Abstract
Objectives
Increasingly, surgeons are performing hepatectomies in older patients. This study was designed to analyse the incidences of and risk factors for post-hepatectomy morbidity and mortality in elderly patients.
Methods
All elective hepatectomies for the period 2005–2010 recorded in the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database were evaluated. Factors associated with 30-day rates of morbidity and mortality were compared between patients aged ≥75 years and those aged <75 years.
Results
Elderly patients accounted for 894 of 7621 (11.7%) hepatectomies. These patients more frequently had comorbidities (diabetes, cardiovascular or lung disease, lower albumin, elevated creatinine, anaesthesia risk; all P < 0.05) and were more likely to undergo partial or left rather than right or extended hepatectomies (P = 0.013). Despite the lesser surgical magnitude of these procedures, elderly patients experienced higher rates of severe complications (23.9% versus 18.4%; P < 0.001) and overall postoperative mortality (4.8% versus 2.0%; P < 0.001). The occurrence of any severe complication was associated with a mortality rate of 20.1% in elderly patients and 10.8% in non-elderly patients (P < 0.001). This disparity in mortality was more pronounced in patients with two or more (31.7% versus 20.2%; P < 0.001) and three or more (46.3% versus 31.1%; P < 0.001) severe complications. Independent risk factors for severe complications and/or mortality included an albumin level of < 4 g/dl, lung disease, intraoperative transfusion, a concurrent intra-abdominal operation, and an operative time of >240 min (all P < 0.05).
Conclusions
Given their lower physiologic reserve, elderly patients are at much greater risk for mortality after severe complications. To improve outcomes, surgeons should balance age and preoperative comorbidities with magnitude of hepatectomy.
Introduction
Over the past three decades, as post-hepatectomy mortality rates have improved, surgeons have extended the limits of tumour resectability and patient medical fitness for liver resection. Currently, expected mortality rates for all but the most complex hepatectomies are reported to be < 2.5%1 and fall to < 1% at specialized centres of excellence.2–4 These favourable outcomes are the result of improvements in patient selection,1 optimization of the future liver remnant with portal vein embolization,5 limiting preoperative chemotherapy duration,6,7 advanced surgical techniques,8 specialized perioperative care,9 personalized multidisciplinary treatment sequencing,10–13 and improved strategies for rescue in the event of complications.14 Given these advances, surgeons are increasingly considering elderly patients as potential candidates for liver surgery.
Past single-institution studies have suggested that it is safe to perform major hepatopancreatobiliary surgery in elderly patients.15,16 These reports suggested equivalent morbidity and mortality rates in elderly and matched non-elderly patients, but close inspection reveals that the similarity between study groups was the result of patient selection bias and limits on the magnitude of procedures performed in the elderly patient group.17 In contrast to these studies, larger studies based on multi-institution national databases have demonstrated that older age is an independent risk factor for morbidity and mortality after major abdominal surgery.9,18–20 Thus, the use of the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database, which samples patients from over 400 hospitals of various sizes and academic or private backgrounds, might be expected to provide a more generalizable view of surgical outcomes, potentially avoiding some of the traditional limitations and biases of single-institution studies.20–22
The hypothesis of the present study was that elderly patients, in comparison with their non-elderly counterparts, were at greater risk for post-hepatectomy morbidity and mortality. To address this question, the current study was designed to analyse national rates of post-hepatectomy morbidity and mortality in the growing population of elderly patients and to compare these with rates in non-elderly patients. Within this context, the primary study aim was to identify risk factors for morbidity and mortality in order to find potentially modifiable risk factors and to suggest methods by which surgeons might improve surgical outcomes in elderly patients undergoing hepatectomy.
Materials and methods
Data acquisition and patients
All hepatectomy procedures recorded in the ACS-NSQIP Participant Use File for 2005–2010 were identified. Emergency operations and wedge resections [current procedural terminology (CPT) code 47100] were excluded and all remaining elective hepatectomies were included for analysis. The extent of hepatectomy was classified by the primary CPT code and included the following liver resections in order of increasing magnitude: partial (CPT 47120); left (47125); right (47130), and extended (47122) hepatectomies. Risk factors for major morbidity and mortality were derived from an analysis of NSQIP-collected perioperative clinical factors, as previously described.1,23
The preoperative NSQIP-collected variables assessed included age, race, sex, weight, body mass index, albumin, haematocrit, platelets, white blood cell count, partial thrombin time, international normalized ratio, blood urea nitrogen, creatinine, alkaline phosphatase, aspartate aminotransferase, bilirubin, performance status, American Society of Anesthesiologists (ASA) class, smoking status, chronic obstructive pulmonary disease (COPD), pneumonia, sepsis, disseminated cancer, diabetes, bleeding disorder, ascites, preoperative transfusion, previous operation within 30 days, preoperative hospitalization, preoperative chemotherapy, and preoperative radiation therapy.
Intraoperative variables included in the analysis were intraoperative transfusion, extent of hepatectomy, operative time, concurrent major surgery and radiofrequency ablation. Concurrent major operations included gastrointestinal resection or anastomosis, biliary resection or reconstruction, thoracic operation, and ventral hernia repair. The definition of major operations excluded cholecystectomy, vena cava repair, diaphragm repair, lymphadenectomy and diagnostic laparoscopy.
Analysed postoperative outcomes included transfusion, bleeding transfusion (transfusion of >4 units of blood within 72 h after surgery), renal insufficiency or failure, respiratory failure, return to the operating room (ROR), cardiac arrest, stroke, coma, myocardial infarction, pneumonia, postoperative sepsis or septic shock, surgical site infection, organ space infection (OSI), fascial dehiscence, length of stay, and 30-day mortality.
