes was observed from the immediate postoperative period through to day 5. This has particular clinical significance: firstly, higher levels of thrombin–antithrombin complexes within the first 48 h after surgery were associated with an increased risk for subsequent thrombotic complications, and, secondly, it questions what the most appropriate thrombotic prophylactic might be, given the acquired antithrombin deficiency.12 Further evidence to support the development of a procoagulant state following partial hepatic resection has included the observation of increased factor VIII and von Willebrand factor between days 1 and 5.12
Fibrinogen is an acute phase protein synthesized within the liver. It is required for clot formation. Fibrinogen is expected to increase following surgical trauma.13 After hepatic resection, this increase has been found to be dampened proportional to the magnitude of the hepatic resection.13 In addition, a temporal failure to increase in the postoperative period is a predictor of complications.13
Thus, it is clear that the balance between an anticoagulated and thrombotic state is dependent on a complex interaction of multiple factors. Standard clotting profiles would not seem to offer an accurate or real-time assessment of the true underlying situation or predict the likelihood of bleeding or complications. As more detailed methods of making a real-time assessment of the clotting pathways become commercially available, it may be appropriate to use these to tailor therapies to combat individually identified deficiencies.
Clinical trials of thromboembolic prophylaxis specifically after liver resection are lacking. However, patients undergoing major abdominal surgery benefit from thromboembolic prophylaxis and available evidence suggests that this is safe and effective in patients undergoing liver resection,14 which is consistent with the known effects of liver resection on both the pro- and anticoagulation pathways.
Metabolic changes
Energy consumption by the liver following hepatic resection increases to support protein synthesis for the acute phase response to injury.15 However, hepatocyte mitosis during liver regeneration incurs an additional burden in the maintenance of metabolic function despite a decrease in liver mass.1,15 As a result of competing metabolic demands, an economic energy crisis occurs, with a fall in energy state or ‘energy charge’, and some synthetic and excretory functions are sacrificed.1 As energy production capacity increases with successful regeneration, liver function recovers.15
Serum calcium and phosphate levels drop significantly in the early postoperative period (Table 1).16–19 The routine treatment of these remains controversial because although severe hypophosphataemia can cause life-threatening complications, it is not clear whether routine treatment reduces complications16,18 in healthy patients. It would seem appropriate to institute phosphate replacement should levels fall to <0.7 mg/dl.
Glucose homeostasis is maintained to a significant extent by hepatic gluconeogenesis. The liver has a key role in the regulation of plasma concentrations of lactate, a precursor of glucose,20 and maintains up to 50% of the decomposition of lactate primarily via gluconeogenesis.20 The liver can change from a lactate consumer to a lactate producer by switching to anaerobic metabolism when there is a reduced oxygen supply, such as during vascular clamping in hepatic resection.20–22 Hyperglycaemia (Table 1) induced by surgical stress is also recognized following liver resection.23 This, in turn, impairs liver metabolism and immune function, thus impacting on postoperative recovery.24
The liver plays an important role in amino acid metabolism, protein synthesis and protein breakdown. It detoxifies end-products of the intestinal metabolism, such as ammonia. Hyperammonaemia is probably a causal component of hepatic encephalopathy.25 An imbalance in plasma amino acids, which is closely associated with hyperammonaemia,25 during liver failure has also been implicated, and can be measured by the ratio between branched-chain amino acids (BCAAs) and the aromatic amino acids (AAAs), otherwise known as Fischer's ratio.26 Fischer's ratio is a useful indicator of the severity of hepatic parenchymal injury in patients with liver disease and severe hepatic insufficiency.26 The pathophysiology of hyperammonaemia in liver disease is complex. In 2008, van de Poll et al. reported that the net splanchnic contribution of ammonia is only minor because ammonia acid supply and metabolism per gram of liver increase significantly immediately following hepatectomy.27 It has been shown that hepatocellular ammonia clearance is also likely to be preserved in the diseased liver.27 Therefore, even the diseased liver has substantial reserve capacity. The kidneys also have a significant role in the regulation of systemic ammonia release and thus therapies aiming to reduce high ammonia levels may need to target renal ammonia production and excretion in addition to intestinal ammonia production.27
Amino acid imbalance with a reduced Fischer's ratio in the diseased or injured liver is caused by an increase in BCAA catabolism in muscle and decreased AAA breakdown in the liver.25 A reduction in the insulin/glucagon ratio may play a role in this metabolic imbalance25 and may be reversed by supplemental BCAA.28 In addition, the administration of BCAAs after major hepatectomy has been shown to support protein synthesis and regeneration of the remnant liver.26 Use of molecular adsorbent recirculating systems (MARS) during liver failure suggests that a low Fischer ratio can be corrected by recirculating albumin dialysis; however, experience with MARS for PLF is limited and there is no evidence to date that clinical outcome is altered.25,29
The effects of the Pringle manoeuvre on amino acid metabolism alter individual amino acid levels, but the Fischer ratio remains unchanged.25 In 2007, Dejong et al. found that in patients who underwent hepatic resection without a Pringle manoeuvre the Fischer ratio remained unchanged, but plasma AAA levels were inversely correlated with RLV.25
Previous studies have shown a correlation of the ratio between BCAAs and tyrosine (BTR) with liver function parameters such as prothrombin and albumin in patients with liver disease.26 Increased BTR levels after hepatectomy were found to be associated with the prolonging of jaundice after liver surgery.26
Immune function
Rates of postoperative infection may reach 50% after major liver resection30 and bacterial infection may develop in up to 80% of patients with PLF in comparison with 20% of patients who do not have PLF.30 Sepsis is the most common immediate cause of death in patients with PLF. Several factors contribute to increased susceptibility to infection and the development of sepsis in patients following liver resection. These include technical factors such as wounds and collections of bile or blood, reduced capacity of the reticuloendothelial system,31 and increased exposure to enteric microorganisms, all of which occur in the setting of a surgical stress response.32
Sepsis can also precipitate or exacerbate PLF as a result of the inhibitory effect of sepsis on hepatic regeneration.1 Systemic inflammatory response syndrome (SIRS) is likely to be implicated in sepsis and end-organ dysfunction in PLF.32 This syndrome is an index of surgical stress early after surgery and is predictive of complications.33 Patients with sepsis can develop circulatory dysfunction as a result of haemodynamic instability and decreased systemic vascular resistance.2,34 This may be further complicated by the rapid development of multi-organ failure, such as in acute renal failure and acute respiratory dysfunction.2,34 The majority of PLF patients will develop SIRS and multi-organ failure, and an 80% mortality rate has been documented in patients who undergo resection of >50% of the liver.30,35
The reticuloendothelial system or Kupffer cell mass and function are reduced and show an S-shaped correlation to the extent of resection.31 Kupffer cells play a significant role in the innate immune system and produce cytokines [tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6)], which induce proliferation of hepatocytes.1 Thus the remnant liver is dependent on Kupffer cells for regeneration as well as for the clearance of blood-borne enteric microorganisms and endotoxins. Recovery of Kupffer cell function may not occur for >2 weeks after resection.1
The increased rate of bacterial translocation to the liver from portal and arterial blood, mesenteric lymph nodes and other organs may be attributed to reduced clearance and possibly to the application of temporary hepatic inflow occlusion.1 Bacterial endotoxins directly affect Kupffer cells by impairing the initiator cytokines needed for regeneration.1 They also disrupt hepatocyte transport mechanisms, membrane function and metabolism, leading to hepatic necrosis.2
