Abstract
Introduction
Xanthogranulomatous cholecystitis (XGC) is often mistaken for, and may predispose to, gallbladder carcinoma (GB Ca). This study reviews the worldwide variation of the incidence, investigations, management and outcome of patients with XGC.
Methods
Data from 29 studies, cumulatively containing 1599 patients, were reviewed and results summarized by geographical region (Europe, India, Far East and Americas) with 95% confidence intervals (CIs) to present variability within regions. The main study outcomes were incidence, association with GB Ca and treatment of patients with XGC.
Results
Overall, the incidence of XGC was 1.3–1.9%, with the exception of India where it was 8.8%. The incidence of GB Ca associated with XGC was lowest in European studies (3.3%) varying from 5.1–5.9% in the remaining regions. Confusion with or undiagnosed GB Ca led to 10.2% of patients receiving over or under treatment.
Conclusions
XGC is a global disease and is associated with GB Ca. Characteristic pathological, radiological and clinical features are shared with GB Ca and contribute to considerable treatment inaccuracy. Tissue sampling by pre-operative endoscopic ultrasound or intra-operative frozen section is required to accurately diagnose gallbladder pathology and should be performed before any extensive resection is performed.
Introduction
Xanthogranulomatous cholecystitis (XGC) is an uncommon form of cholecystitis1,2 which presents with non-specific symptoms and signs, making it indistinguishable from typical acute or chronic cholecystitis.3–5 XGC is traditionally a histopathological diagnosis of focal or diffuse acute and chronic cholecystitis. Microscopically, lipid containing histiocytes infiltrating into the outer layer of the muscle lining the gall bladder wall may be seen to form xanthogranulomatous foci and fibrosis owing to extravasation of bile into the gallbladder wall.6,7
XGC is frequently misdiagnosed as gallbladder carcinoma (GB Ca) on presentation,8,9 ultrasound,10,11 computed tomography (CT)12 and intra-operatively.8 This is confounded by reports that GB Ca is more frequently associated with XGC than other forms of cholecystitis.6,8,13–19 Rates of co-existing carcinoma vary from 2–15% of patients with XGC,8,20,21 suggesting a causal association.8 An incorrect diagnosis, which may be as high as 25%,3 results in inappropriate surgery in the form of open exploration or gallbladder bed resection for GB Ca3,22,23 rather than simple laparoscopic cholecystectomy for XGC or a missed diagnosis of GB Ca in patients undergoing a laparoscopic cholecystectomy8,9 who require open exploration or gallbladder bed resection. XGC also is associated with an increased rate of conversion from laparoscopic to open operative procedures5,9,24,25 owing to procedural difficulty related to local inflammation creating dense adhesions9,26,27 or gallbladder wall thickening.4
A pre-operative ultrasound and CT in patients with XGC may display specific features which are suggestive of XGC. These include the presence of intramural echogenic nodules,1,4,10,28,29 see Fig. 1, a ‘halo sign’ (a hypodense curvilinear band),1,4,10,15,29 intra-hepatic duct dilatation30 and a loss of interface between the gallbladder and the liver.4,7,11,31–34 Although these features are also seen in GB Ca, visualization of a continuous mucosal line,4,9,14,34,35 see Figs 1, 2 and 3, and the absence pericholecystic fluid,34 shown in Fig. 3, which both suggest a diagnosis of XGC rather than GB Ca, may be then used to differentiate between XGC and GB Ca. Although these features have been demonstrated to be effective at detecting XGC and differentiating XGC from GB Ca it is unclear, to date, how frequently these features are reported in routine clinical practice.
Figure 1.
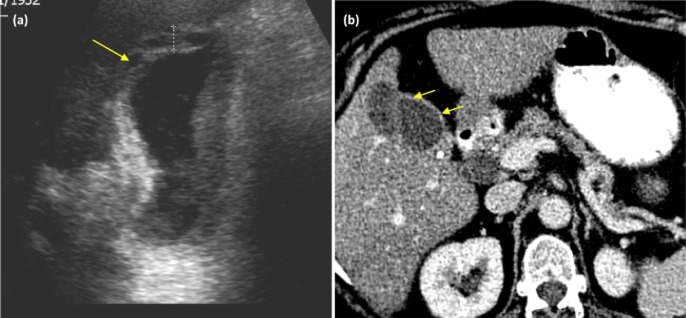
Imaging from patients with xanthogranulomatous cholecystitis (XGC). (a) An ultrasound scan demonstrating diffuse wall thickening (cursor) and intramural echogenic nodules (arrow). (b) Computed tomography (CT) post contrast image demonstrating continuous enhancement of the mucosal wall (small arrows)
Figure 2.
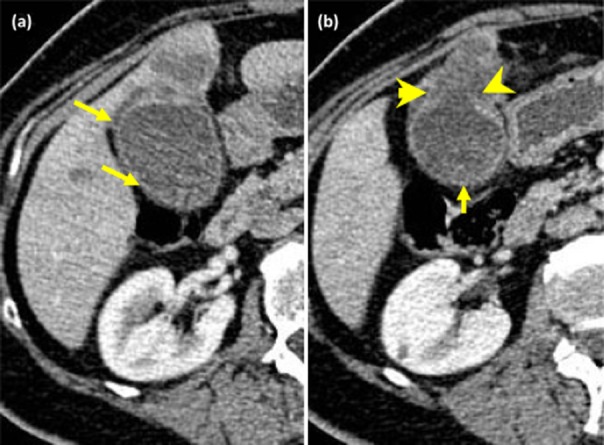
Computed tomography (CT) scans from patients with biopsy-proven gall bladder cancer. (a) Circumferential enhancement of the mucosa with focal wall thickening (small arrows). (b) Circumferential enhancement of the mucosa with focal thickening (small arrow) and breach of the mucosa (arrowheads) with invasion into segment 4b of the liver (contrast with the figure showing XGC)
Figure 3.