Definitions
Elderly patients were defined as those aged ≥75 years; non-elderly patients were defined as those aged < 75 years. This cut-off was chosen based on past NSQIP studies on elderly cancer surgery patients, as well as in order to define a statistically analysable cohort of at least 10% of all patients based on the age distribution around the national median age of hepatectomy patients.20 Post-hepatectomy ‘severe’ complications, or major morbidity, included occurrences of the following NSQIP-collected variables: OSI; dehiscence; re-intubation; ventilator dependence or failure to wean for >48 h; acute renal insufficiency or failure; pneumonia; stroke; coma; cardiac arrest; myocardial infarction; venous thromboembolism; sepsis or septic shock; ROR, and death. In general, therapeutic interventions to manage each of these complications would classify them as being of at least Grade II and frequently as of Grade III or IV in severity.24 Based on the follow-up limits intrinsic to NSQIP data collection, post-hepatectomy mortality was defined as death within 30 days of surgery or death at a later date if the patient's hospital admission extended from surgery to the date of mortality.
Statistical analysis
The relationships between risk factors (comorbidities, extent of hepatectomy, age) and outcomes (severe complications, mortality) were compared for non-elderly and elderly patients. The chi-squared test or Fisher's exact test were used to compare categorical data. The Mann–Whitney U-test was used to compare non-parametric continuous data. After univariate analysis, significant risk factors (P < 0.05 and >1% of all patients) were entered into a multivariate logistic regression model to determine independent associations with study outcomes. Statistical analyses were performed using spss Statistics 19 (IBM Corp., Armonk, NY, USA). All tests were two-sided. Statistical significance was indicated by a P-value of < 0.05.
Results
Patients, age distribution, comorbidities and extent of hepatectomy
Of the 7621 hepatectomy patients who met the study inclusion criteria, 894 (11.7%) were aged ≥75 years; 847 (11.1%) patients were aged 70–74 years, 2135 (28.0%) were aged 60–69 years, 1920 (25.2%) were aged 50–59 years, and 1825 (23.9%) were aged < 50 years. Elderly patients were more likely to have the following characteristics: poor performance status; diabetes; dyspnoea; history of stroke; cardiopulmonary disease; lower protein levels; uraemia; elevated creatinine; thrombocytopenia, and an ASA class of ≥3 (Table 1). Only 129 of 7621 (1.7%) study patients were noted to have symptomatic chronic liver disease, including the presence of ascites and/or varices. Regarding the magnitude of surgery, elderly patients had shorter median operative times. In accordance with the differences in operative times, elderly patients were slightly less likely to undergo right or extended hepatectomies (Fig. 1). Elderly patients were more likely to undergo surgery for malignant diagnoses. However, elderly and non-elderly patients underwent similar rates of concomitant major surgery [114/894 (12.8%) versus 805/6727 (12.0%); P = 0.500]. Based on an expansion in the number of hospitals contributing data to the NSQIP over time, half of all the elderly patients in this study (447 of 894) were operated upon during 2005–2008 and represented 11.3% (447 of 3968) of all liver resection patients. The other half were operated upon during 2009–2010 and accounted for 12.2% (447 of 3653) of all liver resection patients, which does not indicate a trend towards an increased proportion of elderly subjects in the later time period (P = 0.188).
Table 1.
Preoperative differences between elderly (≥75 years) and non-elderly (<75 years) hepatectomy patients
| Clinical characteristic | All patients | Non-elderly patients | Elderly patients | P-value | |||
|---|---|---|---|---|---|---|---|
| Patients, n (%) | 7621 | (100%) | 6727 | (88.3%) | 894 | (11.7%) | |
| Preoperative factors, n (%) | |||||||
| Race, White | 5735 | (75.3%) | 5028 | (74.7%) | 707 | (79.1%) | 0.005 |
| Gender, male | 3690 | (48.4%) | 3206 | (47.7%) | 484 | (54.1%) | <0.001 |
| Diabetes | 1223 | (16.0%) | 1012 | (15.0%) | 211 | (23.6%) | <0.001 |
| Dyspnoea | 669 | (8.8%) | 566 | (8.4%) | 103 | (11.5%) | 0.002 |
| Lack of independent function | 148 | (1.9%) | 122 | (1.8%) | 26 | (2.9%) | 0.026 |
| History of stroke | 161 | (2.1%) | 119 | (1.8%) | 42 | (4.7%) | <0.001 |
| COPD | 239 | (3.1%) | 185 | (2.8%) | 54 | (6.0%) | <0.001 |
| Previous coronary stent | 322 | (4.2%) | 233 | (3.5%) | 89 | (10.0%) | <0.001 |
| Previous cardiac surgery | 303 | (4.0%) | 216 | (3.2%) | 87 | (9.7%) | <0.001 |
| Medical hypertension | 3595 | (47.2%) | 2969 | (44.1%) | 626 | (70.0%) | <0.001 |
| Albumin < 4 g/dl | 2837 | (37.2%) | 2426 | (36.1%) | 411 | (46.0%) | <0.001 |
| INR >1.0 | 2656 | (34.9%) | 2269 | (33.7%) | 387 | (43.3%) | <0.001 |
| Haematocrit < 39% | 3435 | (45.1%) | 2987 | (44.4%) | 448 | (50.1%) | 0.001 |
| Blood urea nitrogen ≥20 mg/dl | 1205 | (15.8%) | 906 | (13.5%) | 299 | (33.4%) | <0.001 |