The liver responds to injury and infection by producing an acute phase response in preparation for change in metabolic processes, the removal of necrotic tissue, the mobilization of immune cells and the reparative process.32,35 An excessive inflammatory response with subsequent transient immunosuppression can lead to increased susceptibility to infection, SIRS and consequent multi-organ failure.35 Portal venous congestion associated with hepatic inflow occlusion can upregulate the production of acute inflammatory cytokines.36 A higher portal vein pressure correlates with an increased inflammatory response and repeated intermittent clamping has been proposed as a method to overcome this.36
Plasma IL-6, IL-10, IL-8, monocyte chemotactic protein-1 (MCP1), cortisol and leptin have been positively correlated with infection and organ dysfunction following hepatic resection.32 Surgical stress causes an increase in energy requirements; however, there is a decrease in hepatic energy levels in the first 48 h after resection,37 which impairs liver regeneration. The ongoing inflammatory activity in patients with chronic liver failure further increases the risk for acute liver failure38 as a result of these patients' reduced ability to adapt to surgical stress.39
Several randomized controlled trials (RCTs) indicate that high-dose steroid administration attenuates the surgical stress response while reducing immune suppression in patients undergoing hepatectomy.35,40,41 However, there is no evidence that this leads to an improvement in clinically important endpoints.35,40,41
Strey et al. confirmed the role of complement activation in the systemic inflammatory response whereby complement products return to baseline by day 7.42 Early increases in C3a and soluble terminal complex sC5b9 were found to be accompanied by an early reduction in C4a and C5a.42 The same study found a decrease in neutrophil chemotaxis and function, which suggests the downregulation of neutrophil activity following hepatic resection. However, only a reduction in C5L2 on monocytes was associated with the impairment of postoperative liver function (bilirubin and thromboplastin time) and no other postoperative clinical data, such as incidence of complications, were given.42
Renal function
Two large series have shown postoperative acute kidney injury (AKI) occurs in 15% of patients undergoing hepatic resection (Table 1).43,44 Postoperative AKI is strongly associated with mortality, which reaches 22.5% in patients with and 0.8% in those without AKI (P < 0.001), as well as extended requirements for intensive care unit (ICU) and hospital care.43 The four strongest predictors43 of postoperative AKI are preoperatively elevated ALT, pre-existing cardiovascular disease, chronic renal failure45,46 and diabetes.43 Such patients have an impaired ability to autoregulate glomerular blood flow during surgery and thus have an increased risk for acute tubular necrosis.45
Increased blood loss during hepatic resection has been associated with greater risk for AKI.47 Low (<5 mmHg) intraoperative central venous pressure (CVP) and fluid restriction are applied in many centres to reduce blood loss.43,44,48 However, the use of low CVP needs to be balanced against the risk for AKI.43,48 If mean arterial blood pressure (MABP) falls to <80 mmHg, the glomerular filtration rate (GFR) drops significantly, leading to an increased risk for the development of AKI.43,44 Postoperative renal function may be impaired by a low arterial blood pressure43 arising from the hypovolaemic state caused by low CVP anaesthesia and portal inflow occlusion. A low perfusion state can also occur secondary to cardiac dysfunction or distributive circulatory changes, such as sepsis or hepatorenal failure.45 Patients who develop PLF show haemodynamic changes similar to those in patients with cirrhosis or acute liver failure of other causes and are at risk for hepatorenal syndrome, particularly when bacterial sepsis ensues.43,44 Cardiac output is increased and blood flow is redistributed towards the splanchnic circulation and away from the systemic vascular bed in response to increased levels of nitric oxide (NO).43,44 These changes lead to reduced central and arterial blood volume, low pulmonary capillary wedge pressure, low CVP, low systemic vascular resistance, increased carbon monoxide, and reduced MABP.43,44 Hepatorenal syndrome results from reduced renal blood flow and activation of homeostatic mechanisms that produce renal arteriolar vasoconstriction.43,44 Perioperative management to prevent mortality from hepatorenal syndrome includes optimizing the patient's fluid status,43 and remaining vigilant for and providing early management of sepsis.30 Terlipressin is a vasopressin analogue that redistributes blood flow from the splanchnic to the renal circulation.49 In the setting of chronic liver disease, terlipressin, alone or with albumin, reduces the mortality associated with hepatorenal syndrome.49 The role of terlipressin in PLF has not been tested, but it is reasonable to consider that it may be beneficial in patients who develop hepatorenal failure in this context.
Hepatic blood flow
Hepatic blood flow is complex and regulated at three levels in normal physiological situations: the systemic circulation; the regional (macro) circulation, and the micro circulation.50 Factors controlling each of these levels and the effects of interventions are shown in Table 3.
Table 3.
Factors controlling and interventions affecting hepatic blood flow (HBF)
| Level | Flow | Regulation | Effect of intervention |
|---|---|---|---|
| Systemic50,98,99 | Total HBF | ↓ in HBF seen with: ▪ ↓ CO ▪ ↓ SVR (other than splanchnic) ▪ ↑ RAP > PVP |
Inflow occlusion results in: ▪ MAP and ↓ CVP ▪ ↓ Blood loss Hepatic venous exclusion: ▪ Well tolerated ▪ ↓ CVP and CO ▪ MAP maintained >55 mmHg as a result of ↑ in SVR Infrahepatic caval clamping: ▪ Well tolerated ▪ ↓ Blood loss |
| Regional50,66,100,101 | Total HBF | Total HBF (constant) = HAF + PVF (hepatic arterial buffer response) | Inflow occlusion followed by reperfusion results in ▪ ↓ SVR: ▪ Total HBF constant When preconditioning used: ▪ Total HBF Laparoscopic surgery: ▪ No difference in effect of inflow occlusion using laparoscopic or open surgery |
| PVF | Passively drains splanchnic arterial bed so ↓ splanchnic flow results in ↓ PVF | Inflow occlusion followed by reperfusion results in: ▪ 29% ↓ in PVF on reperfusion lasting >30 min When preconditioning used: ▪ No change in PVF |
|
| HAF | No auto-regulatory activity ↓ HAF with ↑ arterial resistance Arterial resistance ↑ with ↑ PVF (hepatic arterial buffer response) |
Inflow occlusion followed by reperfusion results in: ▪ 8% ↑ in HAF on reperfusion lasting >30 min When preconditioning used: ▪ 200% ↑ in HAF |
|
| Micro50,66 | HSF | Flow controlled by diameter of the sinusoids Sinusoidal diameter controlled by sinusoidal endothelial cells, hepatic stellate cells and Kupffer cells |
Ischaemia and subsequent reperfusion create a mismatch in time and space of mediator release so that homeostasis of the mediators is lost |
| Mediators released by above cells alter degree of contraction or relaxation of the cells, thus altering sinusoidal diameter and hence resistance to flow |
CO, cardiac output; CVP, central venous pressure; HAF, hepatic artery flow; HSF, hepatic sinusoidal flow; MAP, mean arterial pressure; PVF, portal blood flow; PVP, portal blood pressure; RAP, right atrial pressure; SVR, systemic vascular resistance.
Ischaemia and reperfusion
Ischaemia–reperfusion injury (IRI) occurs following liver resection with hepatic inflow occlusion. The severity of hepatic IRI correlates with the time of ischaemia and clinical factors, such as the duration of the procedure, periods of intraoperative hypotension and the degree of splanchnic ischaemia, most of which are under the surgeon's control.51 In addition to ischaemic injury to the liver, hepatic portal triad clamping causes intestinal venous congestion.36,51 Stagnant portal venous blood stimulates inflammatory mediators that exacerbate IRI to the liver.36 The reflow of stagnant portal venous blood into the ischaemic liver is detrimental to hepatic energy metabolism and therefore subsequently affects the liver's ability to maintain function and regenerate.51
Diseased hepatic parenchyma is more susceptible to IRI51–53 and should not be exposed to >60 min of continuous warm ischaemia,40 although periods of up to 90 min have been shown to be well tolerated by normal liver.51,54 A recent systematic review found that intermittent portal triad occlusion provides better protection against IRI in patients with chronic liver disease than does continuous portal triad occlusion.55
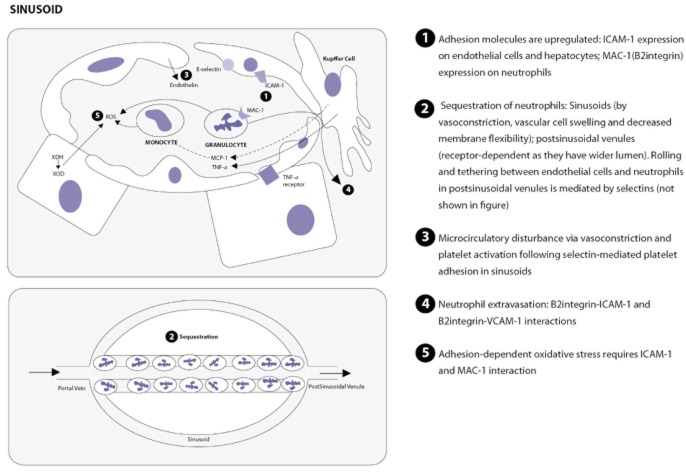
There are three main components of IRI.56 The first concerns cellular activation; the second refers to the expression of adhesion molecules (Fig. 2), and the third to microcirculatory dysfunction. These are linked by various mediators including cytokines, chemokines, lipid mediators and complement factors. The mechanisms of IRI leading to cellular injury are summarized in Fig. 3.
