Abstract
Objectives
Prevalences of bile duct injury (BDI) following laparoscopic cholecystectomy (LC) remain unacceptably high. There is no standardized method for performing an LC. This study aims to describe a standardized technique for LC that will allow for the development of a concept LC checklist, the use of which, it is hoped, will decrease the prevalence of BDI.
Methods
A standardized method for LC was developed based on previously published expert analysis supplemented by video error analysis of operations in which BDI occurred. Established checklist methodology was then used to construct an LC-specific concept checklist.
Results
A five-step technique for the safe establishment of the critical view was created to guide the development of the checklist. The five steps are: (i) confirm the gallbladder lies in the hepatic principal plane and is retracted to the 10 o'clock position; (ii) confirm Hartmann's pouch is lifted up and toward the segment IV pedicle; (iii) identify Rouvière's sulcus; (iv) confirm the release of the posterior leaf of the peritoneum covering the hepatobiliary triangle, and (v) confirm the critical view with or without intraoperative cholangiography.
Conclusions
A standardized approach to LC would allow for the creation of an LC-specific checklist that has the potential to lower the prevalence of BDI.
Introduction
Laparoscopic cholecystectomy (LC) is one of the most widely performed general surgical operations. Its uptake into surgical practice some 20 years ago was rapid, unregulated and associated with an increase in the incidence and severity of bile duct injury (BDI).1–4 Although the frequency of BDI is relatively low (<0.5%), the large number of LCs performed across communities mean prevalences remain unacceptably high.5
Bile duct injury is a catastrophic complication for the individual patient, resulting in significant reductions in the quality and quantity of life.6–9 However, there is no widely accepted standardized method for performing LC and over 70% of surgeons regard BDI as an unavoidable complication associated with the procedure.10 A change in attitude among surgeons is required if improvements in patient care are to be achieved.
Several authors1,11–22 have studied the mechanism of BDI and recommended a number of steps (Table 1) that may help to reduce the risk of incurring BDI, but these have not led to a systematic standardization of the technique of LC. Perhaps the most widely accepted technique refers to the critical view popularized by Strasberg et al.,14,15 although it is thought that up to 80% of BDIs occur while the surgeon is attempting to safely establish the critical view.11,23 Thus, the major issues concern misidentification and loss of awareness of the surroundings, which are subsequently reinforced by cognitive fixation and plan continuation.11,20
Table 1.
Steps recommended to reduce risk for bile duct injury in laparoscopic cholecystectomy (LC)
| Step | Reference(s) |
|---|---|
| Use a 30-degree scope | 12 |
| Use an experienced assistant | 11 |
| Ensure the lateral retraction of the fundus of the gallbladder | 12,13,15 |
| Ensure dissection is lateral to the cystic node | 12,15 |
| Stay on the border of the gallbladder within the window between the cystic artery and the cystic duct | 13,15 |
| Dissect the cholecysto–cystic duct junction toward the common bile duct | 12,13,15 |
| Avoid the use of diathermy | 13 |
| Release the anterior and posterior peritoneum | 11,15 |
| Use Rouvière's sulcus and the base of segment IV as fixed landmarks to aid orientation | 11,16,33 |
| Avoid dissection on the left side of the hepatoduodenal ligament | 13,22 |
| Ensure the routine use of intraoperative cholangiography | 1,12,19 |
| Perform subtotal cholecystectomy rather than fundus-first cholecystectomy in the event of a hostile hepatobiliary triangle | 17,18 |
| Develop a culture of safety when performing LC | 20–22 |
Checklists have been used widely throughout many industries to improve the completion of complex tasks, enhance communication and teamwork, and reduce error rates.24 Their recent introduction into surgery includes the widespread adoption of the World Health Organization (WHO) surgical checklist,25 which has been shown to reduce postoperative mortality across a wide range of hospitals and health systems internationally.25,26 Based on this success, several specialties have begun to develop similar tools.27,28
The present authors hypothesize that the development of a standardized method for LC might provide the basis for a checklist tool that will ultimately decrease the prevalence of BDI. The principal objectives would be to improve communication among the operating surgeon, assistant(s) and operating theatre staff, and to provide a clear and systematic check of the key steps involved in the safe completion of LC.
This paper therefore aims to set out a standard method for safe LC based on the safe achievement of the critical view. It then introduces a concept operation-specific checklist for LC.