| Creatinine >1.3 mg/dl | 436 | (5.7%) | 333 | (5.0%) | 103 | (11.5%) | <0.001 |
| Platelets < 150 000/μl | 942 | (12.4%) | 807 | (12.0%) | 135 | (15.1%) | 0.008 |
| Disseminated cancer | 2896 | (38.0%) | 2599 | (38.6%) | 297 | (33.2%) | 0.002 |
| Bleeding disorder | 255 | (3.3%) | 214 | (3.2%) | 41 | (4.6%) | 0.028 |
| Chemotherapy within 30 days | 551 | (7.2%) | 525 | (7.8%) | 26 | (2.9%) | <0.001 |
| ASA class ≥3 | 5074 | (66.6%) | 4352 | (64.7%) | 722 | (80.8%) | <0.001 |
| Admitted ≥1 day before surgery | 701 | (9.2%) | 600 | (8.9%) | 101 | (11.3%) | 0.021 |
| Malignant (versus benign) disease | 4978 | (85.4%) | 4364 | (84.4%) | 614 | (93.2%) | <0.001 |
| Smokera | 1244 | (16.3%) | 1181 | (17.6%) | 63 | (7.0%) | <0.001 |
| Alcohol usea | 210 | (2.8%) | 195 | (2.9%) | 15 | (1.7%) | 0.036 |
| Body mass index ≥30 kg/m2a | 2865 | (37.6%) | 2607 | (38.8%) | 258 | (28.9%) | <0.001 |
| Intraoperative factors | |||||||
| Operative time, min, median (range) | 220 | (7–1029) | 223 | (7–1029) | 204 | (30–693) | <0.001 |
| Operative time >240 min, n (%) | 3283 | (43.1%) | 2954 | (43.9%) | 329 | (36.8%) | <0.001 |
| Any intraoperative transfusion, n (%) | 1514 | (26.6%) | 1319 | (26.1%) | 195 | (29.9%) | 0.043 |
| Extent of hepatectomy, n (%) | 0.038 | ||||||
| Partial | 4553 | (59.7%) | 3994 | (59.4%) | 559 | (62.5%) | |
| Left | 802 | (10.5%) | 701 | (10.4%) | 101 | (11.3%) | |
| Right | 1494 | (19.6%) | 1342 | (19.9%) | 152 | (17.0%) | |
| Extended | 772 | (10.1%) | 690 | (10.3%) | 82 | (9.2%) | |
| Right/extended versus left/partial | 0.013 | ||||||
| Right/extended, n (%) | 2266 | (29.7%) | 2032 | (30.2%) | 234 | (26.2%) | |
| Left/partial, n (%) | 5355 | (70.3%) | 4695 | (69.8%) | 660 | (73.8%) | |
| Postoperative factors, n (%) | |||||||
| Postoperative pneumonia | 274 | (3.6%) | 219 | (3.3%) | 55 | (6.2%) | <0.001 |
| Re-intubation | 284 | (3.7%) | 220 | (3.3%) | 64 | (7.2%) | <0.001 |
| Ventilator >48 h | 320 | (4.2%) | 262 | (3.9%) | 58 | (6.5%) | <0.001 |
| Renal insufficiency/failure | 169 | (2.2%) | 139 | (2.1%) | 30 | (3.4%) | 0.014 |
| Urinary tract infection | 301 | (3.9%) | 252 | (3.7%) | 49 | (5.5%) | 0.012 |
| Septic shock | 232 | (3.0%) | 183 | (2.7%) | 49 | (5.5%) | <0.001 |
| Any postoperative transfusion | 494 | (6.5%) | 410 | (6.1%) | 84 | (9.4%) | <0.001 |
| Venous thromboembolism | 210 | (2.8%) | 176 | (2.6%) | 34 | (3.8%) | 0.042 |
| Any severe complication, n (%) | 1450 | (19.0%) | 1236 | (18.4%) | 214 | (23.9%) | <0.001 |
| Postoperative LoS, days, median (range) | 6 | (1–138) | 6 | (1–120) | 7 | (1–138) | <0.001 |
| Death within 30 days, n (%) | 177 | (2.3%) | 134 | (2.0%) | 43 | (4.8%) | <0.001 |
The incidence of these risk factors was lower in the elderly group compared with the non-elderly group.
Not significant: year of operation; sodium; white blood cells; partial thrombin time; total bilirubin; aspartate aminotransferase; alkaline phosphatase; recent sepsis; ascites; steroids; preoperative open wound; preoperative transfusion; operation in preceding 30 days; chief resident involvement; preoperative weight loss of >10%; other abdominal organ operation; additional radiofrequency ablation; any surgical site infection or wound disruption; dehiscence; return to operating room, and organ space infection.
Fewer than 0.9% of all patients had the following risk factors and thus these factors were excluded from further analyses: preoperative pneumonia; ascites; varices; preoperative heart failure; preoperative myocardial infarction; preoperative angina; preoperative dialysis; preoperative transfusion of >4 units; postoperative coma, and postoperative stroke.
COPD, chronic obstructive pulmonary disease; INR, international normalized ratio; ASA, American Society of Anesthesiologists; LoS, length of stay.
Figure 1.
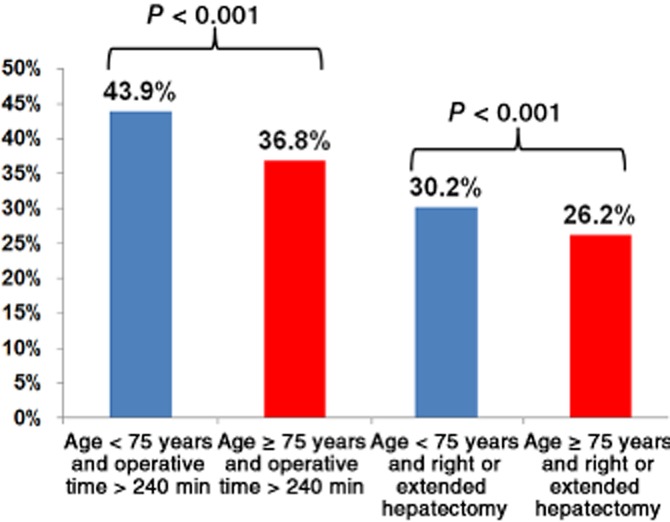
Proportions of patients aged < 75 years and ≥75 years in whom operative time was >240 min and undergoing right or extended hepatectomy, showing that operative time and magnitude of hepatectomy are lower in elderly patients
Severe complications after hepatectomy
Despite lower frequencies of comorbidities and the lesser magnitude of surgeries, elderly patients more frequently experienced severe complications (Fig. 2). Major morbidity rates were strongly associated with the magnitude of hepatectomy in elderly patients: severe complication rates for extended, right, left and partial hepatectomies were 34.1% (28/82), 32.9% (50/152), 23.8% (24/101) and 20.0% (112/559), respectively (Fig. 2). The univariate analysis of risk factors associated with severe complications in elderly patients is detailed in Table 2. In multivariate analysis, risk factors independently associated with severe complications were COPD [odds ratio (OR) 3.12, 95% confidence interval (CI) 1.53–6.33; P = 0.002], intraoperative transfusion (OR 2.43, 95% CI 1.61–3.68; P < 0.001), operative time of >240 min (OR 2.76, 95% CI 1.82–4.18; P = 0.047) and concomitant major abdominal surgery (OR 2.24, 95% CI 1.31–3.82; P = 0.003).