Figure 2.
Role of adhesion molecules in ischaemia–reperfusion injury. ICAM-1, intercellular adhesion molecule-1; MAC-1, macrophage-1 antigen; MCP1, monocyte chemotactic protein-1; ROS, reactive oxygen species; TNF-α, tumour necrosis factor-α; VCAM-1, vascular cell adhesion molecule-1; XDH, xanthine dehydrogenase; XOD, xanthine oxidase. References: 56,89–91
Figure 3.
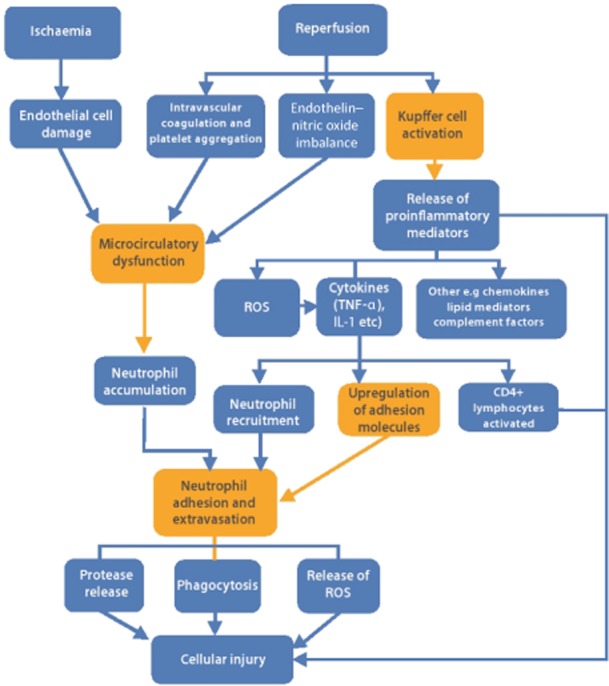
Summary of mechanisms in ischaemia–reperfusion injury. IL-1, interleukin-1; ROS, reactive oxygen species; TNF-α, tumour necrosis factor-α. References: 56,89
In two RCTs involving, respectively, 66 and 60 patients and evaluating the effects of a synthetic protease inhibitor, gabexate mesilate, postoperative serum transminases were markedly reduced, IL-6 production was suppressed and rates of postoperative complications were lowered.57,58
Ischaemic preconditioning is a process by which the liver develops increased protection against IRI through the release of adenosine, decreased endothelin-1 (ET-1) expression, altered nitric oxide synthase (NOS) induction, suppressed cytokine and free radical release, and transcriptional activation of stress genes, such as heat shock proteins, and adenosine triphosphate (ATP) preservation.59–63 An increase in carbon monoxide is protective because it helps to maintain microvascular and portal blood flow by regulating NOS and vasodilation.61 Studies show that the ultrastructure of parenchymal and non-parenchymal cells can be maintained even when the accumulated ischaemic duration with intermittent portal clamping is extended to 120 min.64
Efforts to improve hepatic tolerance of IRI have included the use of high-concentration glucose administered i.v. 24 h prior to surgery with the aim of increasing hepatocellular glycogen and ATP content.65 Heizmann et al. found that improved hepatic macrocirculation (Table 3) in addition to hepatic microcirculation following ischaemic preconditioning offered protection against IRI.66 A systematic review of RCTs discussing pharmalogical interventions that aim to ameliorate IRI, many of which were small, individual, clinical studies, shows that none so far demonstrate any clinical benefit.67 Ischaemic preconditioning may be achieved surgically by exposing the liver to a brief period (often 10 min) of ischaemia followed by 10 min of reperfusion prior to a prolonged ischaemic period.63 It is thought that the first period of portal clamping in the intermittent portal clamping method provides similar effects through a form of ischaemic preconditioning.62 Ischaemic preconditioning also appears to have a protective effect when the selective hepatic vascular exclusion (SHVE) manoeuvre (with inflow and outflow occlusion) is applied.68 However, the effects of ischaemic preconditioning under hepatic inflow occlusion in normal liver were not found to translate into significant reductions in mortality, liver failure, perioperative morbidity, hospital stay and ICU stay in a systematic review of five trials.69 In addition, a systematic review of four RCTs comparing hemihepatic with total hepatic inflow occlusion reported no significant difference in the clinical outcomes of blood loss, transfusion requirement, mortality, morbidity, operating time and hospital stay.70 In patients with livers at increased risk for the effects of ischaemia and reperfusion (cirrhotic, fibrotic or steatotic livers), the potential of the hemihepatic inflow occlusion approach remains to be explored.70,71
Liver regeneration
Liver regeneration is particularly significant in the context of major hepatic resections and small-for-size syndrome.72 The elevation in portal pressure that results from extended liver resection causes excess shear stress on the remnant liver with a reduction in portal vein flow and ultimately impaired regeneration.1,73 Much of the current understanding of physiological pathways in liver regeneration stems from extensive research in animal models.72,74 There are few data on the precise timing of the processes involved in humans.72 By contrast, the volumetric and functional recovery of the liver has been studied more extensively in patients following donor hepatectomy. In a series of 27 donors who underwent right hepatectomy, the remnant liver increased to 74% of its original volume at postoperative day 10 and continued to reach 83% of its original volume at 12 months.75
The liver will regenerate in response to loss of liver mass or liver function in order to restore functional liver volume.72,76 A larger resection or greater tissue damage will result in an increased replication rate.76 Proliferation of all liver cell types occurs, but non-parenchymal cells (endothelial, Kupffer and biliary duct cells) will have a more delayed course.74 Cells undergo cell cycle reactions (G1–G1) until a liver mass matched to body size is restored.72 Signalling pathways lead to an important G1–S transition in which liver cells commit to division and the time from G0 to S phase will depend on cell type.72 The role of the ‘oval cell compartment’ originating from ductular cells in generating hepatocytes and bile duct cells post-hepatectomy has not been established as it has in toxic liver injury and other forms of liver damage.72 Bone marrow-derived cells appear to contribute to further cellular resources capable of restoring liver mass.77
Factors triggering or influencing hepatic regeneration are shown in Table 4. Insulin and other growth factors that facilitate hepatic regeneration are delivered via portal blood to the liver. Other factors, such as glucagons, transforming growth factor-β (TGF-β) and glucocorticoids will inhibit regeneration.72 Signals of the cascade leading to proliferation are integrated to allow regeneration to occur in a synchronized fashion.74 The signal for cells to return to G0 (quiescent phase) is unknown and may be related to TGF-β or activin, potent inhibitors of hepatocyte growth.72 Impaired biliary drainage or biliary obstruction impedes liver hypertrophy and regeneration78,79 (Table 4). Portal hyper-perfusion and/or impaired venous outflow have been implicated in the failure of normal regeneration to occur in small-for-size syndrome.5
Table 4.