Materials and methods
Previously published analyses of the mechanisms of BDI by recognized experts (Table 1) were reviewed. A critical appraisal of videos of LC in which BDI occurred and of videos of LC without BDI performed by surgeons who had recently incurred a BDI in another case was carried out by one of the authors (SJC). The critical steps leading to the BDI were identified by applying the principles of objective error analysis as described by Troidl.13 Specifically, the investigating author repetitively explored ‘why’ the critical view14,15 had not been achieved safely and how it could have been. This investigation specifically focused on manoeuvres specific to LC and assumed the operating team possessed the competence in generic skills required for safe laparoscopy and the provision of competent patient care. The critical steps identified were then reviewed by three surgeons (TJH, TBH, LN), who are recognized regional and international experts in the technical aspects of LC, and the aetiology, management and prevention of BDI.
The hepatobiliary triangle (Fig. 1) was defined as the space delineated by three borders: the common hepatic duct; the cystic duct, and the inferior border of the liver. This space is covered by anterior and posterior layers of peritoneum. The contents include the cystic artery and branches thereof and the cystic node. On occasion, the right hepatic artery traverses this space. This definition differs from that of the triangle described by Calot.29 It is incorrect and confusing to attach Calot's name to the hepatobiliary triangle (also referred to by some authors as the ‘cystohepatic triangle’), which is the surgically relevant anatomical area in LC.
Figure 1.
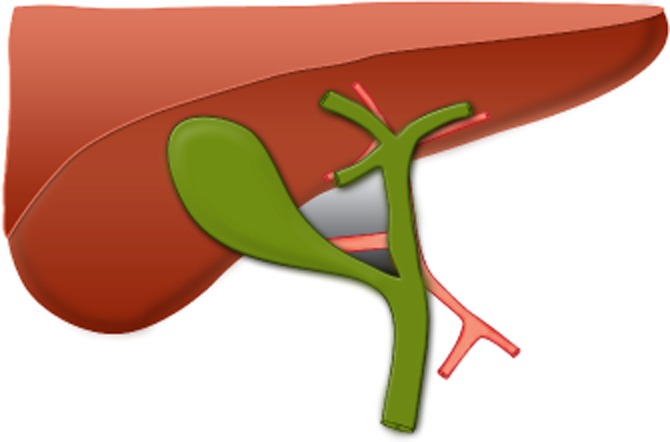
Landmarks of the hepatobiliary triangle. The grey area shows the hepatobiliary triangle, bounded by the common hepatic duct, cystic duct and inferior border of the liver. The smaller dark grey area shows Calot's triangle, bounded by the cystic duct and cystic artery and common hepatic duct
Having defined a standardized method for LC, a concept checklist was developed based on established checklist methodology.30
Results
Defining a standard method
This approach is set out in five parts. A visual explanation is shown in a video available through a hepatopancreatobiliary virtual journal club.31 It is recommended that this video is watched in conjunction with reading this article.
Confirm the gallbladder lies in the hepatic principal plane and is retracted cephalad to the 10 o'clock position
Cephalad retraction of the gallbladder is the first step in facilitating visualization of the gallbladder. It is important that the fundus of the gallbladder is displaced to the 10 o'clock position (Fig. 2a). When combined with Step 2, this will expose the maximum surface area of the posterior peritoneum of the hepatobiliary triangle to the operating surgeon. If the fundus is pushed cephalward, roughly parallel to the falciform ligament, the gallbladder will be placed in the 12 o'clock position (Fig. 2b). This reduces the surface area of the hepatobiliary triangle exposed posteriorly and obliges the surgeon to operate ‘front on’ to the gallbladder–cystic duct–common hepatic duct junction.
Figure 2.