Figure 2.
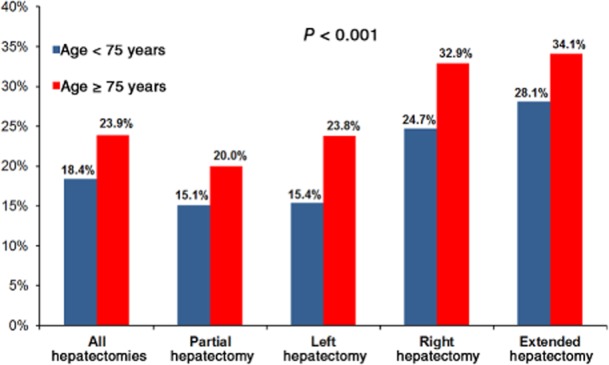
Proportions of patients affected by severe complications across all extents of hepatectomy, showing that greater age increases the risk for severe complications
Table 2.
Factors associated with severe complications in elderly (≥75 years) hepatectomy patients
| Clinical characteristic | All elderly patients | No severe complications | Severe complications | P-value | |||
|---|---|---|---|---|---|---|---|
| Patients, n (%) | 894 | (100%) | 680 | (76.1%) | 214 | (23.9%) | |
| Preoperative factors, n (%) | |||||||
| Gender, male | 484 | (54.1%) | 354 | (52.1%) | 130 | (60.7%) | 0.026 |
| COPD | 54 | (6.0%) | 34 | (5.0%) | 20 | (9.3%) | 0.020 |
| Haematocrit < 39% | 448 | (50.1%) | 328 | (48.2%) | 120 | (56.1%) | 0.045 |
| AST ≥30 IU/l | 334 | (37.4%) | 239 | (35.1%) | 95 | (44.4%) | 0.015 |
| Alkaline phosphatase >93 IU/l | 388 | (43.4%) | 275 | (40.0%) | 113 | (52.8%) | 0.001 |
| INR ≥1.2 | 98 | (11.0%) | 66 | (9.7%) | 32 | (15.0%) | 0.032 |
| White blood cells >11 000/μL | 48 | (5.4%) | 30 | (4.4%) | 18 | (8.4%) | 0.024 |
| ASA class 4 | 56 | (6.3%) | 35 | (5.1%) | 21 | (9.8%) | 0.014 |
| Admitted ≥1 day before surgery | 101 | (11.3%) | 65 | (9.6%) | 36 | (16.8%) | 0.003 |
| Malignant (versus benign) disease | 614 | (93.2%) | 472 | (92.9%) | 142 | (94.0%) | 0.630 |
| Intraoperative factors | |||||||
| Operative time, min, median (range) | 204 | (30–693) | 188 | (30–693) | 253 | (73–693) | <0.001 |
| Operative time >240 min, n (%) | 329 | (36.8%) | 207 | (30.4%) | 122 | (57.0%) | <0.001 |
| Any intraoperative transfusion, n (%) | 195 | (29.9%) | 120 | (23.8%) | 75 | (50.3%) | <0.001 |
| RBC ≥2 units, n (%) | 142 | (21.7%) | 79 | (15.7%) | 63 | (42.3%) | <0.001 |
| RBC ≥4 units, n (%) | 56 | (8.6%) | 28 | (5.6%) | 28 | (18.8%) | <0.001 |
| Extent of hepatectomy, n (%) | <0.001 | ||||||
| Partial | 559 | (62.5%) | 447 | (65.7%) | 112 | (52.3%) | |
| Left | 101 | (11.3%) | 77 | (11.3%) | 24 | (11.2%) | |
| Right | 152 | (17.0%) | 102 | (15.0%) | 50 | (23.4%) | |
| Extended | 82 | (9.2%) | 54 | (7.9%) | 28 | (13.1%) | |
| Partial versus left/right/extended, n (%) | 559 | (62.5%) | 447 | (65.7%) | 112 | (52.3%) | <0.001 |
| Right/extended versus left/partial | <0.001 | ||||||
| Right/extended, n (%) | 234 | (26.2%) | 156 | (22.9%) | 78 | (36.4%) | |
| Left/partial, n (%) | 660 | (73.8%) | 524 | (77.1%) | 136 | (63.6%) | |
| Biliary repair/reconstruction, n (%) | 57 | (6.4%) | 30 | (4.5%) | 27 | (12.7%) | <0.001 |
| Simultaneous colorectal, n (%) | 18 | (2.0%) | 5 | (0.7%) | 13 | (6.1%) | <0.001 |
| Another abdominal organ, n (%) | 115 | (12.9%) | 64 | (9.4%) | 51 | (23.8%) | <0.001 |
| Postoperative factors | |||||||
| Postoperative LoS, days, median (range) | 7 | (1–138) | 6 | (1–138) | 7 | (1–73) | <0.001 |
| Postoperative LoS ≥7 days, n (%) | 469 | (52.5%) | 286 | (42.1%) | 183 | (85.5%) | <0.001 |
| Death within 30 days, n (%) | 43 | (4.8%) | 0 | (0%) | 43 | (20.1%) | <0.001 |
Not significant: race; year of operation; body mass index; diabetes; smoking status; alcohol use; dyspnoea; preoperative independent function; preoperative stroke history; previous coronary stent/angioplasty; cardiac surgery; steroids; medical hypertension; surgical peripheral vascular disease; disseminated cancer; bleeding disorder; chemotherapy within 30 days; sodium; blood urea nitrogen; creatinine; platelets; albumin; partial thrombin time; total bilirubin; operation in preceding 30 days; chief resident involvement, and preoperative weight loss of >10%.