Factors affecting regeneration following hepatic resection
| Factors | Effect |
|---|---|
| Hepatic arterial buffer response | Same total portal flow through smaller hepatic volume carries hepatotrophic factors72,78 |
| Hepatocyte injury | Increase in circulating cytokines, IL-6, TNF-α stimulates priming factors for cell cycle activation72,78 Increased energy requirement triggers release of hepatocyte growth factor, TGF-α and epidermal growth factor72,78 |
| Ischaemic preconditioning | There are few clinical data on the beneficial effects of ischaemia preconditioning on liver regeneration compared with IRI;102 however, it has been shown to protect against IRI, which impairs liver regeneration following hepatectomy |
| Biliary obstruction | Reduces hypertrophy rate and regeneration following PVE or hepatectomy through a restricted portal venous flow caused by hepatic fibrosis, altered signalling pathways associated with regeneration, and an impaired enterohepatic circulation78 Patients with obstructive jaundice treated by external biliary drainage prior to hepatic resection may have impaired liver regeneration as a result of the disruption of the enterohepatic circulation79 |
| Diabetes | Patients with diabetes have reduced synthesis of RNA, DNA and protein on day 1 post-hepatectomy, as well as reduced hypertrophy caused by lack of insulin, a hepatotrophic factor78 |
| Nutrition | Patients with malnutrition have decreased regeneration following hepatectomy and higher postoperative mortality as regeneration capacity depends largely on availability of energy37,78,82 Enteral feeding will help to produce a better portal venous flow favouring regeneration78 NASH has not been shown to affect liver regeneration, which shows over-nutrition probably has a small effect on regeneration capacity78,103 |
| Cirrhosis | Recovery of lost cell mass is delayed and achieved incompletely, which is consistent with a higher risk for liver failure and mortality in the early postoperative period82 A postoperative fall in hepatic ATP energy state is more sustained in cirrhotic liver82 The normal liver will modulate energy metabolism or ‘hepatic energy economy’ in order to accommodate an increased metabolic load, but nonetheless develops a declined energy state around postoperative day 4, at the time when the liver reaches its maximal regenerative capacity, while curtailing its normal differentiated functions81,82 This recovery process is delayed in cirrhotic liver and is probably related to its decreased flexibility in making economic adjustments post-hepatectomy82 |
| Gender | The female gender may be more favourable towards regeneration as a result of the differential effects between the genders of sex hormones on regeneration78 |
ATP, adenosine triphosphate; IL-6, interleukin-6; IRI, ischaemia–reperfusion injury; NASH, non-alcoholic steatohepatitis; PVE, portal vein embolization; TGF-α, transforming growth factor-α; TNF-α, tumour necrosis factor-α.
The increase in liver volume is an indicator of regeneration; computed tomography (CT) is an easy, non-invasive imaging modality for measuring liver volume.79 However, volume restoration may be overestimated in CT as a result of postoperative vascular engorgement or oedema.79,80 Nagino et al. found a poor correlation between regeneration measured by CT volumetry and postoperative liver dysfunction.79 Liver volume may not accurately reflect the quantity of hepatocytes, especially in cirrhotic livers.80 Galactosyl-human serum albumin scintigraphy (GSA) is a method of estimating functional liver mass and was compared with CT liver volume in 32 patients undergoing liver resection.80 Whereas volumetric regeneration reached 90% at 1 month,75,80 6 months were required for functional regeneration to reach the same level.80 Surprisingly, functional gain in the diseased liver (chronic hepatitis or cirrhosis) was more rapid and more complete than volumetric recovery.80
Postoperative changes in phosphoester biochemistry or energy metabolism as detected by proton-decoupled 31P magnetic resonance spectroscopic imaging relate to the rate of liver regeneration.81,82 The derangement in phosphoester metabolism is increased in cirrhotic liver following resection.82 Zakian et al. showed that following the time course of the recovery of phosphoester metabolism may aid in making a clinical judgement of the appropriate timing for adjuvant chemotherapy.81
Pharmacological approaches to improve liver regeneration have so far mostly been investigated in animal studies.74,83 In a double-blind RCT, pentoxifylline was found to improve the regeneration of small remnant livers (ratio of remnant liver to body weight: ≤ 1.2%) based on three-dimensional volumetry by magnetic resonance tomography at postoperative day 8. This effect was believed to have been mediated by IL-6.83 However, morbidity and mortality outcomes did not differ significantly from those in the control group.83 Luo et al. studied the effects of the provision of BCAA and recombinant human growth factor for 5 days on the remnant liver in 24 hepatocellular carcinoma (HCC) patients with liver cirrhosis and found improved recovery of liver function, protein synthesis and regeneration.84
Portal vein embolization (PVE) and two-stage hepatectomy represent two strategies which have been used in clinical practice to increase the volume of the future liver remnant (FLR) and reduce the risk for postoperative liver insufficiency. The reductions in postoperative mortality and morbidity following PVE in both healthy and diseased liver have been documented in many observational studies, although most of these were retrospective.78,85,86 Portal vein embolization is usually considered when the FLR is estimated to be <25–35% of the whole liver in patients with a normal liver and <40% in patients with underlying parenchymal disease and patients who are undergoing preoperative chemotherapy.86 The haemodynamic changes that occur after PVE are not as dramatic as those following partial hepatectomy and therefore activation of liver proliferation occurs less rapidly.78 Despite this difference, hepatic regeneration occurs via similar mechanisms.78
After PVE, hepatic arterial flow to the embolized and non-embolized lobes increases via the ‘hepatic arterial buffer response’.1,78 Portal venous flow is diverted to the non-embolized lobe to maintain drainage from the splanchnic circulation.1,78 The non-embolized lobe thus receives a total increase in blood supply, which results in hypertrophy, while the embolized lobe atrophies in response to its reduction in total blood supply.78 The differentiated function of the embolized lobe is likely to be maintained.78 Failure of the non-embolized lobe to regenerate after contralateral PVE is considered by some to be a risk factor for poor function of the FLR and a relative contraindication to proceeding with resection.87 Dual embolization (PVE followed by arterial embolization) has been proposed as a method to further increase blood flow to the non-embolized lobe and thus to improve the hypertrophy rate.78 A retrospective study comparing two-stage hepatectomy and PVE with one-stage hepatectomy in 43 hepatectomy patients with multiple bilobar colorectal metastases showed that the FLR hypertrophy ratio relative to pre-procedure volume in the two-stage group (50.2%) was twice that in the PVE group (25.3%).88 However, the difference in longterm survival is unknown and a multi-institution prospective study of two-stage hepatectomy is yet to be carried out.
Discussion
Limitations
Because of the breadth of the current review, the range of study types and the essentially descriptive nature of much of the information reviewed, no assessment of bias or the grade quality of individual studies was performed in this review. In addition, because of the lack of high-level evidence, no objective grading was performed for any specific intervention; however, Table 5 provides a narrative description of potential interventions and considers areas for further research. The literature search was limited to English-language studies within the search period and included less recent papers that remain highly relevant. Because of the wide range of subtopics, it is possible that some relevant literature may not have been identified by the electronic search strategy. Therefore, any landmark studies or other relevant studies identified through hand-searching were considered for their eligibility for inclusion.