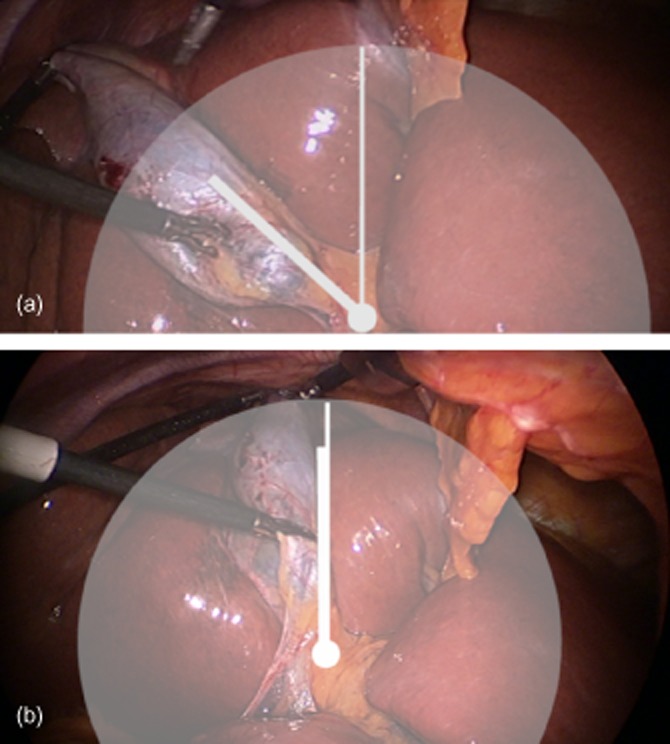
(a) Retracting the gallbladder to the 10 o'clock position assists in the eventual exposure of the posterior peritoneum covering the hepatobiliary triangle. (b) If the gallbladder is retracted cephalward and medially (12 o'clock), the surgeon is obliged to dissect the cystic duct front on
An important step in helping to achieve the 10 o'clock retraction involves ensuring that the lateral port is sited as far laterally as possible when it is inserted through the abdominal wall. If it is difficult to achieve this position as a result of underlying gallbladder neck obstruction, aspiration of the gallbladder may be helpful. In the elective setting, in patients with significant hepatic steatosis, a preoperative very low calorie diet should be considered in an effort to reduce hepatic volume.32
Confirm the lifting and positioning of Hartmann's pouch
Hartmann's pouch should be grasped and lifted up and across toward the origin of the segment IV pedicle (Fig. 3). If this step is prevented by a large impacted stone, it may be possible to ‘milk’ the stone back into the gallbladder or alternatively to open the gallbladder and remove the stone.
Figure 3.
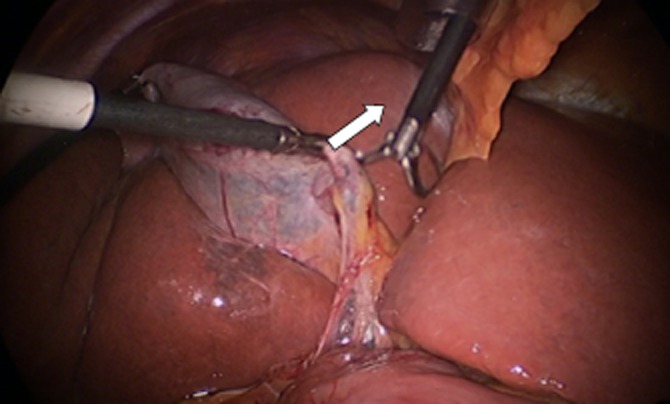
Hartmann's pouch should be lifted up and across toward the segment IV pedicle to maximize the exposure of the posterior peritoneum of the hepatobiliary triangle to the operating surgeon
Combining the retraction of Hartmann's pouch with Step 1 maximizes the exposure of the posterior peritoneum of the hepatobiliary triangle. Failure to perform this step and instead to simply lift and push Hartmann's pouch into the gallbladder fossa will minimize the size of the hepatobiliary triangle exposed to the surgeon. This will have two significant effects. Firstly, it will force the surgeon to dissect the hepatobiliary triangle ‘front on’ even if a 30-degree scope is used. Secondly, combined with the gallbladder's retraction to the 12 o'clock position, this creates a dangerous situation as the surgeon may be unaware of the location of the junction between the cystic duct and the common hepatic duct. This often obliges the surgeon to dissect the anterior peritoneal surface of the hepatobiliary triangle first, which may result in the inadvertent encirclement of the common bile duct.