COPD, chronic obstructive pulmonary disease; AST, aspartate aminotransferase; INR, international normalized ratio; ASA, American Society of Anesthesiologists; RBC, packed red blood cells; LoS, length of stay.
Mortality after hepatectomy
The overall post-hepatectomy mortality rate was 2.0%. Mortality rates in all patients according to the extent of hepatectomy correlated with the magnitude of hepatectomy [extended: 4.8% (37/772); right: 3.7% (56/1494); left: 1.0% (8/802); partial: 1.7% (76/4553); P < 0.001]. Compared with non-elderly patients, elderly patients experienced a higher overall 30-day mortality rate [4.8% (43/894) versus 2.0% (134/6727); P < 0.001] (Fig. 3). The age-related mortality rate difference was most pronounced after extended [9.8% (8/82) versus 4.2% (29/690); P < 0.001] and right [9.9% (15/152) versus 3.1% (41/1342); P < 0.001] hepatectomies (Fig. 3). Mortality rates in elderly patients did not differ significantly between the early and late portions of the study period [3.8% (17/447) versus 5.8% (26/447); P = 0.160]. The univariate analysis of risk factors associated with mortality in elderly patients is documented in Table 3. Independently associated risk factors for mortality in elderly patients identified in multivariate analysis included an albumin level of < 4 g/dl (OR 2.85, 95% CI 1.20–6.74; P = 0.017), intraoperative transfusion (OR 2.37, 95% CI 1.01–5.54; P = 0.047) and operative time of >240 min (OR 4.02, 95% CI 1.60–10.06; P = 0.008).
Figure 3.
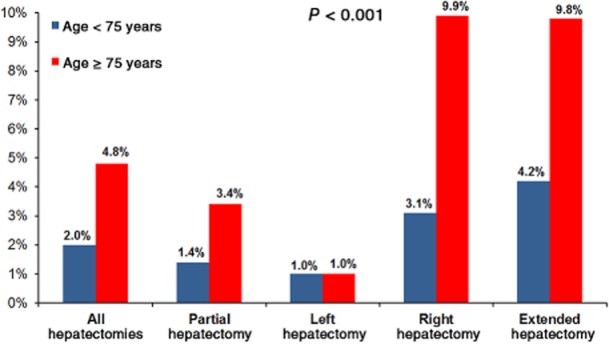
Mortality rates in patients aged < 75 years and ≥75 years showing that mortality increases with extent of hepatectomy and older patient age, with the greatest disparity observed after right and extended hepatectomies. Statistical comparison is between right/extended and partial/left hepatectomies
Table 3.
Factors associated with postoperative death in elderly (≥75 years) hepatectomy patients
| Clinical characteristic | All elderly patients | No death within 30 days | Postoperative death within 30 days | P-value | |||
|---|---|---|---|---|---|---|---|
| Patients, n (%) | 894 | (100%) | 851 | (95.2%) | 43 | (4.8%) | |
| Preoperative factors, n (%) | |||||||
| AST ≥30 IU/l | 334 | (37.4%) | 310 | (36.4%) | 24 | (55.8%) | 0.010 |
| Alkaline phosphatase >93 IU/l | 388 | (43.4%) | 359 | (42.2%) | 29 | (67.4%) | 0.001 |
| Total bilirubin >1 | 116 | (13.0%) | 104 | (12.2%) | 12 | (27.9%) | 0.003 |
| Albumin < 4 g/dl | 411 | (46.0%) | 384 | (45.1%) | 27 | (62.8%) | 0.023 |
| INR ≥1.2 | 98 | (11.0%) | 86 | (10.1%) | 12 | (27.9%) | <0.001 |
| Haematocrit < 39% | 448 | (50.1%) | 420 | (49.4%) | 28 | (65.1%) | 0.044 |
| White blood cells >11 000/μl | 48 | (5.4%) | 41 | (4.8%) | 7 | (16.3%) | 0.001 |
| Disseminated cancer | 297 | (33.2%) | 292 | (34.3%) | 5 | (11.6%) | 0.002 |
| Weight loss >10% | 49 | (5.5%) | 43 | (5.1%) | 6 | (14.0%) | 0.012 |
| ASA class 4 | 56 | (6.3%) | 49 | (5.8%) | 7 | (16.3%) | 0.005 |
| Admitted ≥1 day before surgery | 101 | (11.3%) | 90 | (10.6%) | 11 | (25.6%) | 0.002 |
| Malignant (versus benign) disease | 614 | (93.2%) | 589 | (93.2%) | 25 | (92.6%) | 0.903 |
| Intraoperative factors | |||||||
| Operative time, min, median (range) | 204 | (30–693) | 199 | (30–693) | 287 | (73–567) | <0.001 |
| Operative time >240 min, n (%) | 329 | (36.8%) | 301 | (35.4%) | 28 | (65.1%) | <0.001 |
| Any intraoperative transfusion, n (%) | 195 | (29.9%) | 178 | (28.4%) | 17 | (63.0%) | <0.001 |
| RBC ≥2 units, n (%) | 142 | (21.7%) | 129 | (20.6%) | 13 | (48.1%) | 0.001 |
| RBC ≥4 units, n (%) | 56 | (8.6%) | 48 | (7.7%) | 8 | (29.6%) | <0.001 |
| Extent of hepatectomy, n (%) | 0.001 | ||||||
| Partial | 559 | (62.5%) | 540 | (63.5%) | 19 | (44.2%) | |
| Left | 101 | (11.3%) | 100 | (11.8%) | 2 | (2.3%) | |
| Right | 152 | (17.0%) | 137 | (16.1%) | 15 | (34.9%) | |
| Extended | 82 | (9.2%) | 74 | (8.7%) | 8 | (18.6%) | |
| Partial versus left/right/extended, n (%) | 559 | (62.5%) | 540 | (63.5%) | 19 | (44.2%) | 0.011 |
| Right/extended versus left/partial | <0.001 | ||||||
| Right/extended, n (%) | 234 | (26.2%) | 211 | (24.8%) | 23 | (53.5%) | |
| Left/partial, n (%) | 660 | (73.8%) | 640 | (75.2%) | 20 | (46.5%) | |
| Biliary repair/reconstruction, n (%) | 57 | (6.4%) | 48 | (5.7%) | 9 | (20.9%) | <0.001 |