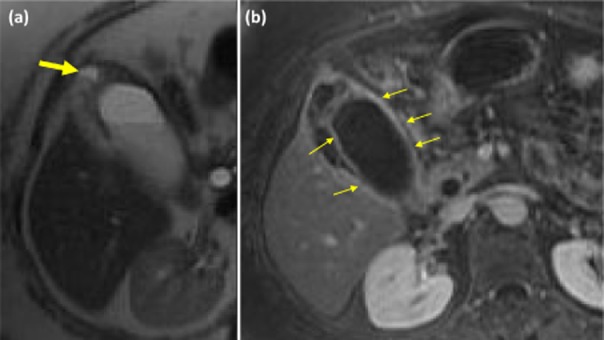
Magnetic resonance imaging demonstrating features of xanthogranulomatous cholecystitis (XGC). (a) A T2 weighted magnetic resonance image (MRI) demonstrating fluid within the wall of the gall bladder (large arrow). (b) A T1 weighted MRI demonstrating continuous enhancement of the mucosal line (small arrows)
The incidence of GB Ca is higher in Indian and Far Eastern populations than in Western populations.36 As there are a greater number of studies originating from Indian and Far Eastern groups there may also be geographical variation in the incidence, treatment and outcomes of patients with XGC. The aim of this study was to identify the incidence of XGC within a European cohort of patients and to pool these data with published studies to explore geographical variation in rates of XGC and associated GB Ca with the intention of providing the most accurate indication of the true incidence of XGC, its association with GB Ca and outcomes. Secondary outcomes of the study aim to review the proportion of patients who receive correct treatment and the findings and reporting frequency of radiographical investigations for XGC and GB Ca.
Methods
This study was a retrospective study of all patients who underwent a cholecystectomy at St James's University Hospital (SJUH), Leeds, United Kingdom, from June 2005 to December 2010. Patients were identified from the department of pathology database and those with XGC selected for detailed review. Patient notes were reviewed to obtain the presenting signs and symptoms, patient demographic variables, findings at radiological intervention (mucosal line continuity, nodule echogenicity, halo sign, visible loss of interface between the gallbladder and liver, pericholecystic fluid and hepatic duct dilation), the planned and actual operation type, complications, if GB Ca was suspected and histopathological data (gall stones, gallbladder thickening, gallbladder fibrosis and the presence of GB Ca).
Pooling data with other studies
Previously published studies were identified by review of PubMed, Medline, the Cochrane Library and Embase using the search terms ‘xanthogranulomatous cholecystitis’ and ‘fibroxanthogranulomatous fibrosis’ (Fig. 4). The reference lists of these studies were searched to identify further studies of relevance. Case reports and case series containing five or less patients with XGC were excluded from review. Of 31 suitable studies, three were excluded as the full paper was not able to be accessed16,20,37 leaving 29 cohorts, including the present study, for review. Included studies (Table 1) were reviewed for patient demographics, investigative findings, management, operative findings and outcomes, histopathological details of the excised gallbladder, operative complications and the incidence of XGC in the local population. The diagnostic accuracy was also calculated for each study as [1 – (number of missed GB Ca cases + number inappropriate extended resections performed for incorrectly suspected GB Ca)/ total number of XGC patients] × 100. Studies that reported pre-operative radiological findings were recorded.
Figure 4.
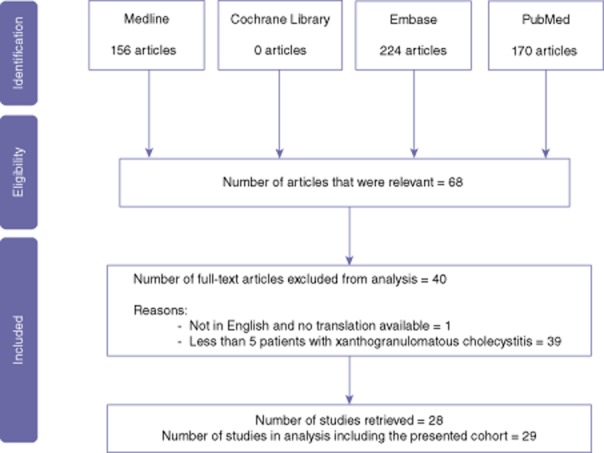
A summary of the search strategy in this review
Table 1.
A summary of the published case series included in the dataset of this study
| Year | Author | Country of origin | Group | N with XGC | Reference |
|---|---|---|---|---|---|
| 1981 | Goodman et al. | USA | Americas | 40 | 11 |
| 2004 | Valdivia et al. | Mexico | Americas | 182 | 5 |
| 2008 | Srivastava et al. | USA | Americas | 11 | 70 |
| 2010 | Goshhima et al. | USA | Americas | 18 | 29 |
| 1987 | Roberts et al. | UK | Europe | 13 | 34 |
| 1994 | Houston et al. | UK | Europe | 31 | 14 |
| 1996 | Casas et al. | Spain | Europe | 12 | 1 |
| 2000 | Parra et al. | Spain | Europe | 26 | 4 |
| 2003 | Karabulut et al. | Turkey | Europe | 12 | 40 |
| 2007 | Srinivas et al. | UK | Europe | 29 | 24 |
| 2013 | Hale et al. | UK | Europe | 42 | Present study |
| 1987 | Hanada et al. | Japan | Far East | 23 | 31 |
| 1999 | Mori et al. | Japan | Far East | 45 | 71 |
| 1999 | Kim et al. | Korea | Far East | 19 | 30 |
| 2004 | Kwon et al. | Japan | Far East | 27 | 21 |
| 2007 | Yang et al. | China | Far East | 33 | 3 |
| 2007 | Kwon et al. | Japan | Far East | 60 | 72 |
| 2007 | Sawada et al. | Japan | Far East | 24 | 73 |
| 2009 | Uchiyama et al. | Japan | Far East | 32 | 7 |
| 2010 | Mekky et al. | Japan | Far East | 6 | 68 |
| 2010 | Chang et al. | South Korea | Far East | 49 | 33 |
| 1998 | Dixit et al. | India | India | 41 | 2 |
| 2004 | Srikanth et al. | India | India | 29 | 23 |
| 2008 | Kansakar et al. | India | India | 33 | 24 |
| 2008 | Kumar et al. | India | India | 620 | 41 |
| 2009 | Kapoor et al. | India | India | 34 | 63 |
| 2010 | Agrawal et al. | India | India | 42 | 74 |
| 2010 | Jayalekshmi et al. | India | India | 9 | 75 |
| 2010 | Rastogi et al. | India | India | 57 | 35 |
Studies were grouped into geographical location of the reporting institution: European (n = 7), Americas (n = 4), Far Eastern (n = 10) and Indian (n = 8), to permit comparison among geographical regions.
A medical statistician (J.H.) provided advice regarding statistical interpretation and preparation of graphical comparison of the reported incidence of: XGC amongst all resected gallbladders, associated gall stones or GB Ca, gender of patients with XGC and the age of patients with XGC. Data for each of the outcomes considered were collected from each of the reported studies. Where possible, 95% confidence intervals (CIs) were produced to give an indication of the degree of variability in the data. The studies within each geographical group were then combined to produce pooled estimates of each outcome, which were also plotted.