Identify Rouvière's sulcus
Often BDI occurs while the surgeon is trying to establish the critical view.11 It is crucial that fixed extrabiliary landmarks are identified early to locate the level at which it is appropriate to commence dissection of the hepatobiliary triangle in order to reduce the risk that the operating surgeon will become spatially disoriented.11 Rouvière's sulcus, which marks the level of the right posterior portal pedicle, is such a landmark and is identifiable in at least 80% of patients.33 When viewed laparoscopically from the umbilicus with normal upward retraction of the gallbladder, an imaginary line drawn along the sulcus and carried across to the base of segment IV shows the level ventral to which dissection is ‘safe’ and dorsal to which it is not (Fig. 4). The importance of taking time to pull back the camera in order to observe the bigger picture and to identify this landmark cannot be overemphasized. This is especially true in the setting of severe acute cholecystitis, in the presence of a shrunken fibrotic gallbladder (common after endoscopic retrograde cholangiopancreatography), or if a large stone impacted in Hartmann's pouch is displacing the cystic duct. Retracting the duodenum downward may help to visualize the sulcus. It is important to note that vital structures can be drawn above Rouvière's line by excessive upward traction pulling Hartmann's pouch and the common bile duct anteriorly, by a shrunken gallbladder with a fibrosed hepatobiliary triangle or by displacement of the cystic duct by stones in Hartmann's pouch.
Figure 4.
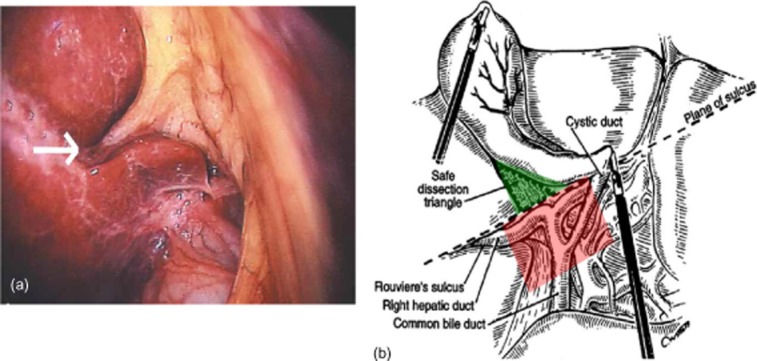
(a) Rouvière's sulcus (arrow) should be used as an extrabiliary landmark to guide the level of safe initial dissection [green in (b)]. (b) The imaginary line drawn between the sulcus and the base of segment IV indicates the level below which dissection should not occur (red). (Modified with permission from Hugh et al.33
Confirm the release of the posterior leaf of the peritoneum covering the hepatobiliary triangle
When checks 1–3 are completed, the posterior leaf of the peritoneum covering the hepatobiliary triangle is presented ‘face on’ to the surgeon; this is aided by the use of a 30-degree scope. This simple but crucial approach allows the hepatobiliary triangle to increase in size as the gallbladder is lifted away from the common hepatic duct. As the dissection proceeds layer by layer close to the gallbladder, the grasper holding Hartmann's pouch can be repositioned to hold the under-surface of the gallbladder, thus tensioning the tissue within the hepatobiliary triangle such that further dissection will expose the posterior surface of the cystic artery (Fig. 5). If diathermy is to be used for this dissection, it is crucial that the surgeon is aware of the danger of collateral damage to the common bile duct caused by arcing or indiscriminate use. In the acute setting, scissor dissection, blunt dissection with a sucker or hydrodissection may well facilitate the development of this plane. A small amount of bleeding may occur, but this usually stops spontaneously. In the presence of chronic inflammation and fibrosis, this plane may be difficult to dissect. This should alert the surgeon to the consideration of whether it is safe to continue laparoscopically or whether conversion to open surgery is advisable. This will be determined by the surgeon's experience in complex biliary surgery. Safe options include opening the gallbladder, evacuating the stones, controlling the cystic duct from within and subsequently leaving a drain.17 A fundus-first cholecystectomy in this setting is not recommended as it may result in extreme vasculobiliary injuries.18
Figure 5.