| Another abdominal organ, n (%) | 115 | (12.9%) | 103 | (12.1%) | 12 | (27.9%) | 0.003 |
| Postoperative factors | |||||||
| Any severe complication, n (%) | 214 | (23.9%) | 171 | (20.1%) | 43 | (100%) | <0.001 |
| Postoperative LoS, days, median (range) | 7 | (1–138) | 7 | (1–138) | 10 | (1–30) | 0.003 |
| Postoperative LoS ≥8 days, n (%) | 329 | (36.8%) | 304 | (35.7%) | 25 | (58.1%) | 0.003 |
Not significant: race; gender; year of operation; body mass index; diabetes; smoking status; alcohol use; dyspnoea; preoperative independent function; preoperative stroke history; chronic obstructive pulmonary disease; previous coronary stent/angioplasty; cardiac surgery; steroids; medical hypertension; surgical peripheral vascular disease; bleeding disorder; chemotherapy within 30 days; operation in preceding 30 days; sodium; blood urea nitrogen; creatinine; platelets; partial thrombin time; chief resident involvement, and radiofrequency ablation.
AST, aspartate aminotransferase; INR, international normalized ratio; ASA, American Society of Anesthesiologists; RBC, packed red blood cells; LoS, length of stay.
Failure to rescue: post-complication mortality in elderly patients
Severe complications resulted in mortality rates of 20.1% (43 of 214) in elderly patients and 10.8% (134 of 1236) in non-elderly patients (P < 0.001) (Fig. 4). As the number of severe complications increased, ability to clinically rescue patients (i.e. prevent mortality after complications) declined. As the burden (number) of severe complications increased, mortality rates were always at least 10 absolute percentage points higher in elderly patients [46.3% (37/80) versus 31.1% (105/338) in patients with three or more severe complications; P < 0.001] (Fig. 4).
Figure 4.
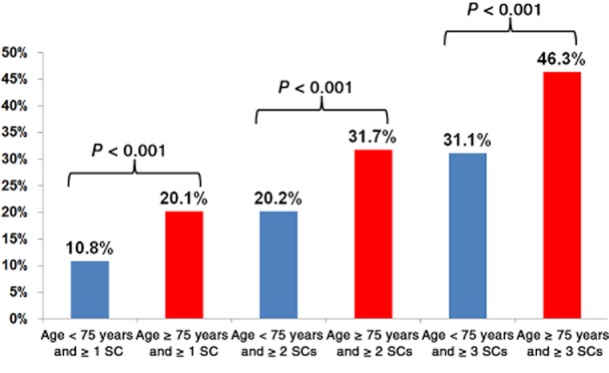
Mortality within 30 days in patients aged < 75 years and ≥75 years according to the number of severe complications (SCs) recorded. Failure to rescue worsens as the burden of severe complications increases. Mortality rates following severe complications increase with the number of concomitant severe complications and older patient age
Discussion
Several convergent factors have increased the number of elderly patients considered for hepatectomy. Firstly, according to the 2010 US Census, population numbers increased at a faster rate in older age groups than in younger age groups over the past decade and a further spike in the elderly population is anticipated as baby-boomers grow older.25 Secondly, rates of most primary and secondary liver cancers increase with age.26 Thirdly, the aforementioned advances in perioperative care and surgical technique have improved the safety of hepatic resection and expanded the definition of an operable patient.6,14,27 Given these factors, this study was designed to test the hypothesis that elderly patients are at greater risk for post-hepatectomy morbidity and mortality. This analysis confirmed that elderly patients experienced more than double the risk for 30-day mortality of non-elderly patients and that this is mostly attributable to a higher rate of mortality after serious complications.
These data differ from those of some previously published single-institution studies which concluded there was no elevation in age-related risk, but which may have been (appropriately) biased by patient selection.16,17 Through the examination of a large, multi-institution, nationwide sample of patients, the current study shows that elderly patients demonstrate higher rates of severe complications and post-hepatectomy mortality after severe complications. Although interesting, these data alone do not help to improve patient care. The aim of this study was to identify potentially modifiable risk factors that surgeons can use to improve post-hepatectomy surgical outcomes in elderly patients. The analysis of this group of elderly patients, which was largely without overt signs of advanced chronic liver disease, determined that pulmonary disease, malnutrition and various aspects of the magnitude of operation, including simultaneous major operations, transfusion needs and prolonged operative time, were independent risk factors for major morbidity and mortality.