Results
Present study cohort
Of the 4773 cholecystectomies performed at SJUH during the study period, 42 (0.9%) were diagnosed as XGC on pathological review; there were no patients with XGC associated with GB Ca. The mean (±standard deviation) age of patients was 63 years (±10.1). Eighteen patients were male. Data detailing presenting features, investigations, treatment, pathological findings and outcomes are given in Table 2. No patients had a pre-operative diagnosis of XGC. Five patients were suspected to have GB Ca on the basis of findings on at least one radiological modality. After further investigation, two patients were treated with a laparoscopic cholecystectomy only. One patient with suspected GB Ca underwent a staging laparoscopy followed by a planned gallbladder bed resection and lymphadenectomy but at open operation no cancer was observed and an open cholecystectomy was performed. In two further patients, both undergoing a hepatectomy for colorectal liver metastases, early GB Ca was suspected owing to macroscopic features and the gallbladder bed was included within the resection. Treatment inaccuracy in this cohort was therefore 3/42.
Table 2.
Pre-operative data, investigations, treatment and outcome of the present cohort
| N | ||
|---|---|---|
| Presenting feature | Generalized abdominal pain | 28 |
| N = 32a | Right upper quadrant pain | 23 |
| Nausea | 9 | |
| Vomiting | 11 | |
| Fever | 7 | |
| Jaundice | 6 | |
| Weight Loss | 4 | |
| Anorexia | 3 | |
| Murphy's sign | 3 | |
| Right upper quadrant mass | 1 | |
| Incidental finding at laparotomy | 2 | |
| Investigation: USS | Suspected cancer | 4 |
| N = 35b | Nodule echogenicity reported | 2 |
| Intramural nodules/bands reported | 1 | |
| Halo sign reported | 0 | |
| Investigation: CT | Suspected cancer | 1 |
| N = 16b | Loss of interface between GB and liver | 2 |
| Pericholecystic fluid | 11 | |
| Intra-hepatic duct dilation | 9 | |
| Pathology | Carcinoma or dysplasia | 0 |
| N = 42 | Gallbladder fibrosis | 25 |
| Gallbladder thickening | 30 | |
| Gall stones | 33 | |
| Choledocholithiasis | 6 | |
| Fistula | 4 | |
| Intervention N = 42 |
Laparoscopic procedure (converted to open) |
36 (7) |
| Planned open cholecystectomy | 2 | |
| Gallbladder bed resection | 2 | |
| Complications N = 42 |
Wound infection | 4 |
10 sets of patients emergency admission records were incomplete or missing.
Every patient received either an USS or CT but not every patient received both.
CT, computed tomography; USS, ultrasound scan; GB, gall bladder.
Review of published studies
Epidemiology
The pooled epidemiological and demographic data of the studies are displayed in Table S1. Of the 42 719 cholecystectomies performed in studies which reported the incidence of XGC (n = 19), 1321 patients (3.1%) were found to have XGC. There appeared to be a higher incidence of XGC in Indian patients (780 of 8913 gallbladders, 8.8%) but a comparable incidence among European, Americas or Far Eastern cohorts (1.3%, 1.5% and 1.9%, respectively, Fig. 5a). There was little variation in patient gender among regions (Fig. 5b). The mean age of all patients for whom data was available (n = 1458) was 53.1 years.2–7,9,15,22,25,26,30–32,34,35,38–44 The mean age varied from 48.7 to 62.4 years among regions (Fig. 5c).1–7,9,15,22,25,26,30–32,38,40–44
Figure 5.
(a) Incidence of xanthogranulomatous cholecystitis (XGC) in resected gallbladders. (b) Proportion of XGC patients that are male. (c) Mean ages of XGC patients. (d) Proportion of XGC patients with gall stones. (e) Proportion of XGC patients with coexisting cancer. The circle represents the mean value for each study, the horizontal bars represent 95% confidence intervals for each study and the diamond represents the pooled data for all studies in each geographical region
Pre-operative symptoms and signs
In studies reporting pre-operative symptoms and signs, the most common symptom was abdominal pain which affected 906 of 1071 patients (84.6%);2,5,9,22,26,31,32,34,40,44 nausea affected 42/164 patients (25.6%);1–3,7,9,26 vomiting affected 34/152 patients (22.4%);2,3,7,9,26 recent weight loss in 10/112 patients (8.9%); and9,15,34 anorexia in 23/128 patients (18%).2,9,34,45 The most common associated clinical feature was a positive Murphy's sign, present in 70/132 patients (53%);2–4,9 jaundice was present in 106/517 patients (20.5%)1,3,5,7,9,15,22,25,26,29,34,38,40 and a right upper quadrant mass was noted in 37/389 patients (9.5%).1–3,5,15,25,34,38 GB Ca was suspected in 190/1195 (15.8%) patients overall (range of 12.1 to 21.8% among regions)1–5,7,15,24,25,32,35,38,40,44 and this study (Table S1).
Pathological data
Within the studies reporting pathological data, gall stones were present in 1176/1366 (86.1%; 69 to 95.9% among regions) (Fig. 5d).1–7,9,15,22,25,30–32,34,38,40–42,44,45 Owing to wide variation reported within geographical regions it was not possible to identify whether there was a significant effect among geographical regions. The incidence of GB Ca coexisting with XGC was observed in 0–20% of patients among all studies.1–7,9,15,22,24–26,31,32,34,35,38,40,44–46 Eleven of 23 studies, representing 326 patients, reported no patients with XGC and GB Ca1,2,7,9,24,32,35,39,45 and this study. The pooled incidence of coexisting GB Ca with XGC across all geographical regions was 75/1449 (5.2%; 3.3 to 5.9% among regions).1–7,9,15,22,24–26,31,32,34,35,38,40,44–46 There was greater variation within, rather than among, regions suggesting reporting bias or local influence instead of geographical variation (Fig. 5e). A grossly thickened GB wall was commonly observed, 974/1209 (80.6%) patients, regardless of the geographical region.1–5,7,9,15,25,26,30–32,34,44,45 A coexisting fistula between the GB and adjacent organ was present in 108/1099 (9.8%; 1.1 to 12.1% among regions) (Table S1).3–5,7,9,15,22,25,26,31,38,44
Surgical data
The accuracy of performing the correct operation, calculated for 11 studies (determined by the frequency of missed GB Ca and incorrect diagnosis of GB Ca), was 338/376 patients (89.9%).3,4,9,15,22,25,26,35,45,46 Of the 38 patients receiving the incorrect treatment, 33 patients (8.8%) received an unnecessary extended operation for incorrectly suspected GB Ca and 5 patients (1.3%) were treated with a cholecystectomy only and were post-operatively diagnosed with GB Ca. There was little variation in the accuracy among geographical regions (Table S1). There was insufficient data in published studies to calculate the accuracy for studies from the Americas. There was wide variation in the proportion of patients for whom treatment was attempted laparoscopically, being lowest in India and the Americas (33.3 and 22.5%, respectively) and highest in Europe and the Far East (74.3% and 79.7%, respectively).4,5,9,22,25,26,38 The conversion rate from a laparoscopic to open cholecystectomy varied widely being lowest in Europe and the Far East but approximately three to four times greater in the Americas and India (Table S1). The proportion of patients who developed post-operative complications varied from 4.9% to 20.1% among regions.