Clinical recommendations and areas of future potential research in patients undergoing hepatic resection
| Purpose | Clinical recommendation | Potential areas of research |
|---|---|---|
| Preoperative identification of patients at high risk for complications | Assessment and optimization of both function and volume of FLR | Standard definition of risk of FLR based on volume and functional status Development of predictive nomograms |
| Prevention of complications Intraoperative techniques |
Optimization of comorbidity including cardiac, renal (Cr > 155 mmol/l) and metabolic43,45,46 | |
| In patients undergoing preoperative biliary drainage ensure bile is returned via enteric tract78,79 | ||
| Cessation of hepatoxic agents (e.g. alcohol, chemotherapy) and period of recovery prior to surgery | Role of reversal of hepatic steatosis prior to hepatic resection | |
| In high-risk patients consider use of perioperative methyl prednisone6,35,40,41 | Large multicentre RCT of steroids with well-defined group of patients at high risk for postoperative complications to determine if improvements occur in clinically significant endpoints | |
| Preoperative manipulation of the FLR in high-risk subgroups78,85,86 | RCTs may not be ethically viable; using predictive nomograms and measuring variance may be a better solution | |
| In patients at high risk for small-for-size syndrome or impaired regeneration, consider use of 5 days of BCAA and human growth factor84 | Determine role of manipulation of splanchnic blood flow either surgically or pharmacologically | |
| Avoid unnecessary invasive foreign bodies such as catheters, drains and i.v. lines | ||
| In patients at risk for AKI, ensure MAP remains at >80 mmHg43,44 In patients in whom IRI is likely to result in an adverse outcome, adopt the following strategy:40,51,54 Intermittent inflow occlusion Hemi-inflow occlusion70,71 Use of gabexate mesilate57,58 Period of ischaemic preconditioning59–63 |
Determine the efficacy of terlipressin in those with hepatorenal syndrome post-hepatic resection | |
| Optimization of coagulation status | In patients undergoing extensive liver resection (>50%) consider: Real-time assessment and correction of coagulation deficits12 |
Further real-time physiological studies on changes to coagulation system as a whole and effect of correcting deficits on clinical outcomes |
| Treatment with AT-3 if coagulation falls to <60% of baseline level12 | Identification of optimal treatment to correct deficits (e.g. factor-specific or generic) | |
| Use of DVT prophylaxis as per international guidelines14 | Identification of patients who benefit from prolonged DVT prophylaxis | |
| Early postoperative identification of patients at risk for complications | In high-risk patients: Early assessment of predictors of PLF: (CRP < 32 mg/l at day 1, α-GST at >490 μg /l at 2 h postoperatively, lactate >4 mmol/l) Monitoring for AKI Early identification of sepsis: ↑ Bilirubin >day 7 ↑ CRP at day 3 |
Further clinical assessment to determine optimal variable and cut-off to determine optimal positive and negative predictive values |
| Treatment of complications | Aggressive management of suspected sepsis as per international guidelines2,30 | |
| Treatment of hypophosphataemia if <0.7 mmol/dl16,18 | Determine if treatment of PO4− improves clinical outcome | |
| Tight glucose control in perioperative period24 | Determine optimal method of lowering serum ammonia | |
| Supplement nutrition with BCAA26 | Determine if MARS has any clinical benefit in patients with PLF |
AKI, acute kidney injury; AT-3, antithrombin-3; BCAA, branched-chain amino acids; CRP, C-reactive protein; DVT, deep venous thrombosis; FLR, future liver remnant; α-GST, α-glutathione S-transferase; IRI, ischaemic–reperfusion injury; MAP, mean arterial blood pressure; MARS, molecular adsorbents recirculation system; PLF, postoperative liver failure; RCT, randomized controlled trial.
Conclusions
Patients undergoing liver resection incur pathophysiological consequences which relate to hepatocellular injury and reduced hepatic mass. Most patients undergo rapid regeneration, but in some the residual mass is either insufficient or regeneration is disordered and these patients are at risk for PLF and other associated complications. These changes are influenced by preoperative factors including liver parenchymal disease, patient age and comorbidities, intraoperative factors including the extent of resection, blood loss and ischaemia, and postoperative factors such as sepsis and other systemic complications. The recognition of any deviation from the expected path to recovery is important because although there is limited high-level evidence for specific treatment modalities for PLF, patients require careful and vigilant supportive care through a prolonged recovery phase. Most interventions tested to date have not been found to have conclusive effects on morbidity and mortality. However, further research and attention to clinical factors and their pathophysiological effects following liver resection may help to improve patient selection, inform the choice of procedure, and recognize and deal with complications at an early stage.
Conflicts of interest
None declared.
References
- Garcea G, Maddern GJ. Liver failure after major hepatic resection. J Hepatobiliary Pancreat Surg. 2009;16:145–155. doi: 10.1007/s00534-008-0017-y. [DOI] [PubMed] [Google Scholar]
- Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of post-resection liver failure. Br J Surg. 2011;98:1188–1200. doi: 10.1002/bjs.7630. [DOI] [PubMed] [Google Scholar]
- Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50-50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissfelder C, Rahbari NN, Koch M, Kofler B, Sutedja N, Elbers H, et al. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. Br J Surg. 2011;98:836–844. doi: 10.1002/bjs.7459. [DOI] [PubMed] [Google Scholar]
- Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605–2610. doi: 10.1111/j.1600-6143.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Takayama T, Yamazaki S, Moriguchi M, Ohkubo T, Nakayama H, et al. Validation of perioperative steroids administration in liver resection: a randomized controlled trial. Ann Surg. 2011;253:50–55. doi: 10.1097/SLA.0b013e318204b6bb. [DOI] [PubMed] [Google Scholar]
- Wada H, Usui M, Sakuragawa N. Haemostatic abnormalities and liver diseases. Semin Thromb Hemost. 2008;34:772–778. doi: 10.1055/s-0029-1145259. [DOI] [PubMed] [Google Scholar]
- Shontz R, Karuparthy V, Temple R, Brennan TJ. Prevalence and risk factors predisposing to coagulopathy in patients receiving epidural analgesia for hepatic surgery. Reg Anesth Pain Med. 2009;34:308–311. doi: 10.1097/AAP.0b013e3181ac7d00. [DOI] [PubMed] [Google Scholar]
- Weinberg L, Scurrah N, Gunning K, McNicol L. Postoperative changes in prothrombin time following hepatic resection: implications for perioperative analgesia. Anaesth Intensive Care. 2006;34:438–443. doi: 10.1177/0310057X0603400405. [DOI] [PubMed] [Google Scholar]
- Tapper EB, Tanaka KA, Sarmiento JM. Evaluation of haemostatic factors in patients undergoing major hepatic resection and other major abdominal surgeries. Am Surg. 2011;77:1188–1193. [PubMed] [Google Scholar]
- Siniscalchi A, Begliomini B, De Pietri L, Braglia V, Gazzi M, Masetti M, et al. Increased prothrombin time and platelet counts in living donor right hepatectomy: implications for epidural anesthesia. Liver Transpl. 2004;10:1144–1149. doi: 10.1002/lt.20235. [DOI] [PubMed] [Google Scholar]