To the extent that the patient's primary disease allows, prehabilitation and preoperative outpatient nutritional support may be modifiable factors that result in fewer complications.28,29 The issues pertaining to the magnitude of surgery are more difficult to address. The data from this study suggest that longer and more complex operations significantly raise the risk for morbidity in elderly patients. For example, a third of elderly patients suffered at least one severe complication after right or extended hepatectomy. These data add to an increasing awareness that the combination of comorbidities (both known and occult) and older age with difficult operations is a potentially volatile mixture which synergistically increases the calculated risk for poor outcomes (Fig. 5). These data indicate that, when it is oncologically feasible, surgeons should limit the magnitude of resection and avoid performing simultaneous major operations (e.g. right hepatectomy combined with proctectomy) in elderly patients.30
Figure 5.
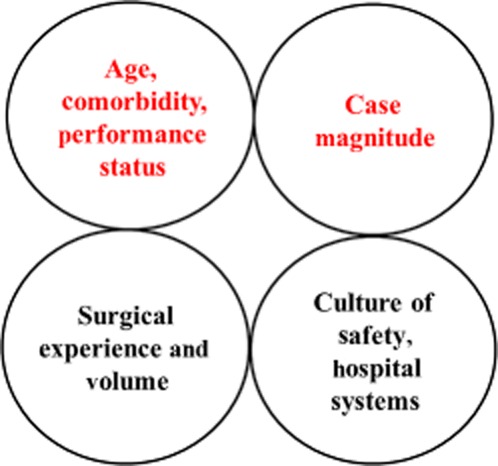
Schematic of the anatomy of a complication. On a given day, a surgeon cannot change the factors in the bottom two circles. However, the two circles at the top represent risk factors for complications which are potentially modifiable. The upper left circle reflects patient selection and medical optimization of operability. The upper right circle reflects the surgeon's judgement in choosing an appropriate magnitude of hepatectomy
A striking issue uncovered by this analysis concerns the age-related difference in the facility with which patients can be rescued after severe complications. Lack of ability to rescue older patients accounted for the majority of the disparity in 30-day death rates between elderly and non-elderly patients. Furthermore, in elderly patients mortality after severe complications rose steeply as the additive burden of severe complications increased, with patients suffering one or more, two or more and three or more complications subject to mortality rates of 20.1%, 31.7% and 46.3%, respectively. These data expose an underlying frailty in elderly patients which may not be initially apparent in the clinic, but is quickly revealed in the setting of inpatient severe complications and ultimately impairs recovery to the point of death. The implication of these findings, for both surgeons and hospitals, is that any improvement in postoperative mortality rates in elderly patients will depend on hypervigilance in the prevention of, early detection of and timely intervention upon post-hepatectomy complications. This knowledge should also be incorporated into the risk : benefit analysis the surgeon discusses with elderly patients so that these patients are better informed when they consent to undergo major hepatectomy.
The intent of this study is not to discourage surgeons from operating on elderly patients. As most surgeons anticipate that the number of operations in elderly patients will increase in the future as a result of the aforementioned population trends, the purpose of this study is to encourage careful medical workup and optimization of preoperative nutrition, conditioning and medical issues in the elderly patient population. The unique risk profile of elderly patients indicates that surgeons need to carefully balance the patient's preoperative issues with the planned extent of operation. For example, the theoretical cost savings31 achieved by performing a simultaneous proctectomy and major hepatectomy in one setting may be outweighed by the significant risks for morbidity and mortality associated with performing such a combination of procedures in an elderly patient. Although complex operations technically can be performed with little to no intraoperative mortality, surgeons should recognize that the higher incidence and multiplicity of postoperative severe complications, combined with the impaired ability of elderly patients to recover from physiologic insults, ultimately result in higher mortality rates.
The limitations of the present study include those typical of research that uses the NSQIP database to assess hepatobiliary-specific surgical outcomes. Although they were not designed for liver surgery,22 the perioperative variables collected by the NSQIP were sufficient for this study's broad analysis of post-hepatectomy morbidity and mortality in elderly patients. In addition, the scale of the patient cohort (n = 7621) reported in the NSQIP database allowed for a robust analysis of risk factors for morbidity and mortality, which is not feasible in smaller single-institution studies. Studies using national databases such as this are not necessarily better than single-institution studies, which often have more granular data on patient- and disease-specific variables, as well as data on longterm outcomes, but multi-institution registries do offer a window into morbidity and mortality rates that may be more representative of surgical practice as it occurs outside major academic institutions that are over-represented in the medical literature and that publish outcomes that may not be generalizable.
Another potential limitation of evaluating post-hepatectomy complications and deaths in the NSQIP dataset is that the data available do not support the study of events that occur beyond 30 days postoperatively.7,32 Some of the practical endpoints that have been proposed as additions to the procedure-targeted NSQIP models for hepatobiliary surgery include 90-day postoperative morbidity and mortality, return to intended oncologic therapy, return to baseline or independent function, and quality of life. It is to be hoped that the inclusion of these more patient-centric endpoints will, in the future, allow clinicians to better characterize the impact of postoperative complications on longterm outcomes that are relevant for both surgeons and patients. Despite these limitations, this NSQIP-based study was able to offer a broader ‘real world’ view of short-term morbidity and mortality in elderly patients which allowed for the development of specific recommendations regarding risk modification and quality improvement.
In conclusion, elderly patients may experience an overall mortality rate after hepatectomy that is more than twice that of non-elderly patients. This mortality differential is not completely explained by the higher rate of severe complications alone. Because they have less physiologic reserve, elderly patients who develop severe complications are at much greater risk for post-hepatectomy death, which reflects their narrow therapeutic window and low tolerance for adverse events in postoperative care. These data suggest that, to improve surgical outcomes in the elderly population, liver surgeons should develop multi-component strategies that address prehabilitation and rescue from complications, while limiting the extent and complexity of resection procedures.
Conflicts of interest
None declared.