Radiological features of XGC
The presence of intramural nodules/bands was reported in six studies (four of which reported the echogenicity of these nodules),4,9,29–31,34 the halo sign in three studies,1,4,32 the interface between the gallbladder and liver in three studies,1,30,31 pericholecystic fluid in five studies,1,15,25,31,38 intra-hepatic duct dilation in three studies4,30,31 and mucosal line continuity in five studies.9,30,31,34,45
Discussion
XGC remains a poorly understood condition and patients risk being over or under treated as a result of confusion with, or the masking of, GB Ca.3 Associated fistulae to adjacent organs and chronic inflammation contribute to the high rate of conversion of laparoscopic into open procedures9 and post-operative complications.4,7 In spite of its importance, however, the true incidence of XGC is still largely unknown with a wide variation reported in the literature.47,48 In addition, although characteristic features on radiological investigation have been shown to be indicative of XGC or discriminative between XGC and GB Ca,1,4 these are infrequently reported on in clinical practice. This study presents the largest European series to date together with all previously published series in order to gain a worldwide perspective of XGC aiming to identify geographical differences in presentation or management.
The most frequent clinical presentation of patients in the pooled cohort was non-specific abdominal pain followed by other symptoms and signs typical of cholecystitis demonstrating that XGC is typically indistinguishable from cholecystitis on clinical assessment.3–5 Gall stones are strongly associated with XGC but are not present in every patient, indicating a role for additional aetiological factors. In the SJUH study, 33 of 42 patients had associated with gall stones, fewer than in previously published European studies which have reported between 92% to 100%.4,38 The prevalence of inflammatory adhesions, fistulae to adjacent organs and post-operative infection rates, which are higher than would be expected after a routine cholecystectomy, reflects the chronic inflammatory nature of this condition. For these reasons a high proportion of laparoscopic operations are converted to an open procedure.3,9,17,26
This study suggests there may be a geographical influence upon the incidence of XGC with rates being approximately three to four times greater in India than in other geographical regions. This has been hypothesized to be because of the increased incidence of gall stones in India44 which was also observed in this study. Gall stones are believed to be causative of XGC owing to causing obstruction of the cystic duct, biliary stasis and gall bladder mucosa ulceration, all of which facilitate local inflammation and extravasation of bile into the gallbladder wall.2,5 There was, however, a wide reported incidence within studies from both the Far East and India. The studies with the smallest number of patients undergoing a cholecystectomy tended to be those reporting the highest incidence of XGC. This suggests there may be reporting bias. However, the largest study from India reviewed 6150 gallbladder specimens in which 620 gallbladders (10.1%) demonstrated XGC, supporting this association. The male-to-female ratio appears to be equal with little geographical influence. A cholecystectomy is most commonly performed in females;49 in 2010–11 43 757 of 58 769 (74.5%) patients undergoing a cholecystectomy in the United Kingdom were female.50 Thus gender does not appear to be a risk factor for XGC while female gender is for biliary colic and uncomplicated cholecystitis.
Several previous authors have suggested an association between XGC and GB Ca.6,8,13–19 The incidence of GB Ca concurrent with XGC displayed little geographical variation, being present in 5.2% of XGC patients in the pooled cohort. This is higher than the reported incidence of GB Ca within cholecystectomy specimens which does vary according to geographical location and has been reported as being: in Europe 0.09 to 1.1% of patients,51–55 in India 0.59 to 1.9%56–61 and in the Far East as 0.3 to 4.7%.62–69 Gall stones were present in the majority of patients with XGC but not in every patient suggesting a causal role together with other as yet unidentified factors. The variation in incidence among regions is difficult to interpret owing to wide variation amongst studies within the same region. The mechanism of a relationship between XGC and GB Ca remains unclear, however, a higher incidence of loss of heterozygosity (LOH) of the fragile histadine triad (FHIT) tumour suppressor gene in XGC compared with chronic cholecystitis implicates a genetic basis for XGC and a link with GB Ca (in which LOH was not significantly greater than in XGC).70 The presence of the association of XGC with GB Ca within each population subgroup indicates this association may be irrespective of the genetic and environmental variation in XGC among the different geographical populations studied.
XGC, or rather its association, with GB Ca is responsible for approximately 1 in 10 patients being either over-treated with unnecessary major surgery or under-treated for missed GB Ca. Identifying diagnostic radiological features of XGC is therefore desirable to avoid unnecessary morbidity. However, many of the characteristic features of XGC demonstrated by radiological investigation are shared with GB Ca.4,9,14,34,35 It has been suggested that the different frequency of these features between XGC and GB Ca can be used to differentiate the two pathological entities.30,34 For example, focal GB wall thickening favours GB Ca while diffuse thickening favours XGC.34 Early GB wall enhancement, loss of a continuous mucosal line and the absence of gall stones similarly favour GB Ca.34 Although the proportion of patients displaying these features is significantly different among those with XGC and GB Ca they are not diagnostic. Reliance upon these characteristics to decide upon treatment may result in patients continuing to receive under or over treatment. Endoscopic ultrasound (EUS) can identify hyperechoic nodules associated with XGC but loss of structure of the multilayered GB wall and local infiltration seen at EUS are also features common with GB Ca.28 It has been suggested that fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) may be effective in diagnosing GB Ca,71–73 however, FDG-PET for GB lesions should be interpreted with caution owing to the inflammatory nature of XGC which is likely to yield a false-positive result.74 One patient in the present cohort had a false-positive FDG-PET result suggesting GB Ca. Thus, on radiological imaging alone, differentiating GB Ca from XGC with a high degree of accuracy is not possible. Targeted tissue sampling at EUS, however, has been shown to identify GB Ca with 93% accuracy in a study of patients with suspected XGC and/or GB Ca.45 Although it is not possible to compare the diagnostic accuracy of patients who were and were not treated with EUS, owing to the data being obtained from different patient cohorts, EUS provides a clear advantage in that it permits assessment of targeted material. Owing to the rarity of both XGC and GB Ca, the cost of performing EUS on patients in whom the diagnosis is uncertain would be small. The increased use of intra-operative frozen section analysis, which is the most effective method of diagnosing XGC,20,75 will also prevent unnecessary extended resections from being performed on XGC patients. A radical cholecystectomy with resection of the liver bed and lymphadenectomy without bile duct resection or hepaticojejunostomy appears to have a slightly greater mortality than open cholecystectomy alone.76,77 The risk of a radical cholecystectomy, however, may be offset by this procedure carrying a lower risk of potentially spilling bile in a patient with GB Ca than an open cholecystectomy alone, which significantly reduces survival in these patients.78 Therefore, in cases of uncertainty a hepatopancreatobiliary surgeon should resect the GB bed and send an intact GB for pathological assessment. If a non-HPB surgeon encounters a case of suspected GB Ca they should abandon the procedure before mobilization or request the attendance of a HPB surgeon. The advised management of cases is detailed in Fig. 6.