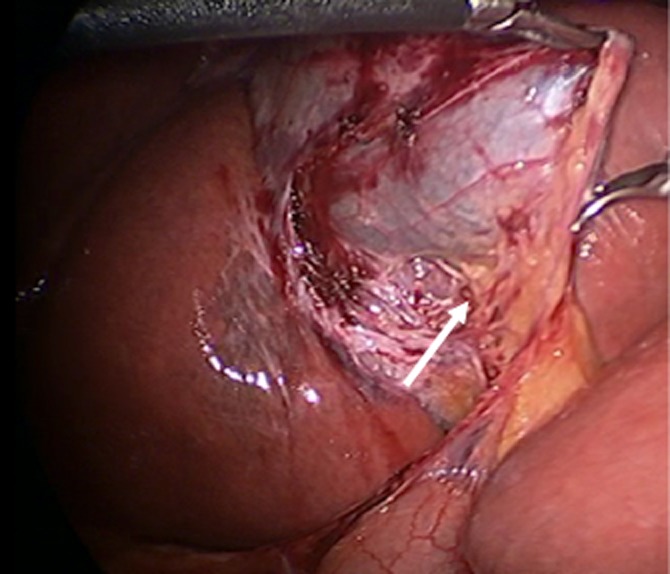
The posterior peritoneum of the hepatobiliary triangle should be released and dissection continued until the posterior surface of the cystic artery can be seen (arrow)
Although this step is not common practice for most surgeons, it is critical because the practice of approaching the posterior wall first prevents the inadvertent encirclement of the common bile duct.
Confirm the critical view and consider intraoperative cholangiography
Once posterior dissection is completed, traction on the fundus can be adjusted, Hartmann's grasper repositioned with traction laterally and inferiorly, and the view of the angled scope adjusted. The anterior leaf of the peritoneum covering the surface of the hepatobiliary triangle can now be dissected from an anterior aspect. By staying close to the gallbladder lateral to the cystic node and sometimes repeating the dissection from the posterior view, the critical view may safely be established. This has usually required the anterior and posterior peritoneum over the lower third of the gallbladder to be released.
Confirmation of the critical view should be achieved before the performance of intraoperative cholangiography (IOC). Those surgeons competent in IOC who wish to use a selective approach to IOC may replace this step by reconfirming the critical view with the assistant prior to clipping the cystic artery and duct (Fig. 6). The correct interpretation of an IOC requires the identification11 of five features: (i) duodenal flow; (ii) proximal filling of the common bile duct; (iii) three proximal hepatic ducts (right anterior, posterior sectoral and left main ducts); (iv) absence of filling defects within the common bile duct, and (v) the presence of spiral valves within the cystic duct. Although these are not always visualized on IOC, these features, when present, offer useful confirmation that it is the cystic duct that has been cannulated (Fig. 7).
Figure 6.
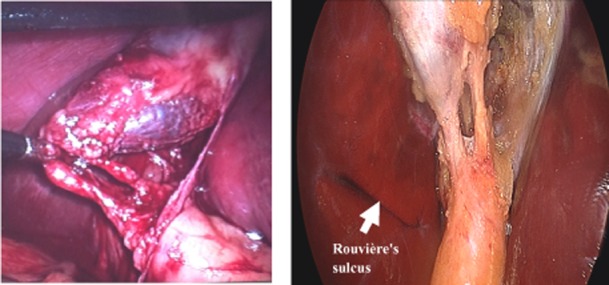
The critical view should be confirmed with the assistant. In these photographs, the gallbladder has been dissected off the cystic plate and only the dissected cystic artery and duct are visible within the hepatobiliary triangle
Figure 7.
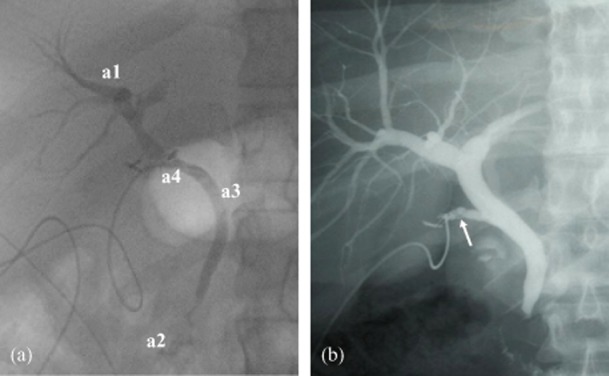
If intraoperative cholangiography (IOC) is performed, critical analysis using the IOC checklist should be applied. (a) This should confirm the presence of three hepatic ducts (right anterior, right posterior, left main) (a1), the filling of the duodenum (a2), the absence of filling defects (a3), and the presence of a cystic duct as indicated by the presence of spiral valves. In this cholangiogram the anatomy appears complete, but note the absence of the cystic duct and the cannulation of the common bile duct (a4), resulting in significant bile duct injury. (b) If present, the spiral valves in the cystic duct (arrow) may provide useful confirmation that the correct duct has been cannulated
To optimize the filling of the proximal ducts, the patient should be positioned head and right side down (opposite to the operating position). In addition, care should be taken not to feed the catheter too far into the common bile duct, which will result in the exit of dye straight into the duodenum. If flow into the duodenum does not occur, 1 mg glucagon may be administered i.v. to induce relaxation of the biliary sphincter. If the spiral cystic duct valves are not visualized, attempts to withdraw the catheter slowly may encourage the filling of the cystic duct.