References
- Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, Galati J, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB. 2009;11:510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres A, Toso C, Moldovan B, Schiffer E, Rubbia-Brandt L, Terraz S, et al. Complications of elective liver resections in a centre with low mortality: a simple score to predict morbidity. Arch Surg. 2011;146:1246–1252. doi: 10.1001/archsurg.2011.175. [DOI] [PubMed] [Google Scholar]
- Zimmitti G, Roses RE, Andreou A, Shindoh J, Curley SA, Aloia TA, et al. Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2628 consecutive resections. J Gastrointest Surg. 2013;17:57–64. doi: 10.1007/s11605-012-2000-9. discussion 64–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779–790. doi: 10.1097/01.RVI.0000159543.28222.73. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Zorzi D, Contreras CM, Maru DM, Kopetz S, Ribero D, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17:2870–2876. doi: 10.1245/s10434-010-1166-1. [DOI] [PubMed] [Google Scholar]
- Tzeng CW, Aloia TA. Colorectal liver metastases. J Gastrointest Surg. 2013;17:195–201. doi: 10.1007/s11605-012-2022-3. [DOI] [PubMed] [Google Scholar]
- Aloia TA, Zorzi D, Abdalla EK, Vauthey JN. Two-surgeon technique for hepatic parenchymal transection of the non-cirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172–177. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farges O, Goutte N, Bendersky N, Falissard B. Incidence and risks of liver resection: an all-inclusive French nationwide study. Ann Surg. 2012;256:697–704. doi: 10.1097/SLA.0b013e31827241d5. discussion 704–705. [DOI] [PubMed] [Google Scholar]
- Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouquet A, Mortenson MM, Vauthey JN, Rodriguez-Bigas MA, Overman MJ, Chang GJ, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934–941. doi: 10.1016/j.jamcollsurg.2010.02.039. [DOI] [PubMed] [Google Scholar]
- Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872–878. doi: 10.1002/bjs.5346. [DOI] [PubMed] [Google Scholar]
- Mentha G, Roth AD, Terraz S, Giostra E, Gervaz P, Andres A, et al. ‘Liver first’ approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg. 2008;25:430–435. doi: 10.1159/000184734. [DOI] [PubMed] [Google Scholar]
- Shindoh J, Tzeng CW, Aloia TA, Curley SA, Zimmitti G, Wei SH, et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol. 2013 doi: 10.1245/s10434-012-2864-7. doi: 10.1245/s10434-012-2864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano Y, Nojiri K, Matsuo K, Tanaka K, Togo S, Ike H, et al. The impact of advanced age on hepatic resection of colorectal liver metastases. J Am Coll Surg. 2005;201:511–516. doi: 10.1016/j.jamcollsurg.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Fong Y, Blumgart LH, Fortner JG, Brennan MF. Pancreatic or liver resection for malignancy is safe and effective for the elderly. Ann Surg. 1995;222:426–434. doi: 10.1097/00000658-199522240-00002. discussion 434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita E, Utsunomiya T, Yamashita Y, Ohta M, Tagawa T, Matsuyama A, et al. Outcome of hepatectomy in hepatocellular carcinoma patients aged 80 years and older. Hepatogastroenterology. 2012;59:1553–1555. doi: 10.5754/hge09485. [DOI] [PubMed] [Google Scholar]
- Kneuertz PJ, Pitt HA, Bilimoria KY, Smiley JP, Cohen ME, Ko CY, et al. Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg. 2012;16:1727–1735. doi: 10.1007/s11605-012-1938-y. [DOI] [PubMed] [Google Scholar]
- Lightner AM, Glasgow RE, Jordan TH, Krassner AD, Way LW, Mulvihill SJ, et al. Pancreatic resection in the elderly. J Am Coll Surg. 2004;198:697–706. doi: 10.1016/j.jamcollsurg.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Al-Refaie WB, Parsons HM, Henderson WG, Jensen EH, Tuttle TM, Vickers SM, et al. Major cancer surgery in the elderly: results from the American College of Surgeons National Surgical Quality Improvement Program. Ann Surg. 2010;251:311–318. doi: 10.1097/SLA.0b013e3181b6b04c. [DOI] [PubMed] [Google Scholar]
- Strasberg SM, Hall BL. Postoperative morbidity index: a quantitative measure of severity of postoperative complications. J Am Coll Surg. 2011;213:616–626. doi: 10.1016/j.jamcollsurg.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Pitt HA, Kilbane M, Strasberg SM, Pawlik TM, Dixon E, Zyromski NJ, et al. ACS-NSQIP has the potential to create an HPB-NSQIP option. HPB. 11:405–413. doi: 10.1111/j.1477-2574.2009.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng CW, Katz MH, Fleming JB, Pisters PW, Lee JE, Abdalla EK, et al. Risk of venous thromboembolism outweighs post-hepatectomy bleeding complications: analysis of 5651 National Surgical Quality Improvement Program patients. HPB. 2009;14:506–513. doi: 10.1111/j.1477-2574.2012.00479.x. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. 2011. Census Briefs: Age and Sex Composition 2010 Available at http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf (last accessed 14 March 2013)
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY, et al. Kinetic growth rate after portal vein embolization predicts post-hepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201–209. doi: 10.1016/j.jamcollsurg.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga M, Gianotti L, Vignali A, Schmid A, Nespoli L, Di Carlo V. Hospital resources consumed for surgical morbidity: effects of preoperative arginine and omega-3 fatty acid supplementation on costs. Nutrition. 2005;21:1078–1086. doi: 10.1016/j.nut.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132:805–814. doi: 10.1067/msy.2002.128350. [DOI] [PubMed] [Google Scholar]
- Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- Abbott DE, Cantor SB, Hu CY, Aloia TA, You YN, Nguyen S, et al. Optimizing clinical and economic outcomes of surgical therapy for patients with colorectal cancer and synchronous liver metastases. J Am Coll Surg. 2012;215:262–270. doi: 10.1016/j.jamcollsurg.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]