- Bezeaud A, Denninger MH, Dondero F, Saada V, Venisse L, Huisse MG, et al. Hypercoagulability after partial liver resection. Thromb Haemost. 2007;98:1252–1256. [PubMed] [Google Scholar]
- Giovannini I, Chiarla C, Giuliante F, Vellone M, Nuzzo G. Modulation of plasma fibrinogen levels in acute-phase response after hepatectomy. Clin Chem Lab Med. 2004;42:261–265. doi: 10.1515/CCLM.2004.048. [DOI] [PubMed] [Google Scholar]
- Tzeng CW, Katz MH, Fleming JB, Pisters PW, Lee JE, Abdalla EK, et al. Risk of venous thromboembolism outweighs post-hepatectomy bleeding complications: analysis of 5651 National Surgical Quality Improvement Program patients. HPB. 2012;14:506–513. doi: 10.1111/j.1477-2574.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DV, Lam WW, Hjelm NM, So NM, Yeung DK, Metreweli C, et al. Metabolic control patterns in acute phase and regenerating human liver determined in vivo by 31-phosphorus magnetic resonance spectroscopy. Ann Surg. 2002;235:408–416. doi: 10.1097/00000658-200203000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomposelli JJ, Pomfret EA, Burns DL, Lally A, Sorcini A, Gordon FD, et al. Life-threatening hypophosphataemia after right hepatic lobectomy for live donor adult liver transplantation. Liver Transpl. 2001;7:637–642. doi: 10.1053/jlts.2001.26287. [DOI] [PubMed] [Google Scholar]
- Salem RR, Tray K. Hepatic resection-related hypophosphataemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2005;241:343–348. doi: 10.1097/01.sla.0000152093.43468.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Suh KS, Kim J, Shin WY, Cho EH, Yi NJ, et al. Hypophosphataemia after live donor right hepatectomy. Surgery. 2008;144:448–453. doi: 10.1016/j.surg.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Nafidi O, Lapointe RW, Lepage R, Kumar R, D'Amour P. Mechanisms of renal phosphate loss in liver resection-associated hypophosphataemia. Ann Surg. 2009;249:824–827. doi: 10.1097/SLA.0b013e3181a3e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietsch UC, Herrmann ML, Uhlmann D, Busch T, Hokema F, Kaisers UX, et al. Blood lactate and pyruvate levels in the perioperative period of liver resection with Pringle manoeuvre. Clin Hemorheol Microcirc. 2010;44:269–281. doi: 10.3233/CH-2010-1276. [DOI] [PubMed] [Google Scholar]
- Orii R, Sugawara Y, Hayashida M, Uchida K, Yamada Y, Takayama T, et al. Lactate is correlated with the indocyanine green elimination rate in liver resection for cirrhotic patients. Anesth Analg. 2001;92:1064–1070. doi: 10.1097/00000539-200104000-00049. [DOI] [PubMed] [Google Scholar]
- Theodoraki K, Arkadopoulos N, Fragulidis G, Voros D, Karapanos K, Markatou M, et al. Transhepatic lactate gradient in relation to liver ischaemia/reperfusion injury during major hepatectomies. Liver Transpl. 2006;12:1825–1831. doi: 10.1002/lt.20911. [DOI] [PubMed] [Google Scholar]
- Okabayashi T, Hnazaki K, Nishimori I, Sugimoto T, Maeda H, Yatabe T, et al. Continuous postoperative blood glucose monitoring and control using a closed-loop system in patients undergoing hepatic resection. Dig Dis Sci. 2008;53:1405–1410. doi: 10.1007/s10620-007-0010-3. [DOI] [PubMed] [Google Scholar]
- Little SA, Jarnagin WR, DeMatteo RP, Blumgart LH, Fong Y. Diabetes is associated with increased perioperative mortality but equivalent longterm outcome after hepatic resection for colorectal cancer. J Gastrointest Surg. 2002;6:88–94. doi: 10.1016/s1091-255x(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Dejong CH, van de Poll MC, Soeters PB, Jalan R, Olde Damink SW. Aromatic amino acid metabolism during liver failure. J Nutr. 2007;137 (Suppl.):1579–1585. doi: 10.1093/jn/137.6.1579S. [DOI] [PubMed] [Google Scholar]
- Nanashima A, Yamaguchi H, Shibasaki S, Abo T, Morino S, Yoshinaga M, et al. Changes of branched chain amino acids and tyrosine ratio (BTR) after hepatectomy. Acta Med Nagasaki. 2003;48:29–33. [Google Scholar]
- van de Poll MC, Ligthart-Melis GC, Olde Damink SW, van Leeuwen PA, Beets-Tan RG, Deutz NE, et al. The gut does not contribute to systemic ammonia release in humans without portosystemic shunting. Am J Physiol Gastrointest Liver Physiol. 2008;295:760–765. doi: 10.1152/ajpgi.00333.2007. [DOI] [PubMed] [Google Scholar]
- Togo S, Tanaka K, Morioka D, Sugita M, Ueda M, Miura Y, et al. Usefulness of granular BCAA after hepatectomy for liver cancer complicated with liver cirrhosis. Nutrition. 2005;21:480–486. doi: 10.1016/j.nut.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Chiu A, Chan LM, Fan ST. Molecular adsorbent recirculating system treatment for patients with liver failure: the Hong Kong experience. Liver Int. 2006;26:695–702. doi: 10.1111/j.1478-3231.2006.01293.x. [DOI] [PubMed] [Google Scholar]
- Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindl MJ, Millar AM, Redhead DN, Fearon KC, Ross JA, Dejong CH, et al. The adaptive response of the reticuloendothelial system to major liver resection in humans. Ann Surg. 2006;243:507–514. doi: 10.1097/01.sla.0000205826.62911.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, et al. Circulating cytokines, chemokines, and stress hormones are increased in patients with organ dysfunction following liver resection. J Surg Res. 2006;133:102–112. doi: 10.1016/j.jss.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Togo S, Tanaka K, Matsuo K, Nagano Y, Ueda M, Morioka D, et al. Duration of antimicrobial prophylaxis in patients undergoing hepatectomy: a prospective randomized controlled trial using flomoxef. J Antimicrob Chemother. 2007;59:964–970. doi: 10.1093/jac/dkm028. [DOI] [PubMed] [Google Scholar]
- Thasler WE, Bein T, Jauch KW. Perioperative effects of hepatic resection surgery on haemodynamic, pulmonary fluid balance, and indocyanine green clearance. Langenbecks Arch Surg. 2002;387:271–275. doi: 10.1007/s00423-002-0327-5. [DOI] [PubMed] [Google Scholar]
- Schmidt SC, Hamann S, Langrehr JM, Höflich C, Mittler J, Jacob D, et al. Preoperative high-dose steroid administration attenuates the surgical stress response following liver resection: results of a prospective randomized study. J Hepatobiliary Pancreat Surg. 2007;14:484–492. doi: 10.1007/s00534-006-1200-7. [DOI] [PubMed] [Google Scholar]
- Kim YI, Song KE, Kim JW, Hwang YJ, Lee JW, Chun BY. Enhanced inflammatory cytokine production at ischaemia/reperfusion in human liver resection. Hepatogastroenterology. 2002;49:1077–1082. [PubMed] [Google Scholar]
- Kooby DA, Zakian KL, Challa SN, Matei C, Petrowsky H, Yoo HH. Use of phosphorus-31 nuclear magnetic resonance spectroscopy to determine safe timing of chemotherapy after hepatic resection. Cancer Res. 2000;60:3800–3806. [PubMed] [Google Scholar]
- Eguchi H, Umeshita K, Sakon M, Nagano H, Ito Y, Kishimoto SI, et al. Presence of active hepatitis associated with liver cirrhosis is a risk factor for mortality caused by post-hepatectomy liver failure. Dig Dis Sci. 2000;45:1383–1388. doi: 10.1023/a:1005564205755. [DOI] [PubMed] [Google Scholar]
- Lan AK, Luk HN, Goto S, Chen SM, Eng HL, Chen YS, et al. Stress response to hepatectomy in patients with a healthy or a diseased liver. World J Surg. 2003;27:761–764. doi: 10.1007/s00268-003-6955-2. [DOI] [PubMed] [Google Scholar]
- Muratore A, Ribero D, Ferrero A, Bergero R, Capussotti L. Prospective randomized study of steroids in the prevention of ischaemic injury during hepatic resection with pedicle clamping. Br J Surg. 2003;90:17–22. doi: 10.1002/bjs.4055. [DOI] [PubMed] [Google Scholar]