A concept checklist
The concept checklist (Fig. 8) was constructed around three pause points. The first is a natural pause pre-incision and concurs with the WHO Surgical Safety Checklist.25 Its function is to alert the surgeon to patients who may be at high risk for BDI and to establish clear communication within the team. The second occurs pre-dissection, and requires a conscious halt in operative flow to establish the critical view. The third occurs at what is often a natural pause point when IOC is used. It reminds the surgeon to confirm the critical view if IOC is not used, and also sets out five factors that should be confirmed on IOC.
Figure 8.

Proposed format for a laparoscopic cholecystectomy checklist
Discussion
After more than two decades of experience with LC, the incidence and prevalence of iatrogenic BDI remain unacceptably high.5,34 Despite this, there is no widely accepted standardized method for performing LC and over 70% of surgeons regard BDI as an unavoidable complication associated with the procedure.10
This paper outlines a standardized method for performing LC and challenges the assumption that BDI is an unavoidable complication. In fact, the profession should expect and aim for a 0% rate of BDI. Such seemingly radical targets have been met in other areas of medicine, most notably in the elimination of central line infections in several intensive care units.35
The present authors propose a checklist for LC that aims to promote a defined standard approach. Adherence to this checklist should allow the critical view to be achieved safely. If the checklist cannot be completed, this failure should indicate that it is dangerous to persist and should prompt a ‘stop’ that generates an alternative approach. The checklist is designed to help overcome the psychological factors associated with BDI20 by involving a third party (such as when the operating surgeon seeks confirmation from the assistant or another surgeon) and by creating heightened awareness of the possibility of BDI when the information available suggests a deviation from the norm; it thus supports the adoption of a culture of safety.21
In addition, such checklists may improve teamwork and communication. However, much of the success of a checklist lies in its implementation. Buy-in on the part of the surgeon, assistant and nursing staff is essential; this is achieved by their early engagement and involvement in design. The issues of who will run the checklist and what physical form it should take must also be considered. This is ultimately a concept checklist and requires further refinement and rapid-cycle testing in order to better elucidate its usability and feasibility. The present authors aim to undertake this process and then to conduct a pilot study that explores pre- and post-implementation compliance with the assistance of video analysis.
High-level evidence suggests that the use of IOC is associated with reduced incidence and severity, and increased intraoperative recognition, of BDI.1,5,19,34,36 Despite this, perhaps no single technical issue in a general surgical procedure has generated as much controversy as IOC. However, IOC will not prevent the occurrence of all BDI.11 It has also been shown that IOCs may often be misinterpreted.37 Nonetheless, at a population level routine IOC is likely to be cost-effective.38 Such debate should not be allowed to distract from the main aim of this paper. The use of IOC is included because it would seem mandatory that a surgeon is competent in performing and interpreting an IOC.
In conclusion, the current incidence of BDI following LC remains unacceptably high. The profession needs to develop a standardized approach to performing LC which would potentially allow the introduction of a checklist with the aim of reducing the incidence of BDI.
Conflicts of interest
None declared.