Figure 6.
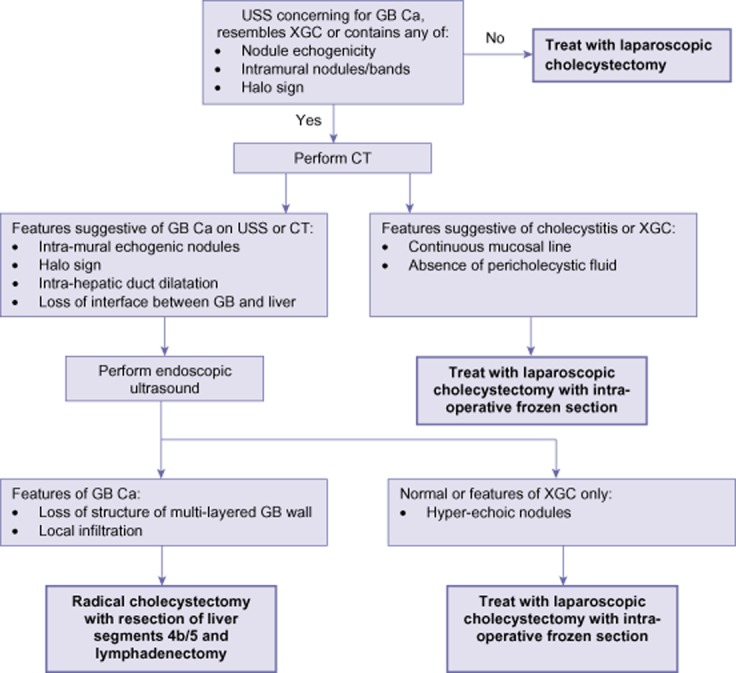
A suggested treatment pathway for patients with ultrasounds features suggesting XGC or GB Ca.CT: computed tomography scan; GB Ca: gall bladder carcinoma; GB: gall bladder; USS: ultrasounds scan; XGC: xanthogranulomatous cholecystitis
This study also demonstrated that patients with XGC exhibit a greater rate of post-operative complications than those undergoing a routine cholecystectomy without XGC49 in all geographical regions, although this is lower in the Americas for reasons which are unclear. This may reflect the presence of chronic inflammation and high rates of open procedures. In the majority of studies the nature of post-operative complications were not defined highlighting the importance of adopting standard definitions and reporting of common clinical complications.
The major limitation of this study was that the completeness of the dataset is subject to the reporting quality of the included studies. Additionally, as this study was unable to access the original data it was not possible to perform statistical significance testing among different geographical groups.
Conclusion
This study has demonstrated that XGC is a difficult condition to diagnose pre-operatively and that confusion between XGC and GB Ca results in incorrect treatment for over 1 in 10 patients with XGC. The association of XGC with an increased rate of GB Ca is highly likely within European, American, far Eastern and Indian populations. It is the recommendation of this study that characteristic features seen at radiological intervention be used to alert clinicians to the possibility of XGC. These radiological findings cannot be relied upon to accurately diagnose XGC or exclude coexisting GB Ca, however, and thus pre-operative investigation with EUS and tissue sampling is also required. Intra-operative frozen section analysis should be considered mandatory in patients with suspected GB Ca to permit accurate operative interventions.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
A summary of the incidence of xanthogranulomatous cholecystitis (XGC) and associated findings overall and by geographical region reported in the 29 studies reviewed.
References
- Casas D, Perez-Andres R, Jimenez J, Mariscal A, Cuadras P, Salas M, et al. Xanthogranulomatous cholecystitis: a radiological study of 12 cases and review of the literature. Abdom Imaging. 1996;460:456–460. doi: 10.1007/s002619900104. [DOI] [PubMed] [Google Scholar]
- Dixit VK, Prakash A, Gupta A, Pandey M, Gautam A, Kumar M, et al. Xanthogranulomatous cholecystitis. Dig Dis Sci. 1998;43:940–942. doi: 10.1023/a:1018802028193. [DOI] [PubMed] [Google Scholar]
- Yang T, Zhang B, Zhang J, Zhang Y, Jiang X, Wu M. Surgical treatment of xanthogranulomatous cholecystitis: experience in 33 cases. Hepatobiliary Pancreat Dis Int. 2007;6:504–508. [PubMed] [Google Scholar]
- Parra J, Acinas O, Bueno J, Güezmes A, Fernández MA. Xanthogranulomatous cholecystitis: clinical, sonographic, and CT findings in 26 patients. AJR Am J Roentgenol. 2000;147:979–983. doi: 10.2214/ajr.174.4.1740979. [DOI] [PubMed] [Google Scholar]
- Guzmán-Valdivia G. Xanthogranulomatous cholecystitis: 15 years' experience. World J Surg. 2004;28:254–257. doi: 10.1007/s00268-003-7161-y. [DOI] [PubMed] [Google Scholar]
- Goodman Z, Ishak K. Xanthogranulomatous cholecystitis. Am J Surg Pathol. 1981;5:653–659. doi: 10.1097/00000478-198110000-00007. [DOI] [PubMed] [Google Scholar]
- Roberts K, Parsons M. Xanthogranulomatous cholecystitis: clinico- pathological study of 13 cases. J Clin Pathol. 1987;40:412–417. doi: 10.1136/jcp.40.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbow EW. Xanthogranulomatous cholecystitis associated with carcinoma of the gallbladder. Postgrad Med J. 1989;65:528–531. doi: 10.1136/pgmj.65.766.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama K, Ozawa S, Ueno M, Hayami S, Hirono S, Ina S, et al. Xanthogranulomatous cholecystitis: the use of preoperative CT findings to differentiate it from gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:333–338. doi: 10.1007/s00534-009-0067-9. [DOI] [PubMed] [Google Scholar]
- Lichtman JB, Varma VA. Ultrasound demonstration of xanthogranulomatous cholecystitis. J Clin Ultrasound. 1987;15:342–345. doi: 10.1002/jcu.1870150509. [DOI] [PubMed] [Google Scholar]
- Düber C, Störkel S, Wagner PK, Müller J. Xanthogranulomatous cholecystitis mimicking carcinoma of the gallbladder: CT findings. J Comput Assist Tomogr. 1984;8:1195–1198. doi: 10.1097/00004728-198412000-00034. [DOI] [PubMed] [Google Scholar]