- Pulitanò C, Aldrighetti L, Arru M, Finazzi R, Soldini L, Catena M, et al. Prospective randomized study of the benefits of preoperative corticosteroid administration on hepatic ischaemia–reperfusion injury and cytokine response in patients undergoing hepatic resection. HPB. 2007;9:183–189. doi: 10.1080/13651820701216984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strey CW, Siegmund B, Rosenblum S, Marquez-Pinilla RM, Oppermann E, Huber-Lang M, et al. Complement and neutrophil function changes after liver resection in humans. World J Surg. 2009;33:2635–2643. doi: 10.1007/s00268-009-0209-x. [DOI] [PubMed] [Google Scholar]
- Slankamenac K, Breitenstein S, Held U, Beck-Schimmer B, Puhan MA, Clavien PA. Development and validation of a prediction score for postoperative acute renal failure following liver resection. Ann Surg. 2009;250:720–728. doi: 10.1097/SLA.0b013e3181bdd840. [DOI] [PubMed] [Google Scholar]
- Saner F. Kidney failure following liver resection. Transplant Proc. 2008;40:1221–1224. doi: 10.1016/j.transproceed.2008.03.068. [DOI] [PubMed] [Google Scholar]
- Armstrong T, Welsh FK, Wells J, Chandrakumaran K, John TG, Rees M. The impact of preoperative serum creatinine on short-term outcomes after liver resection. HPB. 2009;11:622–628. doi: 10.1111/j.1477-2574.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moug SJ, Smith D, Wilson IS, Leen E, Horgan PG. The renal sequelae of a novel triphasic approach to blood loss reduction during hepatic resection. Eur J Surg Oncol. 2006;32:435–438. doi: 10.1016/j.ejso.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Gluud LL, Christensen K, Christensen E, Krag A. Terlipressin for hepatorenal syndrome. Cochrane Database Syst Rev. 2012;(9) doi: 10.1002/14651858.CD005162.pub3. CD005162. [DOI] [PubMed] [Google Scholar]
- Pannen BH. New insights into the regulation of hepatic blood flow after ischaemia and reperfusion. Anesth Analg. 2002;94:1448–1457. doi: 10.1097/00000539-200206000-00012. [DOI] [PubMed] [Google Scholar]
- Kim YI. Ischaemia–reperfusion injury of the human liver during hepatic resection. J Hepatobiliary Pancreat Surg. 2003;10:195–199. doi: 10.1007/s00534-002-0730-x. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Wente MN, Schemmer P, Diener MK, Hoffmann K, Motschall E, et al. Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. Br J Surg. 2008;95:424–432. doi: 10.1002/bjs.6141. [DOI] [PubMed] [Google Scholar]
- Li SQ, Liang LJ, Huang JF, Li Z. Ischaemic preconditioning protects liver from hepatectomy under hepatic inflow occlusion for hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol. 2004;10:2580–2584. doi: 10.3748/wjg.v10.i17.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyrniotis V, Kostopanagiotou G, Lolis E, Theodoraki K, Farantos C, Andreadou I, et al. Effects of hepatovenous back flow on ischaemic–reperfusion injuries in liver resections with the Pringle manoeuvre. J Am Coll Surg. 2003;197:949–954. doi: 10.1016/j.jamcollsurg.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Gurusamy KS, Sheth H, Kumar Y, Sharma D, Davidson BR. Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD006409.pub3. CD007632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga NR, Homer-Vanniasinkam S, Graham A, Al-Mukhtar A, White SA, Prasad KR. Ischaemic preconditioning in transplantation and major resection of the liver. Br J Surg. 2005;92:528–538. doi: 10.1002/bjs.5004. [DOI] [PubMed] [Google Scholar]
- Kim YI, Chung HJ, Song KE, Hwang YJ, Lee JW, Lee YJ, et al. Evaluation of a protease inhibitor in the prevention of ischaemia and reperfusion injury in hepatectomy under intermittent Pringle manoeuvre. Am J Surg. 2006;191:72–76. doi: 10.1016/j.amjsurg.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Kim YI, Hwang YJ, Song KE, Yun YK, Lee JW, Chun BY. Hepatocyte protection by a protease inhibitor against ischaemia/reperfusion injury of human liver. J Am Coll Surg. 2002;195:41–50. doi: 10.1016/s1072-7515(01)01118-8. [DOI] [PubMed] [Google Scholar]
- Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischaemia–reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- Dixon E, Vollmer CMJ, Bathe OF, Sutherland F. Vascular occlusion to decrease blood loss during hepatic resection. Am J Surg. 2005;190:75–86. doi: 10.1016/j.amjsurg.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Patel A, van de Poll MC, Greve JW, Buurman WA, Fearon KC, McNally SJ, et al. Early stress protein gene expression in a human model of ischaemic preconditioning. Transplantation. 2004;78:1479–1487. doi: 10.1097/01.tp.0000144182.27897.1e. [DOI] [PubMed] [Google Scholar]
- Man K, Lo CM, Liu CL, Zhang ZW, Lee TK, Ng IO, et al. Effects of the intermittent Pringle manoeuvre on hepatic gene expression and ultrastructure in a randomized clinical study. Br J Surg. 2003;90:183–189. doi: 10.1002/bjs.4027. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischaemic preconditioning. Ann Surg. 2003;238:843–850. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man K, Liang TB, Lo CM, Liu CL, Ng IO, Yu WC, et al. Hepatic stress gene expression and ultrastructural features under intermittent Pringle manoeuvre. Hepatobiliary Pancreat Dis Int. 2002;1:249–257. [PubMed] [Google Scholar]
- Tang L, Tian F, Tao W, Cui J. Hepatocellular glycogen in alleviation of liver ischaemia–reperfusion injury during partial hepatectomy. World J Surg. 2007;31:2039–2043. doi: 10.1007/s00268-007-9186-0. [DOI] [PubMed] [Google Scholar]
- Heizmann O, Meimarakis G, Volk A, Matz D, Oertli D, Schauer RJ. Ischaemic preconditioning-induced hyperperfusion correlates with hepatoprotection after liver resection. World J Gastroenterol. 2010;16:1871–1878. doi: 10.3748/wjg.v16.i15.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Amara M, Gurusamy KS, Glantzounis G, Fuller B, Davidson BR. Pharmacological interventions for ischaemia–reperfusion injury in liver resection surgery performed under vascular control. Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD008154. CD008154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkadopoulos N, Kostopanagiotou G, Theodoraki K, Farantos C, Theodosopoulos T, Stafyla V, et al. Ischaemic preconditioning confers antiapoptotic protection during major hepatectomies performed under combined inflow and outflow exclusion of the liver. A randomized clinical trial. World J Surg. 2009;33:1909–1915. doi: 10.1007/s00268-009-0117-0. [DOI] [PubMed] [Google Scholar]
- Gurusamy KS, Kumar Y, Pamecha V, Sharma D, Davidson BR. Ischaemic preconditioning for elective liver resections performed under vascular occlusion. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD007629. CD007629. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Yang JY, Yan LN. Hemihepatic versus total hepatic inflow occlusion during hepatectomy: a systematic review and meta-analysis. World J Gastroenterol. 2011;17:3158–3164. doi: 10.3748/wjg.v17.i26.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Lau WY, Li GG, Tang QH, Li AJ, Pan ZY, et al. A prospective randomized controlled trial to compare Pringle manoeuvre, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am J Surg. 2011;201:62–69. doi: 10.1016/j.amjsurg.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Mangnall D, Bird NC, Majeed AW. The molecular physiology of liver regeneration following partial hepatectomy. Liver Int. 2003;23:124–138. doi: 10.1034/j.1600-0676.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi H, Ishimura K, Okano K, Karasawa Y, Goda F, Maeba T, et al. Application of preoperative portal vein embolization before major hepatic resection in patients with normal or abnormal liver parenchyma. Surgery. 2002;131:26–33. doi: 10.1067/msy.2002.118259. [DOI] [PubMed] [Google Scholar]