References
- Fletcher DR, Hobbs MS, Tan P, Valinsky LJ, Hockey RL, Pikora TJ, et al. Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg. 1999;229:449–457. doi: 10.1097/00000658-199904000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM, Young W, Theriault ME, Hernandez R. Has laparoscopic cholecystectomy changed patterns of practice and patient outcome in Ontario? CMAJ. 1996;154:491–500. [PMC free article] [PubMed] [Google Scholar]
- Russell JC, Walsh SJ, Mattie AS, Lynch JT. Bile duct injuries, 1989–1993. A state-wide experience. Connecticut Laparoscopic Cholecystectomy Registry. Arch Surg. 1996;131:382–388. doi: 10.1001/archsurg.1996.01430160040007. [DOI] [PubMed] [Google Scholar]
- Richardson MC, Bell G, Fullarton GM. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: an audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br J Surg. 1996;83:1356–1360. doi: 10.1002/bjs.1800831009. [DOI] [PubMed] [Google Scholar]
- Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289:1639–1644. doi: 10.1001/jama.289.13.1639. [DOI] [PubMed] [Google Scholar]
- Flum DR, Cheadle A, Prela C, Dellinger EP, Chan L. Bile duct injury during cholecystectomy and survival in Medicare beneficiaries. JAMA. 2003;290:2168–2173. doi: 10.1001/jama.290.16.2168. [DOI] [PubMed] [Google Scholar]
- Boerma D, Rauws EA, Keulemans YC, Bergman JJ, Obertop H, Huibregtse K, et al. Impaired quality of life 5 years after bile duct injury during laparoscopic cholecystectomy: a prospective analysis. Ann Surg. 2001;234:750–757. doi: 10.1097/00000658-200112000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DE, Feurer ID, Holzman MD, Wudel LJ, Strickland C, Gorden DL, et al. Longterm detrimental effect of bile duct injury on health-related quality of life. Arch Surg. 2004;139:476–481. doi: 10.1001/archsurg.139.5.476. discussion 481–482. [DOI] [PubMed] [Google Scholar]
- Landman MP, Feurer ID, Moore DE, Zaydfudim V, Pinson CW. The longterm effect of bile duct injuries on health-related quality of life: a meta-analysis. HPB. 2013;15:252–259. doi: 10.1111/j.1477-2574.2012.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoeur JR, Wiseman K, Buczkowski AK, Chung SW, Scudamore CH. Surgeons’ anonymous response after bile duct injury during cholecystectomy. Am J Surg. 2003;185:468–475. doi: 10.1016/s0002-9610(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Hugh TB. New strategies to prevent laparoscopic bile duct injury – surgeons can learn from pilots. Surgery. 2002;132:826–835. doi: 10.1067/msy.2002.127681. [DOI] [PubMed] [Google Scholar]
- Hunter JG. Avoidance of bile duct injury during laparoscopic cholecystectomy. Am J Surg. 1991;162:71–76. doi: 10.1016/0002-9610(91)90207-t. [DOI] [PubMed] [Google Scholar]
- Troidl H. Disasters of endoscopic surgery and how to avoid them: error analysis. World J Surg. 1999;23:846–855. doi: 10.1007/s002689900588. [DOI] [PubMed] [Google Scholar]
- Strasberg SM, Sanabria JR, Clavien PA. Complications of laparoscopic cholecystectomy. Can J Surg. 1992;35:275–280. [PubMed] [Google Scholar]
- Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101–125. [PubMed] [Google Scholar]
- Slater K, Strong RW, Wall DR, Lynch SV. Iatrogenic bile duct injury: the scourge of laparoscopic cholecystectomy. ANZ J Surg. 2002;72:83–88. doi: 10.1046/j.1445-2197.2002.02315.x. [DOI] [PubMed] [Google Scholar]
- Michalowski K, Bornman PC, Krige JE, Gallagher PJ, Terblanche J. Laparoscopic subtotal cholecystectomy in patients with complicated acute cholecystitis or fibrosis. Br J Surg. 1998;85:904–906. doi: 10.1046/j.1365-2168.1998.00749.x. [DOI] [PubMed] [Google Scholar]
- Strasberg SM, Gouma DJ. Extreme vasculobiliary injuries: association with fundus-down cholecystectomy in severely inflamed gallbladders. HPB. 2012;14:1–8. doi: 10.1111/j.1477-2574.2011.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flum DR, Koepsell T, Heagerty P, Sinanan M, Dellinger EP. Common bile duct injury during laparoscopic cholecystectomy and the use of intraoperative cholangiography: adverse outcome or preventable error? Arch Surg. 2001;136:1287–1292. doi: 10.1001/archsurg.136.11.1287. [DOI] [PubMed] [Google Scholar]