- Levy A, Murakata L, Abbott R, Rohrmann C. Benign tumors and tumorlike lesions of the gallbladder and extrahepatic bile ducts: radiologic-pathologic correlation. Radiographics. 2002;22:387–413. doi: 10.1148/radiographics.22.2.g02mr08387. [DOI] [PubMed] [Google Scholar]
- Ros R, Goodman D. Xanthogranulomatous versus Gallbladder Cholecystitis Carcinoma. Radiology. 1997;203:10–12. doi: 10.1148/radiology.203.1.9122374. [DOI] [PubMed] [Google Scholar]
- Kitagawa S, Nakagawa M, Yamada T, Mori Y, Simizu H, Rin S, et al. Clinico-pathological study of xanthogranulomatous cholecystitis. Nihon Geka Gakkai Zasshi. 1990;91:1001–1010. [PubMed] [Google Scholar]
- Houston JP, Collins MC, Cameron I, Reed MW, Parsons MA, Roberts KM. Xanthogranulomatous cholecystitis. Br J Surg. 1994;81:1030–1032. doi: 10.1002/bjs.1800810735. [DOI] [PubMed] [Google Scholar]
- Dao AH, Wong SW, Adkins RB. Xanthogranulomatous cholecystitis. A clinical and pathologic study of twelve cases. Am Surg. 1989;55:32–35. [PubMed] [Google Scholar]
- Benbow EW, Taylor PM. Simultaneous xanthogranulomatous cholecystitis and primary adenocarcinoma of gallbladder. Histopathology. 1988;12:672–675. doi: 10.1111/j.1365-2559.1988.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Shukla VK, Khandelwal C, Roy SK, Vaidya MP. Primary carcinoma of the gall bladder: a review of a 16-year period at the University Hospital. J Surg Oncol. 1985;28:32–35. doi: 10.1002/jso.2930280109. [DOI] [PubMed] [Google Scholar]
- Pandey M, Khatri AK, Sood BP, Shukla RC, Shukla VK. Cholecystosonographic evaluation of the prevalence of gallbladder diseases. A university hospital experience. Clin Imaging. 1996;20:269–272. doi: 10.1016/0899-7071(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Krishnani N, Dhingra S, Kapoor S, Pandey R. Cytopathologic diagnosis of xanthogranulomatous cholecystitis and coexistent lesions. A prospective study of 31 cases. Acta Cytol. 2007;51:37–41. doi: 10.1159/000325680. [DOI] [PubMed] [Google Scholar]
- Lee HS, Joo KR, Kim DH, Park NH, Jeong YK, Suh JH, et al. A case of simultaneous xanthogranulomatous cholecystitis and carcinoma of the gallbladder. Korean J Intern Med. 2003;18:53–56. doi: 10.3904/kjim.2003.18.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon A-H, Matsui Y, Uemura Y. Surgical procedures and histopathologic findings for patients with xanthogranulomatous cholecystitis. J Am Coll Surg. 2004;199:204–210. doi: 10.1016/j.jamcollsurg.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Spinelli A, Schumacher G, Pascher A, Lopez-hanninen E, Al-abadi H, Benckert C, et al. Extended surgical resection for xanthogranulomatous cholecystitis mimicking advanced gallbladder carcinoma: a case report and review of literature. World J Gastroenterol. 2006;12:2293–2296. doi: 10.3748/wjg.v12.i14.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth G, Kumar A, Khare R, Siddappa L, Gupta A, Sikora SS, et al. Should laparoscopic cholecystectomy be performed in patients with thick-walled gallbladder? J Hepatobiliary Pancreat Surg. 2004;11:40–44. doi: 10.1007/s00534-003-0866-3. [DOI] [PubMed] [Google Scholar]
- Srinivas GNS, Sinha S, Ryley N, Houghton PWJ. Perfidious gallbladders – a diagnostic dilemma with xanthogranulomatous cholecystitis. Ann R Coll Surg Engl. 2007;89:168–172. doi: 10.1308/003588407X155833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansakar PBS, Rodrigues G, Khan SA. Xanthogranulomatous cholecystitis: a clinicopathological study from a tertiary care health institution. Kathmandu Univ Med J (KUMJ) 2008;6:472–475. doi: 10.3126/kumj.v6i4.1738. [DOI] [PubMed] [Google Scholar]
- Eriguchi N, Matsunaga A, Tokunaga S, Futamata Y, Hanamoto Y, Tayama K, et al. Xanthogranulomatous cholecystitis mimicking gallbladder cancer: report of a case. Kurume Med J. 2001;48:321–324. doi: 10.2739/kurumemedj.48.321. [DOI] [PubMed] [Google Scholar]
- Muguruma N, Okamura S, Okahisa T, Shibata H, Ito S, Yaki K. Endoscopic sonography in the diagnosis of xanthogranulomatous cholecystitis. J Clin Ultrasound. 1999;27:347–350. doi: 10.1002/(sici)1097-0096(199907/08)27:6<347::aid-jcu7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Kim PN, Ha HK, Kim YH, Lee MG, Kim MH, Auh YH. US findings of xanthogranulomatous cholecystitis. Clin Radiol. 1998;53:290–292. doi: 10.1016/s0009-9260(98)80129-3. [DOI] [PubMed] [Google Scholar]
- Goshima S, Chang S, Wang JH, Kanematsu M, Bae KT, Federle MP. Xanthogranulomatous cholecystitis: diagnostic performance of CT to differentiate from gallbladder cancer. Eur J Radiol. 2010;74:e79–e83. doi: 10.1016/j.ejrad.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Kim P, Lee S, Kim J, Ha H, Lee Y, Lee M, et al. Xanthogranulomatous cholecystitis: radiologic findings with histologic correlation that focuses on intramural nodules. AJR Am J Roentgenol. 1999;172:949–953. doi: 10.2214/ajr.172.4.10587127. [DOI] [PubMed] [Google Scholar]