- Ansari JA, Jamil M. Pathways of hepatic regeneration and pharmacological approaches for hepatoprotection: a brief approach. J Pharmacol Toxicol. 2011;6:24–29. [Google Scholar]
- Nadalin S, Testa G, Malagó M, Beste M, Frilling A, Schroeder T, et al. Volumetric and functional recovery of the liver after right hepatectomy for living donation. Liver Transpl. 2004;10:1024–1029. doi: 10.1002/lt.20182. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Imamura H, Aoki T, Sugawara Y, Kokudo N, Makuuchi M. Morphological regeneration and hepatic functional mass after right hemihepatectomy. Dig Surg. 2006;23:44–50. doi: 10.1159/000093754. [DOI] [PubMed] [Google Scholar]
- Galun E, Axelrod JH. The role of cytokines in liver failure and regeneration: potential new molecular therapies. Biochim Biophys Acta. 2002;1592:345–358. doi: 10.1016/s0167-4889(02)00326-9. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Nagino M, Nimura Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: a review. World J Surg. 2007;31:367–374. doi: 10.1007/s00268-006-0526-2. [DOI] [PubMed] [Google Scholar]
- Nagino M, Ando M, Kamiya J, Uesaka K, Sano T, Nimura Y. Liver regeneration after major hepatectomy for biliary cancer. Br J Surg. 2001;88:1084–1091. doi: 10.1046/j.0007-1323.2001.01832.x. [DOI] [PubMed] [Google Scholar]
- Kwon AH, Matsui Y, Kaibori M, Kamiyama Y. Functional hepatic regeneration following hepatectomy using galactosyl-human serum albumin liver scintigraphy. Transplant Proc. 2004;36:2257–2260. doi: 10.1016/j.transproceed.2004.08.075. [DOI] [PubMed] [Google Scholar]
- Zakian KL, Koutcher JA, Malhotra S, Thaler H, Jarnagin W, Schwartz L, et al. Liver regeneration in humans is characterized by significant changes in cellular phosphorus metabolism: assessment using proton-decoupled 31P-magnetic resonance spectroscopic imaging. Magn Reson Med. 2005;54:264–271. doi: 10.1002/mrm.20560. [DOI] [PubMed] [Google Scholar]
- Mann DV, Lam WWM, Hjelm NM, So NMC, Yeung DKW, Metreweli C, et al. Human liver regeneration: hepatic energy economy is less efficient when the organ is diseased. Hepatology. 2001;34:557–565. doi: 10.1053/jhep.2001.27012. [DOI] [PubMed] [Google Scholar]
- Petrowsky H, Breitenstein S, Slankamenac K, Vetter D, Lehmann K, Heinrich S, et al. Effects of pentoxifylline on liver regeneration: a double-blinded, randomized, controlled trial in 101 patients undergoing major liver resection. Ann Surg. 2010;252:813–822. doi: 10.1097/SLA.0b013e3181fcbc5e. [DOI] [PubMed] [Google Scholar]
- Luo SM, Liang LJ, Lai JM. Effects of recombinant human growth hormone on remnant liver after hepatectomy in hepatocellular carcinoma with cirrhosis. World J Gastroenterol. 2004;10:1292–1296. doi: 10.3748/wjg.v10.i9.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanashima A, Sumida Y, Abo T, Nonaka T, Takeshita H, Hidaka S, et al. Clinical significance of portal vein embolization before right hepatectomy. Hepatogastroenterology. 2009;56:773–777. [PubMed] [Google Scholar]
- Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdalla EK. Portal vein embolization (prior to major hepatectomy) effects on regeneration, resectability, and outcome. J Surg Oncol. 2010;102:960–967. doi: 10.1002/jso.21654. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Shimada H, Matsuo K, Ueda M, Endo I, Togo S. Remnant liver regeneration after two-stage hepatectomy for multiple bilobar colorectal metastases. Eur J Surg Oncol. 2007;33:329–335. doi: 10.1016/j.ejso.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Molecular mechanisms of hepatic ischaemia–reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 284:15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- Kubes P, Payne D, Woodman RC. Molecular mechanisms of leukocyte recruitment in post-ischaemic liver microcirculation. Am J Physiol Gastrointest Liver Physiol. 2003;283:139–147. doi: 10.1152/ajpgi.00058.2002. 2002. [DOI] [PubMed] [Google Scholar]
- Choukèr A, Martignoni A, Schauer R, Dugas M, Rau HG, Jauch KW, et al. Beneficial effects of ischaemic preconditioning in patients undergoing hepatectomy: the role of neutrophils. Arch Surg. 2005;140:129–136. doi: 10.1001/archsurg.140.2.129. [DOI] [PubMed] [Google Scholar]
- Giovannini I, Chiarla C, Giuliante F, Vellone M, Ardito F, Sarno G, et al. Analysis of the components of hypertransaminasaemia after liver resection. Clin Chem Lab Med. 2007;45:357–360. doi: 10.1515/CCLM.2007.078. [DOI] [PubMed] [Google Scholar]
- Chiarla C, Giovannini I, Giuliante F, Vellone M, Ardito F, Masi A, et al. Plasma bilirubin correlations in non-obstructive cholestasis after partial hepatectomy. Clin Chem Lab Med. 2008;46:1598–1601. doi: 10.1515/CCLM.2008.321. [DOI] [PubMed] [Google Scholar]
- Rahman SH, Evans J, Toogood GJ, Lodge PA, Prasad KR. Prognostic utility of postoperative C-reactive protein for post-hepatectomy liver failure. Arch Surg. 2008;143:247–253. doi: 10.1001/archsurg.2007.75. [DOI] [PubMed] [Google Scholar]
- Choukèr A, Martignoni A, Schauer RJ, Dugas M, Schachtner T, Kaufmann I, et al. Alpha-gluthathione S-transferase as an early marker of hepatic ischaemia/reperfusion injury after liver resection. World J Surg. 2005;29:528–534. doi: 10.1007/s00268-004-7431-3. [DOI] [PubMed] [Google Scholar]
- Isaksson B, D'Souza MA, Jersenius U, Ungerstedt J, Lundell L, Permert J, et al. Continuous assessment of intrahepatic metabolism by microdialysis during and after portal triad clamping. J Surg Res. 2011;169:214–219. doi: 10.1016/j.jss.2009.11.720. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Mayumi T, Arishima T, Takahashi H, Shikano T, Nakao A, et al. Hyperlactaemia can predict the prognosis of liver resection. Shock. 2007;28:35–38. doi: 10.1097/shk.0b013e3180310ca9. [DOI] [PubMed] [Google Scholar]
- Eyraud D, Richard O, Borie DC, Schaup B, Carayon A, Vézinet C, et al. Haemodynamic and hormonal responses to the sudden interruption of caval flow: insights from a prospective study of hepatic vascular exclusion during major liver resections. Anesth Analg. 2002;95:1173–1178. doi: 10.1097/00000539-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Chen XP, Zhang ZW, Zhang BX, Chen YF, Huang ZY, Zhang WG, et al. Modified technique of hepatic vascular exclusion: effect on blood loss during complex mesohepatectomy in hepatocellular carcinoma patients with cirrhosis. Langenbecks Arch Surg. 2006;391:209–215. doi: 10.1007/s00423-006-0043-7. [DOI] [PubMed] [Google Scholar]
- Choukèr A, Schachtner T, Schauer R, Dugas M, Löhe F, Martignoni A, et al. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. Br J Anaesth. 2004;93:204–211. doi: 10.1093/bja/aeh195. [DOI] [PubMed] [Google Scholar]
- Decailliot F, Cherqui D, Leroux B, Lanteri-Minet M, Ben Saïd S, Husson E, et al. Effects of portal triad clamping on haemodynamic conditions during laparoscopic liver resection. Br J Anaesth. 2001;87:493–496. doi: 10.1093/bja/87.3.493. [DOI] [PubMed] [Google Scholar]
- Gomez D, Homer-Vanniasinkam S, Graham AM, Prasad KR. Role of ischaemic preconditioning in liver regeneration following major liver resection and transplantation. World J Gastroenterol. 2007;13:657–670. doi: 10.3748/wjg.v13.i5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein O, Szvalb S, Van den Akker-Berman LM, Assy N. Liver regeneration is not altered in patients with non-alcoholic steatohepatitis (NASH) when compared to chronic hepatitis C infection with similar grade of inflammation. Dig Dis Sci. 2002;47:1926–1931. doi: 10.1023/a:1019679603042. [DOI] [PubMed] [Google Scholar]