- Dekker SWA, Hugh TB. Laparoscopic bile duct injury. Understanding the psychology and heuristics of the error. ANZ J Surg. 2008;78:1109–1114. doi: 10.1111/j.1445-2197.2008.04761.x. [DOI] [PubMed] [Google Scholar]
- Strasberg SM. Biliary injury in laparoscopic surgery. J Am Coll Surg. 201:604–611. doi: 10.1016/j.jamcollsurg.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Diamond T, Mole DJ. Anatomical orientation and cross checking – the key to safer laparoscopic cholecystectomy. Br J Surg. 2005;92:663–664. doi: 10.1002/bjs.4992. 2005. [DOI] [PubMed] [Google Scholar]
- Olsen D. Bile duct injuries during laparoscopic cholecystectomy. Surg Endosc. 1997;11:133–138. doi: 10.1007/s004649900315. [DOI] [PubMed] [Google Scholar]
- Gwande A. The Checklist Manifesto: How to Get Things Right. London: Profile Books; 2010. [Google Scholar]
- Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- de Vries EN, Prins HA, Crolla RM, den Outer AJ, van Andel G, van Helden SH, et al. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010;363:1928–1937. doi: 10.1056/NEJMsa0911535. [DOI] [PubMed] [Google Scholar]
- Ziewacz JE, Arriaga AF, Bader AM, Berry WR, Edmondson L, Wong JM, et al. Crisis checklists for the operating room: development and pilot testing. J Am Coll Surg. 2011;213:212–217. doi: 10.1016/j.jamcollsurg.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Spector JM, Agrawal P, Kodkany B, Lipsitz S, Lashoher A, Dziekan G, et al. Improving quality of care for maternal and newborn health: prospective pilot study of the WHO safe childbirth checklist programme. PLoS ONE. 2012;7:e35151. doi: 10.1371/journal.pone.0035151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubriuch WS. Calot of Calot's triangle. Gastroenterology. 2002;123:1440. doi: 10.1053/gast.2002.1231440. [DOI] [PubMed] [Google Scholar]
- Weiser TG, Haynes AB, Lashoher A, Dziekan G, Boorman DJ, Berry WR, et al. Perspectives in quality: designing the WHO Surgical Safety Checklist. Int J Qual Health Care. 2010;22:365–370. doi: 10.1093/intqhc/mzq039. [DOI] [PubMed] [Google Scholar]
- Connor S. 2012. Preventing bile duct injury Available at http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1477-2574/homepage/virtual_journal_club.htm#I2 (last accessed 27 November 2012)
- Lewis MC, Phillips ML, Slavotinek JP, Kow L, Thompson CH, Toouli J. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg. 2006;16:697–701. doi: 10.1381/096089206777346682. [DOI] [PubMed] [Google Scholar]
- Hugh TB, Kelly MD, Mekisic A. Rouviere's sulcus. A useful landmark in laparoscopic cholecystectomy. Br J Surg. 1997;84:1253–1254. doi: 10.1046/j.1365-2168.1997.02769.x. [DOI] [PubMed] [Google Scholar]
- Törnqvist B, Strömberg C, Persson G, Nilsson M. Effect of intended intraoperative cholangiography and early detection of bile duct injury on survival after cholecystectomy: population-based cohort study. BMJ. 2012;345:e6457. doi: 10.1136/bmj.e6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipitz-Snyderman A, Needham DM, Colantuoni E, Goeschel CA, Marsteller JA, Thompson DA, et al. The ability of intensive care units to maintain zero central line-associated bloodstream infections. Arch Intern Med. 2011;171:856–858. doi: 10.1001/archinternmed.2011.161. [DOI] [PubMed] [Google Scholar]
- Ludwig K, Bernhardt J, Steffen H, Lorenz D. Contribution of intraoperative cholangiography to incidence and outcome of common bile duct injuries during laparoscopic cholecystectomy. Surg Endosc. 2002;16:1098–1104. doi: 10.1007/s00464-001-9183-6. [DOI] [PubMed] [Google Scholar]
- Sanjay P, Tagolao S, Dirkzwager I, Bartlett A. A survey of the accuracy of interpretation of intraoperative cholangiograms. HPB. 2012;14:673–676. doi: 10.1111/j.1477-2574.2012.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flum DR, Flowers C, Veenstra DL. A cost-effectiveness analysis of intraoperative cholangiography in the prevention of bile duct injury during laparoscopic cholecystectomy. J Am Coll Surg. 2003;196:385–393. doi: 10.1016/S1072-7515(02)01806-9. [DOI] [PubMed] [Google Scholar]