- Hanada K, Nakata H, Nakayama T, Tsukamoto Y, Terashima H, Kuroda Y, et al. Radiologic findings in xanthogranulomatous cholecystitis. AJR Am J Roentgenol. 1987;148:727–730. doi: 10.2214/ajr.148.4.727. [DOI] [PubMed] [Google Scholar]
- Delamarre J, Capron JP, Sevenet F, Quénum C, Rémond A, Besson P, et al. Xanthogranulomatous cholecystitis. X-ray computed tomographic study of a pseudotumoral form. Gastroenterol Clin Biol. 1985;9:732–737. [PubMed] [Google Scholar]
- Chang BJ, Kim SH, Park HY, Lim SW, Kim J, Lee KH, et al. Distinguishing xanthogranulomatous cholecystitis from the wall-thickening type of early-stage gallbladder cancer. Gut Liver. 2010;4:518–523. doi: 10.5009/gnl.2010.4.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi A, Singh DK, Sakhuja P, Gondal R. Florid xanthogranulomatous cholecystitis masquerading as invasive gallbladder cancer leading to extensive surgical resection. Indian J Pathol Microbiol. 2010;53:144–147. doi: 10.4103/0377-4929.59209. [DOI] [PubMed] [Google Scholar]
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- Reyes CV, Jablokow VR, Reid R. Xanthogranulomatous cholecystitis: report of seven cases. Am Surg. 1981;47:322–325. [PubMed] [Google Scholar]
- Karabulut Z, Besim H, Hamamci O, Bostanoğlu S, Korkmaz A. Xanthogranulomatous cholecystitis. Retrospective analysis of 12 cases. Acta Chir Belg. 2003;103:297–299. doi: 10.1080/00015458.2003.11679427. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Sharma A, Kapoor VK, Nagana Gowda GA. Stones from cancerous and benign gallbladders are different: a proton nuclear magnetic resonance spectroscopy study. Hepatol Res. 2008;38:997–1005. doi: 10.1111/j.1872-034X.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- Mori M, Watanabe M, Sakuma M, Tsutsumi Y. Infectious etiology of xanthogranulomatous cholecystitis: immunohistochemical identification of bacterial antigens in the xanthogranulomatous lesions. Pathol Int. 1999;49:849–852. doi: 10.1046/j.1440-1827.1999.00953.x. [DOI] [PubMed] [Google Scholar]
- Sawada S, Harada K, Isse K, Sato Y, Sasaki M, Kaizaki Y, et al. Involvement of Escherichia coli in pathogenesis of xanthogranulomatous cholecystitis with scavenger receptor class A and CXCL16-CXCR6 interaction. Pathol Int. 2007;57:652–663. doi: 10.1111/j.1440-1827.2007.02154.x. [DOI] [PubMed] [Google Scholar]
- Agrawal V, Goel A, Krishnani N, Pandey R, Agrawal S, Kapoor VK. P53, carcinoembryonic antigen and carbohydrate antigen 19.9 expression in gall bladder cancer, precursor epithelial lesions and xanthogranulomatous cholecystitis. J Postgrad Med. 2010;56:262–266. doi: 10.4103/0022-3859.70933. [DOI] [PubMed] [Google Scholar]
- Jayalakshmi K, Sonkar K, Behari A, Kapoor VK, Sinha N. Lipid profiling of cancerous and benign gallbladder tissues by 1H NMR spectroscopy. NMR Biomed. 2010;4:335–342. doi: 10.1002/nbm.1594. [DOI] [PubMed] [Google Scholar]
- Krishna RP, Kumar A, Singh RK, Sikora S, Saxena R, Kapoor VK. Xanthogranulomatous inflammatory strictures of extrahepatic biliary tract: presentation and surgical management. J Gastrointest Surg. 2008;12:836–841. doi: 10.1007/s11605-008-0478-y. [DOI] [PubMed] [Google Scholar]
- Hijioka S, Mekky MA, Bhatia V, Sawaki A, Mizuno N, Hara K, et al. Can EUS-guided FNA distinguish between gallbladder cancer and xanthogranulomatous cholecystitis? Gastrointest Endosc. 2010;72:622–627. doi: 10.1016/j.gie.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Kwon A-H, Sakaida N. Simultaneous presence of xanthogranulomatous cholecystitis and gallbladder cancer. J Gastroenterol. 2007;42:703–704. doi: 10.1007/s00535-007-2072-6. [DOI] [PubMed] [Google Scholar]
- Fligiel S, Lewin KJ. Xanthogranulomatous cholecystitis: case report and review of the literature. Arch Pathol Lab Med. 1982;106:302–304. [PubMed] [Google Scholar]
- Solanki RL, Arora HL, Gaur SK, Anand VK, Gupta R. Xanthogranulomatous cholecystitis (XGC): a clinicopathological study of 21 cases. Indian J Pathol Microbiol. 1989;32:256–260. [PubMed] [Google Scholar]
- Duca S, Bãlã O, Al-Hajjar N, Lancu C, Puia IC, Munteanu D, et al. Laparoscopic cholecystectomy: incidents and complications. A retrospective analysis of 9542 consecutive laparoscopic operations. HPB. 2003;5:152–158. doi: 10.1080/13651820310015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew C. Main procedures and interventions: 4 character. 2011. pp. 2010–2011.
- Antonakis P, Alexakis N, Mylonaki D, Leandros E, M Konstadoulakis M, Zografos G, et al. Incidental finding of gallbladder carcinoma detected during or after laparoscopic cholecystectomy. Eur J Surg Oncol. 2003;29:358–360. doi: 10.1053/ejso.2002.1402. [DOI] [PubMed] [Google Scholar]
- Contini S, Dalla Valle R, Zinicola R. Unexpected gallbladder cancer after laparoscopic cholecystectomy: an emerging problem? Reflections on four cases. Surg Endosc. 1999;13:264–267. doi: 10.1007/s004649900959. [DOI] [PubMed] [Google Scholar]
- Csendes A, Becerra M, Smok G, Medina E, Maluenda F, Morales E. [Prevalence of gallbladder neoplasms in cholecystectomies] Rev Med Chil. 1991;119:887–890. [PubMed] [Google Scholar]
- Genç V, Kirimker EO, Akyol C, Kocaay F, Karabörk A, Tüzüner A, et al. Incidental gallbladder cancer diagnosed during or after laparoscopic cholecystectomy in members of the Turkish population with gallstone disease. Turk J Gastroenterol. 2011;2011:513–516. doi: 10.4318/tjg.2011.0250. [DOI] [PubMed] [Google Scholar]
- Morera Ocón FJ, Ballestín Vicente J, Ripoll Orts F, Landete Molina F, García-Granero Ximénez M, Millán Tarín J, et al. [Gallbladder cancer in a regional hospital] Cir Esp. 2009;86:219–223. doi: 10.1016/j.ciresp.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Khan MA, Khan RA, Siddiqui S, Maheshwari V. Occult carcinoma of gallbladder: incidence and role of simple cholecystectomy. JK Pract. 2007;14:22–23. [Google Scholar]
- Mittal R, Jesudason MR, Nayak S. Selective histopathology in cholecystectomy for gallstone disease. Indian J Gastroenterol. 2010;29:211. doi: 10.1007/s12664-010-0056-6. [DOI] [PubMed] [Google Scholar]
- Tantia O, Jain M, Khanna S, Sen B. Incidental carcinoma gall bladder during laparoscopic cholecystectomy for symptomatic gall stone disease. Surg Endosc. 2009;23:2041–2046. doi: 10.1007/s00464-008-9950-8. [DOI] [PubMed] [Google Scholar]
- Shrestha R, Tiwari M, Ranabhat SK, Aryal G, Rauniyar SK, Shrestha HG. Incidental gallbladder carcinoma: value of routine histological examination of cholecystectomy specimens. Nepal Med Coll J. 2010;12:90–94. [PubMed] [Google Scholar]
- Ghimire P, Yogi N, Shrestha BB. Incidence of incidental carcinoma gall bladder in cases of routine cholecystectomy. Kathmandu Univ Med J (KUMJ) 2011;9:3–6. doi: 10.3126/kumj.v9i2.6278. [DOI] [PubMed] [Google Scholar]
- Samad A. Gall bladder carcinoma in patients undergoing cholecystectomy for cholelithiasis. J Pak Med Assoc. 2005;55:497–499. [PubMed] [Google Scholar]
- Mori T, Souda S, Hashimoto J, Yoshikawa Y, Ohshima M. Unsuspected gallbladder cancer diagnosed by laparoscopic cholecystectomy: a clinicopathological study. Surg Today. 1997;27:710–713. doi: 10.1007/BF02384982. [DOI] [PubMed] [Google Scholar]
- Zhang W-J, Xu G-F, Zou X-P, Wang W-B, Yu J-C, Wu G-Z, et al. Incidental gallbladder carcinoma diagnosed during or after laparoscopic cholecystectomy. World J Surg. 2009;33:2651–2656. doi: 10.1007/s00268-009-0218-9. [DOI] [PubMed] [Google Scholar]
- Choi SB, Han HJ, Kim CY, Kim WB, Song T-J, Suh SO, et al. Incidental gallbladder cancer diagnosed following laparoscopic cholecystectomy. World J Surg. 2009;33:2657–2663. doi: 10.1007/s00268-009-0249-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Hayakawa N, Kitagawa Y, Katohno Y, Sasaya T, Takara D, et al. Unsuspected gallbladder carcinoma after laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2005;12:391–398. doi: 10.1007/s00534-005-0996-x. [DOI] [PubMed] [Google Scholar]
- Kwon A-H, Imamura A, Kitade H, Kamiyama Y. Unsuspected gallbladder cancer diagnosed during or after laparoscopic cholecystectomy. J Surg Oncol. 2008;97:241–245. doi: 10.1002/jso.20944. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Arima Y, Yokomuro S, Yoshida H, Mamada Y, Nomura T, et al. Incidental gallbladder cancer diagnosed during and after laparoscopic cholecystectomy. J Nippon Med Sch. 2006;73:136–140. doi: 10.1272/jnms.73.136. [DOI] [PubMed] [Google Scholar]
- Yokomuro S, Arima Y, Mizuguchi Y, Shimizu T, Kawahigashi Y, Kannda T, et al. Occult gallbladder carcinoma after laparoscopic cholecystectomy: a report of four cases. J Nippon Med Sch. 2007;74:300–305. doi: 10.1272/jnms.74.300. [DOI] [PubMed] [Google Scholar]
- Weinstein D, Herbert M, Bendet N, Sandbank J, Halevy A. Incidental finding of gallbladder carcinoma. Isr Med Assoc J. 2002;4:334–336. [PubMed] [Google Scholar]
- Priya TP, Kapoor VK, Krishnani N, Agrawal V, Agarwal S. Fragile histidine triad (FHIT) gene and its association with p53 protein expression in the progression of gall bladder cancer. Cancer Invest. 2009;27:764–773. doi: 10.1080/07357900802711304. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Fernández A, Gómez-Río M, Llamas-Elvira JM, Ortega-Lozano S, Ferrón-Orihuela JA, Ramia-Angel JM, et al. Positron-emission tomography with fluorine-18-fluoro-2-deoxy-D-glucose for gallbladder cancer diagnosis. Am J Surg Pathol. 2004;188:171–175. doi: 10.1016/j.amjsurg.2003.12.070. [DOI] [PubMed] [Google Scholar]
- Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90–97. doi: 10.1016/j.gassur.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Oe A, Kawabe J, Torii K, Kawamura E, Higashiyama S, Kotani J, et al. Distinguishing benign from malignant gallbladder wall thickening using FDG-PET. Ann Nucl Med. 2006;20:699–703. doi: 10.1007/BF02984683. [DOI] [PubMed] [Google Scholar]
- Makino I, Yamaguchi T, Sato N, Yasui T, Kita I. Xanthogranulomatous cholecystitis mimicking gallbladder carcinoma with a false-positive result on fluorodeoxyglucose PET. World J Gastroenterol. 2009;15:3691–3693. doi: 10.3748/wjg.15.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C, Hurwitz JL, Schuss A, Katz DS. Radiology-pathology conference: xanthogranulomatous cholecystitis. Clin Imaging. 2003;27:421–425. doi: 10.1016/s0899-7071(02)00589-2. [DOI] [PubMed] [Google Scholar]
- Callum K, Gray A, Hoile R, Ingram G, Martin I, Sherry K, et al. 2000. The 2000 Report of the National Confidential Enquiry into Perioperative Deaths London.
- Abramson MA, Pandharipande P, Ruan D, Gold JS, Whang EE. Radical resection for T1b gallbladder cancer: a decision analysis. HPB. 2009;11:656–663. doi: 10.1111/j.1477-2574.2009.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-M, Kim B-W, Kim WH, Wang H-J, Kim MW. Clinical implication of bile spillage in patients undergoing laparoscopic cholecystectomy for gallbladder cancer. Am Surg. 2011;77:697–701. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A summary of the incidence of xanthogranulomatous cholecystitis (XGC) and associated findings overall and by geographical region reported in the 29 studies reviewed.



